Abstract
We describe a transgenic mouse line, Pax8-rtTA, which, under control of the mouse Pax8 promoter, directs high levels of expression of the reverse tetracycline–dependent transactivator (rtTA) to all proximal and distal tubules and the entire collecting duct system of both embryonic and adult kidneys. Using crosses of Pax8-rtTA mice with tetracycline-responsive c-MYC mice, we established a new, inducible model of polycystic kidney disease that can mimic adult onset and that shows progression to renal malignant disease. When targeting the expression of transforming growth factor-β1 to the kidney, we avoided early lethality by discontinuous treatment and successfully established an inducible model of renal fibrosis. Finally, a conditional knockout of the gene encoding tuberous sclerosis complex-1 was achieved, which resulted in the early outgrowth of giant polycystic kidneys reminiscent of autosomal recessive polycystic kidney disease. These experiments establish Pax8-rtTA mice as a powerful tool for modeling renal diseases in transgenic mice.
Conditional gene expression systems, such as the tetracycline-dependent (Tet) system1–3, provide an exceptional way for studying adult organ physiology in gene-modified mice by exerting stringent control on transgene expression and circumventing any developmental defect or embryonic or early postnatal lethality.
The Tet system consists of a tetracycline-dependent transactivator (tTA) and its associated promoter Ptet. The transactivator binds and activates Ptet, but its binding is directly controlled by tetracycline or the analog doxycycline. In the Tet-off system, tetracycline prevents binding of tTA to Ptet, whereas tetracycline is required for binding of the reverse tTA (rtTA) in the Tet-on system.
To model clinically relevant renal diseases such as polycystic kidney disease (PKD), renal cancer or fibrosis, we set out to adapt the Tet-on system for specific use in renal tubular cells. We considered the promoter of the Pax8 gene as a candidate promoter, because Pax8 is highly expressed in both fetal and adult kidney4,5 but is virtually absent from all other major organs except for the thyroid gland5.
Here we describe the use of genetic control elements derived from the mouse Pax8 locus to target the expression of rtTA to the kidney. Pax8-rtTA–transgenic mice deliver high levels of transgene expression in a uniform and tetracycline-dependent manner to virtually all renal tubular epithelial cells. We show the usefulness of the Pax8-rtTA transgenic platform in three different settings: overexpression of c-MYC, overexpression of transforming growth factor-β1 (Tgf-β1) and conditional knockout of the gene encoding tuberous sclerosis complex-1 (Tsc1). These experiments establish Pax8-rtTA mice as a unique tool for acute and chronic modulation of renal tubular function in vivo.
RESULTS
Generation of Pax8-rtTA mice
We isolated a 5,636–base pair fragment from the mouse Pax8 gene that included 4.3 kilobases (kb) of the putative promoter along with complete exon 1, intron 1, exon 2 and part of intron 2. We modified the region around the translation initiation codon, amplified rtTA2s-M2 (ref. 6) by PCR and inserted it into the Pax8 promoter construct (Supplementary Fig. 1 online).
After pronuclear injection of the 6.6-kb Pax8-rtTA insert DNA, we identified two transgenic founders among 72 offspring mice. Only one of those lines, designated Pax8-rtTA, transmitted the transgene and is described here.
Using multicolor fluorescence in situ hybridization (M-FISH) and two-color FISH analysis, we mapped the site of integration of the Pax8-rtTA construct to mouse chromosome 8 band B2 (Supplementary Fig. 2 online). We then used ligation-mediated PCR similar to detection of integrated papillomavirus sequences (DIPS)-PCR assay7 to amplify the fusion region between integrated Pax8-rtTA and adjacent genomic DNA (Supplementary Methods online). Sequence analysis of 14 individual DIPS products allowed us to deduce that there were five to six copies of the Pax8-rtTA construct integrated into a mouse L1 Line repetitive element (data not shown).
Functional Pax8-rtTA activity in vivo
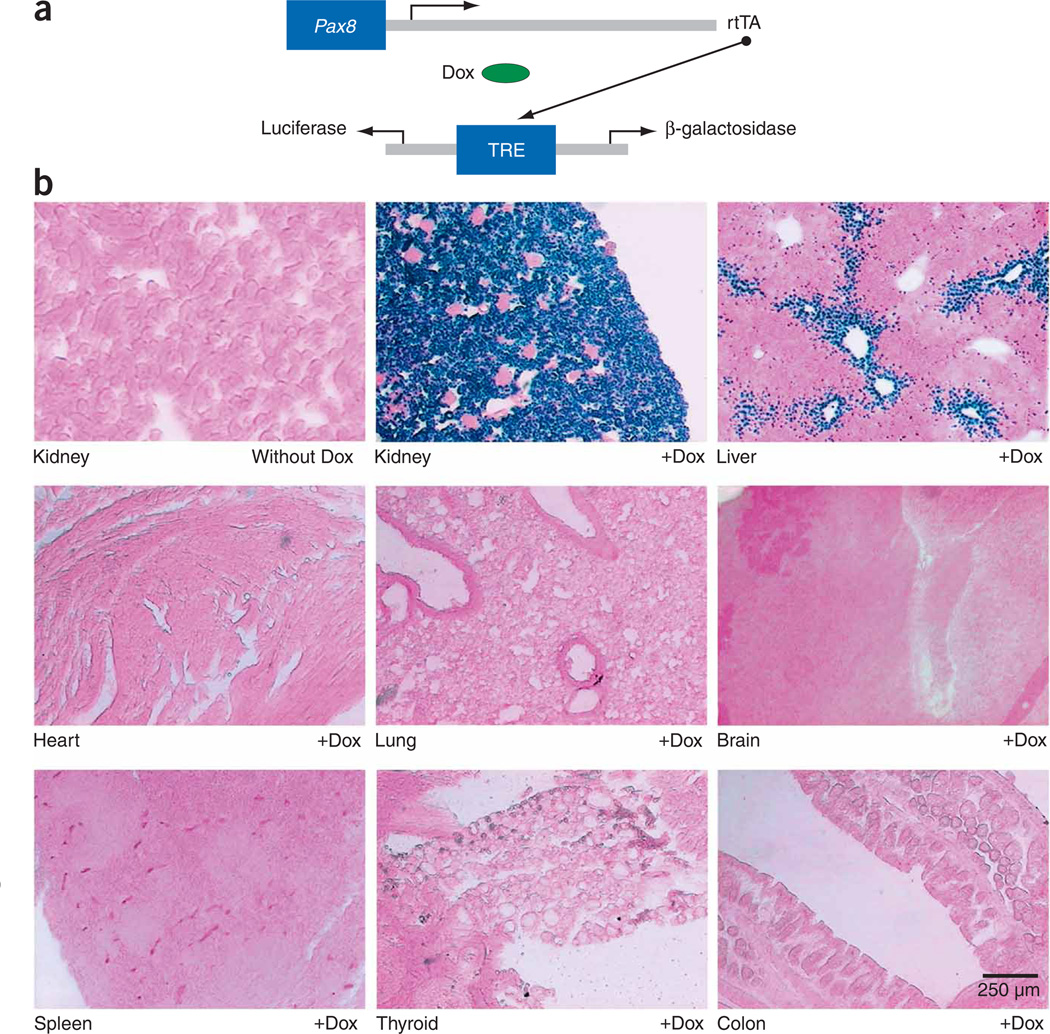
To show functional expression of rtTA in vivo, we crossbred Pax8-rtTA mice with NZL-2 mice, a line with a tetracycline-responsive reporter encoding nuclear β-galactosidase (Fig. 1a). Doxycycline was given to double-transgenic (Pax8-rtTA/NZL-2) mice at 12 weeks of age for 10 d. The kidneys of treated mice but not of control mice showed strong β-galactosidase expression in all renal tubular cells (Fig. 1b). Within the liver, β-galactosidase expression was found in a small subset of hepatocytes that were located periportally. However, no expression was detected in the remaining hepatocytes or in any other tissue tested, including heart, lung, brain, spleen, thyroid and colon (Fig. 1b).
Figure 1.
Pax8-rtTA–mediated, doxycycline-controlled β-galactosidase expression in adult tissues. (a) The Pax8 promoter directs the expression of rtTA, which, in the presence of doxycycline (Dox), binds and transactivates the tetracycline-responsive element (TRE). The TRE is a bidirectional Ptet and allows the tetracycline-controlled expression of two target genes at a time. In the case of NZL-2 mice, these are two reporter genes encoding luciferase and nuclear β-galactosidase. (b) Enzymatic X-gal staining of cryosections of kidneys derived from Pax8-rtTA/NZL-2 mice either uninduced (without Dox) or induced with doxycycline over a period of 10 d (+Dox). β-galactosidase positivity was strictly dependent on the prior administration of Dox, and it was exclusively found in the tubular epithelium of the kidney, leaving glomeruli and blood vessels unstained. Some periportally located hepatocytes in the liver also stained positively; all other major organs, including the thyroid gland, were negative.
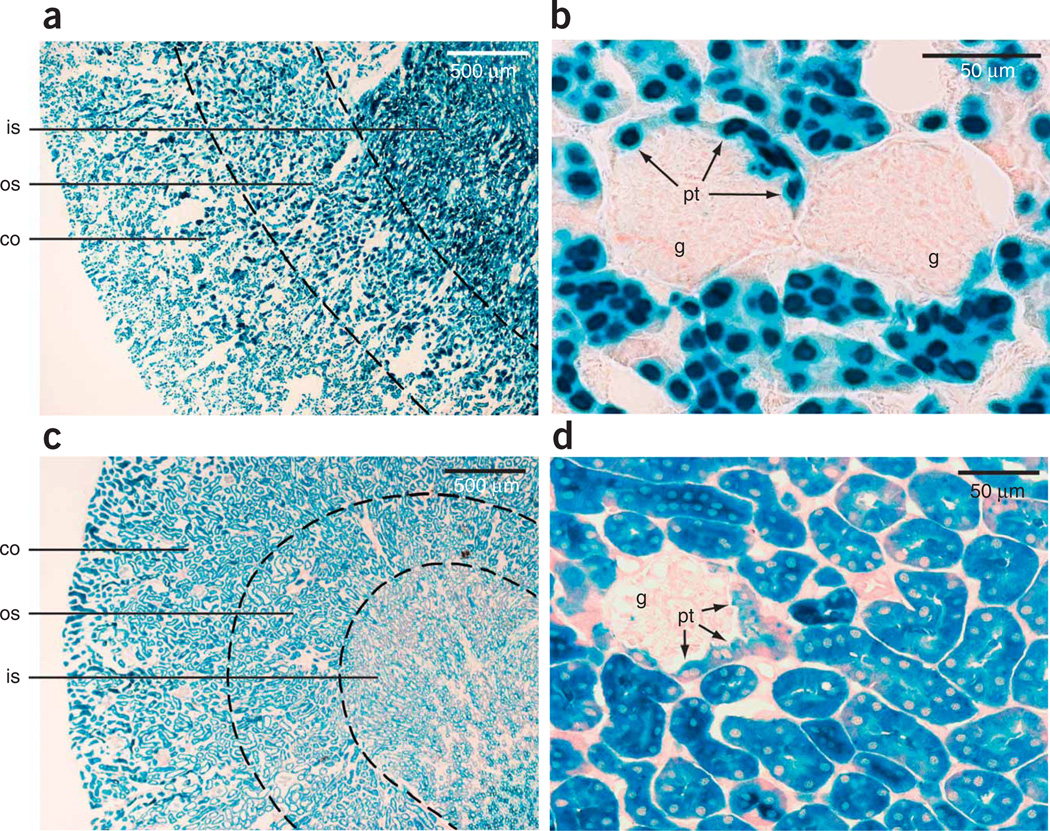
Closer examination of the kidney revealed uniform β-galactosidase positivity in the renal cortex along all proximal and distal tubules and all collecting ducts (Fig. 2a). Additionally, in the medulla, tubules and collecting ducts invariably stained positively (Fig. 2a). In contrast, parietal and visceral epithelial cells of glomeruli, mesangial cells and blood vessels did not stain (Fig. 2b). Only proximal tubular epithelial cells, when extending into the glomerular parietal epithelium, conferred β-galactosidase positivity to glomeruli (Fig. 2b).
Figure 2.
Pax8-rtTA–mediated, doxycycline-controlled β-galactosidase expression in adult renal tubular cells. (a) β-galactosidase positivity (nuclear staining) in Pax8-rtTA/NZL-2 double-transgenic mice induced with doxycycline was found in all renal tubular epithelial cells, including cortical and medullary regions. (b) Parietal and visceral epithelial cells, as well as mesangial and endothelial cells, of the glomeruli were unstained. Only proximal tubular epithelial cells in a parietal position stained positively in Pax8-rtTA/NZL-2 mice. (c,d) A virtually identical staining pattern to that in b in Pax8-rtTA/LC-1/Rosa26R triple-transgenic mice induced with doxycycline. This experiment uses a transgene encoding tetracycline-inducible Cre recombinase (LC-1) and a gene encoding a cytoplasmic β-galactosidase reporter (Rosa26R). Co, cortex; os, outer stripe of medulla; is, inner stripe of medulla; g, glomerulus; pt, proximal tubular cells in parietal position.
Essentially the same pattern of activity was found in triple-transgenic Pax8rtTA/LC-1/Rosa26R mice (Fig. 2c,d), in which the expression of Cre recombinase from the LC-1 transgene8 is triggered and then activates the conditional cytosolic Rosa26 β-galactosidase reporter9.
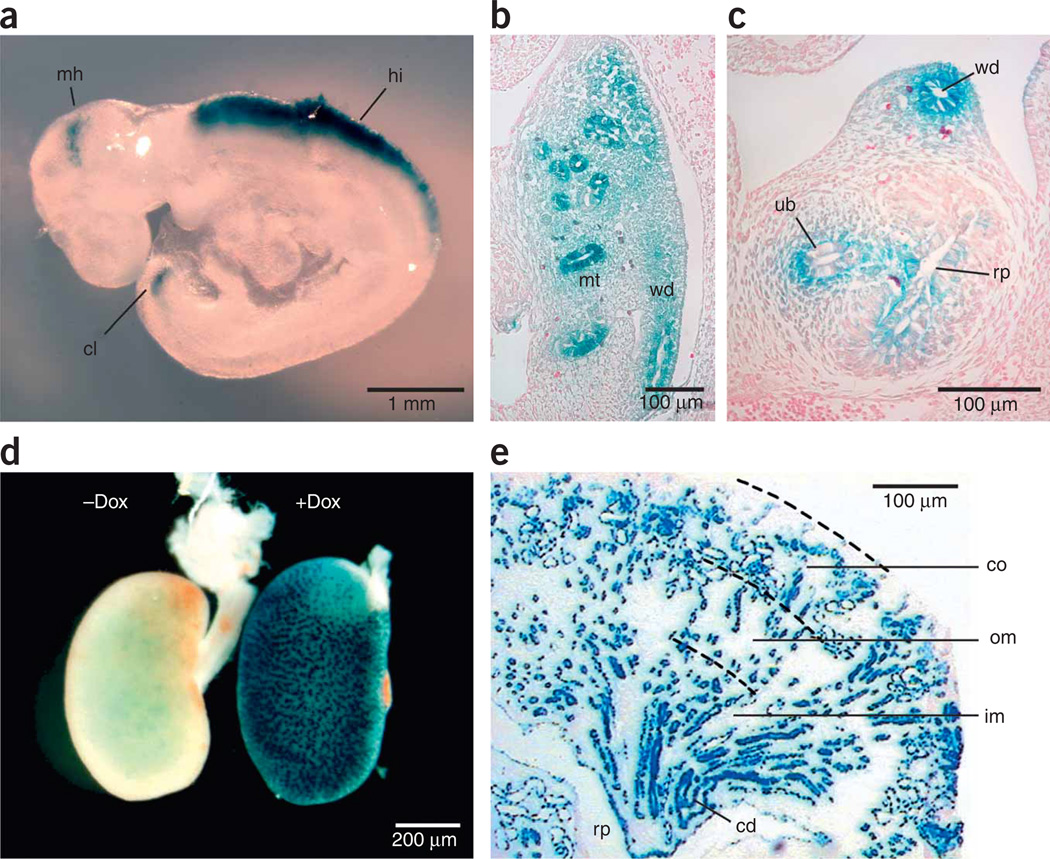
To investigate rtTA expression in mouse embryos, we mated Pax8-rtTA and NZL-2 mice under continuous treatment with doxycycline. Using whole-mount β-galactosidase staining of embryonic day 10.5 (E10.5) embryos, we found weak expression in the mid-hindbrain region and cloaca, whereas we detected strong expression in the caudal part of the hindbrain (Fig. 3a). Cross-sections from the same embryos revealed additional Pax8-rtTA activity in mesonephric tubules, the Wolffian duct and the ureteric bud invading the metanephric mesenchyme (Fig. 3b,c).
Figure 3.
Pax8-rtTA–mediated, doxycycline-controlled β-galactosidase expression in embryonic kidney. (a) Whole-mount embryo (E10.5) stained for β-galactosidase activity. β-galactosidase–positive cells were macroscopically visible in three locations: mid-hindbrain region (mh), hindbrain (hi) and cloaca (cl). (b) Cross-section through whole-mount embryo (E10.5) at abdominal level stained for β-galactosidase activity. β-galactosidase–positive cells were found in mesonephric tubules (mt) and Wolffian duct (wd). (c) Cross-section through whole-mount embryo (E10.5) at a more caudal level than in b. β-galactosidase–positive cells were found in Wolffian duct and the ureteric bud (ub) invading the metanephric mesenchyme. Rp, prospective renal pelvis. (d) Whole-mount embryonic kidney (E17.5) stained for β-galactosidase activity. β-galactosidase positivity was strictly dependent on the prior administration of doxycycline. (e) Longitudinal cross-section of induced embryonic kidney (E17.5) stained for β-galactosidase activity. Expression was found in maturing tubular epithelium and developing collecting ducts (cd). No β-galactosidase activity was detected in the early nephron anlagen or in developing or mature glomeruli. Om, outer medulla; im, inner medulla.
Induction of nephrogenesis within the metanephros starts in the subcapsular nephrogenic zone and maturation progresses along the cortico-medullary axis. Therefore, all stages of nephron development, including the metanephrogenic blastema, renal vesicles, comma- and S-shaped bodies and maturing glomeruli, can be found in a single section. In kidneys of E17.5 embryos, β-galactosidase activity was dependent on doxycycline (Fig. 3d), and it was seen within all stages of tubular epithelial development later than the S-shaped body stage and in all developing collecting duct cells (Fig. 3e). Metanephrogenic blastema, comma- and S-shaped bodies, and maturing glomeruli did not stain positively for β-galactosidase (Fig. 3e).
Time course of transgene induction
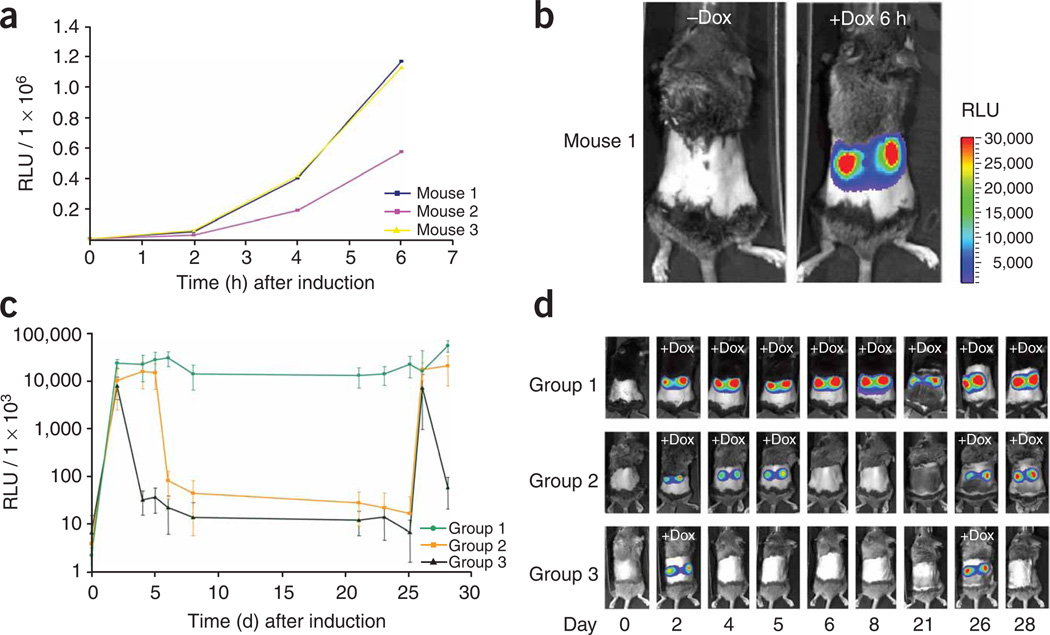
We evaluated the kinetics of transgene induction in Pax8-rtTA/LC-1 double-transgenic mice (n = 3) using a tetracycline-responsive luciferase reporter gene and noninvasive bioluminescence imaging.
In the absence of doxycycline, no luciferase activity was found (Fig. 4a). However, 2 h after the start of induction, luciferase activity (>3 × 104 relative light units (RLU); sampling time, 180 s) could be detected, and it increased by more than tenfold between 2 and 6 h (Fig. 4a). Colored overlay pictures revealed that the kidneys were the major source of the collected bioluminescence signal (Fig. 4b).
Figure 4.
Time course of transgene induction in Pax8-rtTA/LC-1–transgenic mice upon doxycycline administration. (a) Time course of luciferase induction in three individual double-transgenic mice (exposure time, 180 s). Signal intensities are plotted relative to the time after doxycycline treatment. (b) Whole-body bioluminescence of kidney-specific luciferase expression in a representative double-transgenic mouse before and after 6 h of treatment with doxycycline. The relative luminescence intensity is indicated by a color scale bar. (c) Cycle times of luciferase gene activation and deactivation via doxycycline was monitored for 28 d in Pax8-rtTA/LC-1–transgenic mice (exposure time, 1 s). The signal intensities from four mice per group were averaged and plotted relative to the time. Mice were treated with doxycycline as follows: group 1, at days 1–28; group 2, at days 1–5 and 25–28; group 3, at days 1 and 2 and 25 and 26. On day 25, treatment with doxycycline was started just after imaging. (d) One representative mouse for each of the three experimental groups in c is presented (signal intensity scale bar as in b). Data represent means ± s.d.
To study reversibility of transgene expression, we assayed bioluminescence in three groups of mice over 4 weeks (n = 4 per group). One group was given doxycycline continuously, the second and third group for periods of 5 d and 2 d, respectively. Again, the luminescent signal rapidly developed and reached maximum levels (>1 × 107 RLU; sampling time, 1 s) within 3 d in each group (Fig. 4c,d). The signal remained high as long as the mice were given doxycycline (group 1, Fig. 4c,d) but dropped sharply when treatment was stopped (groups 2 and 3, Fig. 4c,d). Multiple cycles of induction were feasible.
PKD and renal cancer induced by overexpression of c-MYC
Mice overexpressing the proto-oncogene product c-MYC in renal epithelial cells develop morphological features similar to autosomal dominant polycystic kidney disease10, but with a markedly earlier onset. To generate a model of adult onset of PKD, we crossed Pax8-rtTA mice with TetO-MYC mice, which express c-MYC under control of the Ptet11.
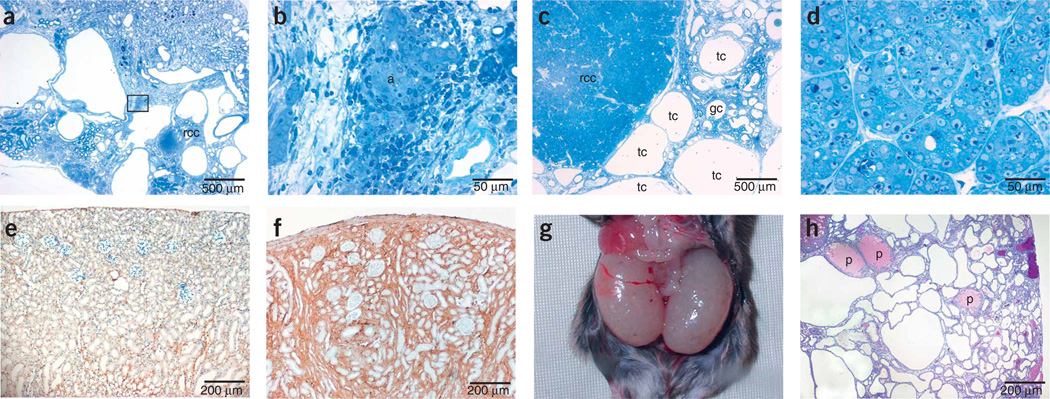
Pax8-rtTA/TetO-MYC double-transgenic mice were healthy, with no detectable phenotype. The same mice treated with doxycycline, however, rapidly developed cysts in all renal tubular compartments (Fig. 5a), leading to renal failure within 3–4 months of treatment. We also observed adenomas (Fig. 5a,b), glomerular cysts (Fig. 5c) and renal cell carcinomas (Fig. 5a,c,d) in this model.
Figure 5.
Renal disease models. (a–d) Histological analysis of polycystic kidneys showing different stages of malignant progression induced in Pax8-rtTA/tetO-MYC double-transgenic mice. Doxycycline was continuously administered to 3-month-old Pax8-rtTA/tetO-MYC double-transgenic mice over a period of 4 months. (a) Polycystic kidney with renal adenoma and renal cell carcinoma (rcc). (b) Renal adenoma (a) magnified from marked box in a. (c) Polycystic kidney with tubular (tc) and glomerular (gc) cysts and renal cell carcinoma. (d) Renal cell carcinoma cells magnified from c. (e,f) Renal fibrosis induced in Pax8-rtTA/tetO–Tgf-β1 double-transgenic mice. Pax8-rtTA/tetO–Tgf-β1 double-transgenic mice (3 months old) were treated for six consecutive cycles (2 d with doxycycline, 5 d without doxycycline). Overexpression of Tgf-β1 led to massive extracellular matrix accumulation and prominent renal fibrosis, as indicated here by collagen I immunohistochemistry in noninduced (e) or induced (f) mice. (g,h) Severe PKD induced in Pax8-rtTA–mediated, tetracycline-induced Tsc1-knockout mice. Pregnant Pax8-rtTA/LC-1/Tsc1flox/flox mice were treated with doxycycline from the first day of mating until delivery. After birth, doxycycline treatment was stopped. Giant polycystic kidneys developed in 3-week-old Pax8-rtTA/LC-1/Tsc1flox/flox offspring mice as shown in g. PAS staining in h revealed extensive cyst formation in all tubular segments and occasional protein deposits (p) caused by proteinuria.
Renal fibrosis caused by overexpression of Tgf-β1
Tgf-β1 has a key role in the onset of renal fibrosis and progression of chronic renal disease. To generate a mouse model of Tgf-β1 production in renal tubular epithelial cells, we bred Pax8-rtTA mice with TetO–Tgf-β1 mice12. Upon induction with doxycycline, double-transgenic mice became severely ill within only a few days. The kidneys were not yet severely affected in these mice, but the toxicity may result from high systemic levels of Tgf-β1, as similar toxicity has been reported with the Tet-off system in the liver13. To avoid lethality, we applied a protocol of discontinuous treatment. Mice were given doxycycline for only 2 d and then pure water for 5 d a week before they were induced again. This kind of treatment leads to maximum serum levels of 504 ng ml−1 (± 68 ng ml−1) doxycycline and complete clearance (<5 ng ml−1) within 24 h of doxycycline deprivation (assayed in nontransgenic C57BL/6 mice; data not shown). We carried out ELISAs to determine Tgf-β1 abundance. After 2 d of treatment with doxycycline, we found high amounts of Tgf-β1 in Pax8-rtTA/TetO–Tgf-β1 double-transgenicmice (plasma: 325.2 ng ml−1 (± 55.1 ng ml−1), n = 3; kidney: 4.5 ng per mg protein (± 0.4 ng per mg protein), n = 3), whereas Tgf-β1 was undetectable (<100 pg ml−1) in single-transgenic control mice or in double-transgenic mice before induction. Pax8-rtTA/TetO–Tgf-β1 mice treated for six cycles with doxycycline showed a distinct renal fibrosis phenotype, as we could show by collagen I immunohistochemistry (Fig. 5e,f). Doxycycline alone did not have any adverse effect on renal histomorphology or function even after ten cycles of treatment in control mice (data not shown).
Severe PKD in conditional knockout mice of Tsc1
We tested the ability of Pax8-rtTA mice to drive the expression of Cre recombinase in a conditional knockout model of Tsc1, one of the many genes that are embryonically lethal when constitutively inactivated in conventional knockout mice14,15.
Pax8-rtTA mice were interbred with LC-1 (ref. 8) and Tsc1flox/flox (ref. 16) mice to generate Pax8-rtTA/LC-1/Tsc1flox/flox triple-transgenic mice. Treatment with doxycycline in these experiments was confined to pregnancy and was stopped upon delivery.
In the absence of doxycycline, Pax8-rtTA/LC-1/Tsc1flox/flox mice were healthy, fertile and showed no obvious phenotype. When, however, Pax8-rtTA/LC-1/Tsc1flox/flox mice were exposed to doxycycline in utero, newborn mice showed massive hyperproliferation of proximal and distal tubules and collecting duct epithelial cells, which resulted in fulminant cyst formation. These mice died 3–4 weeks after birth with giant polycystic kidneys reminiscent of autosomal recessive polycystic kidney disease (Fig. 5g,h).
DISCUSSION
Here we report a transgenic mouse line, Pax8-rtTA, that allows kidney tubule–specific modulation of transgene expression in a highly efficient and tetracycline-dependent manner. Pax8-rtTA mice combine high activity with complete penetrance of the renal tubular compartment from early development throughout adult life. In contrast, currently available tools rely on the use of promoters that are either monospecific for distinct subcompartments, such as the kidney androgen-regulated promoter (proximal tubules)17, γ-glutamyl transpeptidase promoter (cortical tubules)18 and the aquaporin-2 and homeobox B7 promoter (collecting ducts)19,20, or are not fully penetrant, such as the Ksp-cadherin promoter21.
Pax8-rtTA mice show high organ specificity for functional rtTA expression. Extrarenal activity occurs only in a minority of hepatocytes, with all other major organs being essentially negative for rtTA activity. The absence of rtTA activity even in the thyroid gland, a known site of Pax8 gene expression22, may result from lack of a thyroid-specific enhancer in the particular Pax8 promoter fragment used.
Although Pax8-rtTA mice were derived from only one founder, the many similarities between the observed Pax8-rtTA activity and known Pax8 gene expression suggest that it is the promoter fragment and not the integration site that directs rtTA expression to the kidney. We found expression in the kidney but not in other adult organs. Within the kidney, there was expression in tubular cells, but there was an absence of expression in podocytes. During development, expression was found in mid-hindbrain, caudal hindbrain, Wolffian duct, mesonephros, metanephros and cloaca. All of these different embryonic structures are known sites of endogenous Pax8 gene expression.
Pax8-rtTA mice can be used to derive both overexpression and kidney-specific knockout models. The inducibility of the transgene helps to avoid early lethality and further allows for the study of immediate early changes caused by ectopic gene expression. The reversibility of the transgene’s expression (in overexpression models) could even make the course of cure of renal diseases accessible to medical research. Owing to the extended window of expression specified by Pax8-rtTA mice, all of these useful features can be exploited equally well in embryonic, adolescent and adult mouse kidneys.
In the present study, Pax8-rtTA mice have been successfully used to promote fibrosis, drive hyperproliferation and cause loss of gene function in a quantitative manner in the mouse kidney. These experiments identify Pax8-rtTA mice as a powerful new tool for modeling renal diseases in transgenic mice.
METHODS
Transgenic mice
Animal experimental procedures were performed according to German and European Legislation and approved by the Governing Office for Baden-Württenberg (Regierungspräsidium, Karlsruhe, Germany).
We established Pax8-rtTA transgenic mice by conventional methods (Supplementary Methods online). Pax8-rtTA mice express the gene encoding rtTA under the control of the 4.3 kb upstream regulatory region of the mouse Pax8 promoter. LC-1 is a transgenic line in which the expression of luciferase and Cre recombinase is under control of the bidirectional Ptet promoter8. NZL-2 is a reporter line similar to LC-1, but the gene encoding Cre recombinase has been replaced by a gene encoding nuclear β-galactosidase23. Rosa26R is a Cre reporter line in which a gene encoding cytosolic β-galactosidase is under control of the ubiquitously active Rosa26 promoter. However, expression is conditional on the prior removal of a neomycin expression cassette that is flanked by loxP sites9. TetO–Tgf-β1 is a transgenic line in which the expression of a constitutively active porcine Tgf-β1 is under control of the Ptet promoter12. TetO-MYC is a transgenic line in which the expression of human c-MYC is under control of the Ptet promoter11. Tsc1flox is a transgenic line harboring a wild-type allele of the Tsc1 tumor suppressor gene flanked by loxP sites15. Cre-mediated DNA excision generates a null Tsc1 allele through deletion of exons 17 and 18.
Animal housing
We housed mice in groups of up to 5. They received regular laboratory chow, and water was supplied ad libitum. We maintained a 12-h day and night cycle.
Genotyping
We prepared high molecular weight DNA from the tails of mice with the QIAamp DNA mini kit (Qiagen) and performed genotyping by PCR (Supplementary Methods).
Doxycycline administration
We exposed mice to doxycycline (Sigma) at a concentration of 0.2 mg ml−1 in drinking water supplemented with 5% sucrose. For prenatal induction, we administered doxycycline from the first day of mating on.
β-galactosidase staining
To reveal β-galactosidase activity, we prepared whole-mount E10.5 embryos or dissected tissues. We washed the samples in PBS, fixed them in ice-cold 3% paraformaldehyde for 1 h, washed them in PBS again and incubated them overnight in 18% sucrose in PBS at 4 °C. The next day, we froze the samples on dry ice and stored them at −80 °C. We incubated whole-mount embryos or 5-µm cryosections overnight in the dark in 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (X-gal) solution (50 mM Tris HCl pH 7.5, 2.5 mM potassium ferriferrocyanide, 15 mM NaCl, 1 mM MgCl2 and 0.5 mg ml−1 X-gal). We counterstained cryosections with eosin and mounted them in glycerol-gelatine.
In vivo bioluminescence imaging
We evaluated expression of the luciferase reporter with an In vivo Imaging System (Xenogen). We assayed mice before (time point = 0) and after doxycycline administration. We anesthetized the mice, removed their back fur and performed imaging after intraperitoneal application of 50 µl luciferin (30 mg ml−1 in 0.9% NaCl).
Histomorphology and immunocytochemistry
We killed mice by retrograde total body perfusion-fixation as previously described24 using 3% glutardialdehyde, 0.1 M cacodylate and 0.1% picric acid as fixative. We processed tissue by standard procedures and embedded it into EPON. We cut 1-µm sections with an Ultracut microtome (Leica) and stained as previously published25. For H&E staining, periodic acid–Schiff (PAS) staining or immunostaining, we perfused mice with 2% paraformaldehyde in PBS and immersed the organs in the same fixative for 2–4 h before embedding them in paraffin. We performed H&E and PAS staining according to standard protocols.
For the immunocytochemical detection of collagen I, we used a polyclonal rabbit antibody to rat collagen (Biotrend) at a dilution of 1:100. For detection, we applied Vectastain Elite ABC-Kit Peroxidase (Vector Labs) and diaminobenzidine as substrate-chromogen (Vector Labs).
Determination of serum doxycycline
We determined serum abundance of doxycycline by measuring the activity of a tetracycline-dependent luciferase reporter in HeLa cells (Supplementary Methods).
Determination of Tgf-β1 by enzyme-linked immunosorbent assay
We collected blood samples by intracardiac puncture into heparinized tubes after killing the mice with CO2. We centrifuged heparanized blood for 5 min and took the supernatant plasma. To obtain protein extracts from kidneys, we homogenized the tissues in PBS buffer containing 0.5 M NaCl and 0.01% Triton X-100 with an Ultra Turrax homogenizer (Janke and Kunkel). We centrifuged the homogenate for 3 min at 10,000g and collected the supernatant. We normalized results to milligrams of total protein as determined by a standard Bradford assay.
We assayed Tgf-β1 protein abundance by ELISA without acid pretreatment with commercially available kits (Promega).
Strain availability
Pax8-rtTA transgenic mice will be available for academic researchers under the stock number 7176 from The Jackson Laboratory (http://jaxmice.jax.org/query/) and under the stock number EM:02144 from the European Mouse Mutant Archive (http://www.emmanet.org/).
Statistical analyses
Quantitative data on relative light units, Tgf-β1 and doxycycline levels are provided as arithmetic means ± s.d.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Deutsche Forschungsgemeinschaft grants FOR406 (to W.K. and R.K.) and SFB 405 B10 (to H.-J.G.), by Schweizerische Forschungsstiftung Kind und Krebs (O.G.), by Prof. Dr. Karl und Gerhard Schiller-Stiftung (W.K.) and by US National Institutes of Health grants R01-CA85610, R01-CA105102, 3R01CA089305-03S1, NIH/NCI ICMIC P50 and NIH/NCI 1P20 CA112973 (D.W.F.). We thank P. Soriano (Fred Hutchinson Cancer Research Center) for providing Rosa26R mice; F. Zimmermann and S. Dlugosz for DNA microinjection; I. Voehringer, J. Charon-Alvarez, H. Hosser and B. Hahnel for technical assistance; the teams of the animal facilities at Deutsches Krebsforschungszentrum and Interfakultäre Biomedizinische Forschungseinrichtung Heidelberg for animal caretaking; T.P. Sijmonsma for skillful and expert handling of the mice; S. Wang for help with tissue preservation; M. Schorpp-Kistner and C. Gebhardt for expert advice in mouse embryology; R. Nonnenmacher for graphical work; and W.A. Grandy for carefully reading the manuscript.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
References
- 1.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gossen M, et al. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 3.Kistner A, et al. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc. Natl. Acad. Sci. USA. 1996;93:10933–10938. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poleev A, et al. PAX8, a human paired box gene: isolation and expression in developing thyroid, kidney and Wilms’ tumors. Development. 1992;116:611–623. doi: 10.1242/dev.116.3.611. [DOI] [PubMed] [Google Scholar]
- 5.Plachov D, et al. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development. 1990;110:643–651. doi: 10.1242/dev.110.2.643. [DOI] [PubMed] [Google Scholar]
- 6.Urlinger S, et al. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc. Natl. Acad. Sci. USA. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luft F, et al. Detection of integrated papillomavirus sequences by ligation-mediated PCR (DIPS-PCR) and molecular characterization in cervical cancer cells. Int. J. Cancer. 2001;92:9–17. [PubMed] [Google Scholar]
- 8.Schonig K, Schwenk F, Rajewsky K, Bujard H. Stringent doxycycline dependent control of Cre recombinase in vivo. Nucleic Acids Res. 2002;30 doi: 10.1093/nar/gnf134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 10.Trudel M, D’Agati V, Costantini F. C-myc as an inducer of polycystic kidney disease in transgenic mice. Kidney Int. 1991;39:665–671. doi: 10.1038/ki.1991.80. [DOI] [PubMed] [Google Scholar]
- 11.Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol. Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, et al. Conditional epidermal expression of TGFβ 1 blocks neonatal lethality but causes a reversible hyperplasia and alopecia. Proc. Natl. Acad. Sci. USA. 2001;98:9139–9144. doi: 10.1073/pnas.161016098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueberham E, et al. Conditional tetracycline-regulated expression of TGF-β1 in liver of transgenic mice leads to reversible intermediary fibrosis. Hepatology. 2003;37:1067–1078. doi: 10.1053/jhep.2003.50196. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi T, et al. A germ-line Tsc1 mutation causes tumor development and embryonic lethality that are similar, but not identical to, those caused by Tsc2 mutation in mice. Proc. Natl. Acad. Sci. USA. 2001;98:8762–8767. doi: 10.1073/pnas.151033798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwiatkowski DJ, et al. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum. Mol. Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 16.Meikle L, et al. A mouse model of cardiac rhabdomyoma generated by loss of Tsc1 in ventricular myocytes. Hum. Mol. Genet. 2005;14:429–435. doi: 10.1093/hmg/ddi039. [DOI] [PubMed] [Google Scholar]
- 17.Lavoie JL, Lake-Bruse KD, Sigmund CD. Increased blood pressure in transgenic mice expressing both human renin and angiotensinogen in the renal proximal tubule. Am. J. Physiol. Renal Physiol. 2004;286:F965–F971. doi: 10.1152/ajprenal.00402.2003. [DOI] [PubMed] [Google Scholar]
- 18.Sepulveda AR, Huang SL, Lebovitz RM, Lieberman MW. A 346–base pair region of the mouse γ-glutamyl transpeptidase type II promoter contains sufficient cis-acting elements for kidney-restricted expression in transgenic mice. J. Biol. Chem. 1997;272:11959–11967. doi: 10.1074/jbc.272.18.11959. [DOI] [PubMed] [Google Scholar]
- 19.Nelson RD, et al. Expression of an AQP2 Cre recombinase transgene in kidney and male reproductive system of transgenic mice. Am. J. Physiol. 1998;275:C216–C226. doi: 10.1152/ajpcell.1998.275.1.C216. [DOI] [PubMed] [Google Scholar]
- 20.Srinivas S, et al. Expression of green fluorescent protein in the ureteric bud of transgenic mice: a new tool for the analysis of ureteric bud morphogenesis. Dev. Genet. 1999;24:241–251. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<241::AID-DVG7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 21.Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J. Am. Soc. Nephrol. 2002;13:1837–1846. doi: 10.1097/01.asn.0000016444.90348.50. [DOI] [PubMed] [Google Scholar]
- 22.Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require Pax8 gene function. Nat. Genet. 1998;19:87–90. doi: 10.1038/ng0598-87. [DOI] [PubMed] [Google Scholar]
- 23.Schonig K, Bujard H. Generating conditional mouse mutants via tetracycline-controlled gene expression. Methods Mol. Biol. 2003;209:69–104. doi: 10.1385/1-59259-340-2:69. [DOI] [PubMed] [Google Scholar]
- 24.Kaissling B, Kriz W. Variability of intercellular spaces between macula densa cells: a transmission electron microscopic study in rabbits and rats. Kidney Int. Suppl. 1982;12:S9–S17. [PubMed] [Google Scholar]
- 25.Richardson KC, Jarett L, Finke EH. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.