Abstract
Cannabinoids have emerged as brain protective agents under neurodegenerative conditions. Many neuroprotective actions of cannabinoids depend on the activation of specific receptors, cannabinoid receptor type 1 (CB1R) and type 2 (CB2R). The aim of the present study was to determine whether the CB2R and CB1R agonist WIN 55,212-2 (WIN) protects neonatal brain against focal cerebral ischemia-reperfusion and whether anti-inflammatory mechanisms play a role in protection. 7-day-old rats were subjected to 90 minutes middle cerebral artery occlusion (MCAO) and injured rats identified by diffusion-weighted MRI during the occlusion. After reperfusion rats were subcutaneously administered 1mg/kg of WIN or vehicle twice daily until sacrifice. MCAO led to increased mRNA expression of CB2R (but not CB1R), chemokine receptors (CCR2 and CX3CR1) and cytokines (IL-1β and TNFα), as well as increased protein expression of chemokines MCP-1 and MIP-1α and microglial activation 24 hours after MCAO. WIN administration significantly reduced microglial activation at this point and attenuated infarct volume and microglial accumulation and proliferation in the injured cortex 72 hours after MCAO. Cumulatively, our results show that the cannabinoid agonist WIN protects against neonatal focal stroke in part due to inhibitory effects on microglia.
The endocannaboinoid system has been identified as an endogenous neuroprotective system in different animal models of acute and chronic neurodegenerative diseases (reviewed in Martínez-Orgado et al., 2007; Mechoulam et al., 2002; Sagredo et al., 2007). Numerous cannabinergic ligands have been proven to be efficacious neuroprotectants against traumatic and ischemic brain damage in adult animals (Nagayama et al., 1999; Shohami et al., 1997). Several protective mechanisms have been proposed through activation of the two major cannabinoid receptors (CBRs) in the brain, but the exact mechanisms and possible adverse effects have not been fully elucidated. Cannabinoid receptor type-1 (CB1R) is predominantly located on neurons and is involved, among many other functions, in the modulation of synaptic transmission and prevention of excitotoxicity (Kim et al., 2006; Marsicano et al., 2003). Cannabinoid receptor type-2 (CB2R) expression is induced in immune cells under pathological conditions, and its activation in the brain has been associated with the modulation of several inflammatory functions of microglia and macrophages (Baker et al., 2007; Marchalant et al, 2007; Walter et al., 2003). The combined activation of both cannabinoid receptors has been shown to exert synergistic protective effects in different models of CNS diseases, including stroke, consistent with the better prevention of neural cell death when mixed CB1R and CB2R agonists are used, rather than individual CB1R or CB2R agonists (Docagne et al., 2007; Fernández-López et al., 2006; Fernández-López et al., 2007). However, there is conflicting evidence regarding the neuroprotective versus neurotoxic effect of CB1R ligands (Sarne et al., 2011) that may limit the protective effect of the simultaneous activation of both receptors in the brain.
Arterial stroke in neonates is frequent, with an incidence of 1/2800 to 1/5000 live-births (Chabrier et al., 2011). Unfortunately there are no effective therapies for neonatal stroke (Kirton and deVeber, 2009). It is well known that neuroinflammation, in part due to microglial activation, is an important factor that modulates brain injury after neonatal stroke (reviewed in Vexler and Yenari, 2009). The mixed cannabinergic ligand WIN 55,212-2 (WIN), which binds to both CB1R and CB2R with high affinity (Pertwee, 2006), significantly reduced injury in brain slices subjected to oxygen-glucose deprivation (Fernández-López et al., 2006) and long-term brain injury in animal models of acute severe asphyxia and hypoxia-ischemia in neonatal rodents, models relevant to asphyxia in human newborns (Fernández-López et al., 2007; Fernández-López et al., 2010; Martínez-Orgado et al., 2003). We also showed that WIN enhances neural repair after neonatal hypoxia-ischemia (Fernández-López et al., 2010). However, it is unknown if long-term protective potential for WIN against brain ischemia during the neonatal period occurs via suppression of inflammation during the acute injury phase. It is also unknown if WIN can protect the developing brain against pure ischemia-reperfusion. In this study we asked if a CB1R/CB2R agonist WIN protects against arterial focal stroke in the neonate by its effects on microglial activation.
1. EXPERIMENTAL PROCEDURES
1.1. Animal model of neonatal stroke
All animal research was approved by the University of California San Francisco Institutional Animal Care and Use Committee and was performed in accordance with the Guide for the Care and Use of Laboratory Animals (U.S. Department of Health and Human Services, Publication Number 85–23, 1985). Sprague-Dawley dams with a dated litter of pups were purchased from Charles River Laboratories (Wilmington, Mass). Transient 1.5-hour right middle cerebral artery occlusion (MCAO) was performed in 7-day-old pups (P7) as previously described (Derugin et al., 2000). Sham animals were used as controls.
1.2. DWI
Spin-echo echo planar diffusion-weighted MRI (DWI) was performed during MCAO to identify injured animals (Derugin et al., 2005) only animals with injury extending throughout the MCA territory on DWI were used. Injury volume on DWI was determined in five consecutive 2-mm thick coronal sections.
1.3. Administration of WIN and 5-bromo-2′-deoxyuridine (BrdU)
Pups received first injection of either WIN (1 mg/kg/dose, Sigma-Aldrich) or vehicle (2% DMSO, 5% BSA in PBS) immediately after reperfusion, followed by a second injection 4 hours later. Then the pups were injected twice daily with WIN or vehicle until sacrifice at 24 or 72 hours after MCAO. To label proliferating cells, pups received BrdU (50 mg/kg in 0.9% saline, i.p.) in three injections given every two hours starting from the 6 hours prior to sacrifice.
1.4. Stereological determination of infarct volume
Animals were sacrificed by transcardiac perfusion with 4% paraformaldehyde in 0.1M phosphate buffer. Brains were removed, post-fixed, cryoprotected by inmersion in 30% sucrose 0.1M phosphate buffer and cut in 50 μm-thick slices using a vibratome. Eight serial brain sections per brain (350 μm apart, Bregma 2.2mm to 0.6mm) were stained with Nissl and the contralateral and non-injured ipsilateral areas were traced under a Zeiss microscope at 10x magnification using StereoInvestigator (MicroBright Fields, Inc). Volumes were estimated using the Cavalieri probe with an average coefficient of error for the estimation of (Gundersen m=0) of 0.046. Infarct size was expressed as the percentage of injured ipsilateral hemisphere corrected for brain edema, as calculated by the formula [(Volcontra−Volipsi not injured)/Volcontra] × 100
1.5. Immunofluorescence and image analysis
Free-floating brain serial sections (50 μm) were immunostained for BrdU (ABD Serotec, 1:100) and the microglial/macrophage marker Iba1 (Wako, 1:500) as previously described (González et al., 2007). A minimum of 6 images from 3 different sections per brain were captured in the ischemic core, ischemic penumbra and corresponding areas in the contralateral cortex using a 20x objective on a Zeiss microscope. The ischemic penumbra was defined based on the appearance of DWI during MCAO (Manabat et al., 2003) with additional confirmation on Nissl-stained sections adjacent to immunostained sections (Figure 1). Images were thresholded and analyzed using Openlab software (Improvision, Inc.). Data were expressed as the ipsilateral/contralateral ratio of Iba1+ cell density in the ischemic core and penumbra.
Figure 1. WIN protects the neonatal brain during the subacute injury phase after stroke.
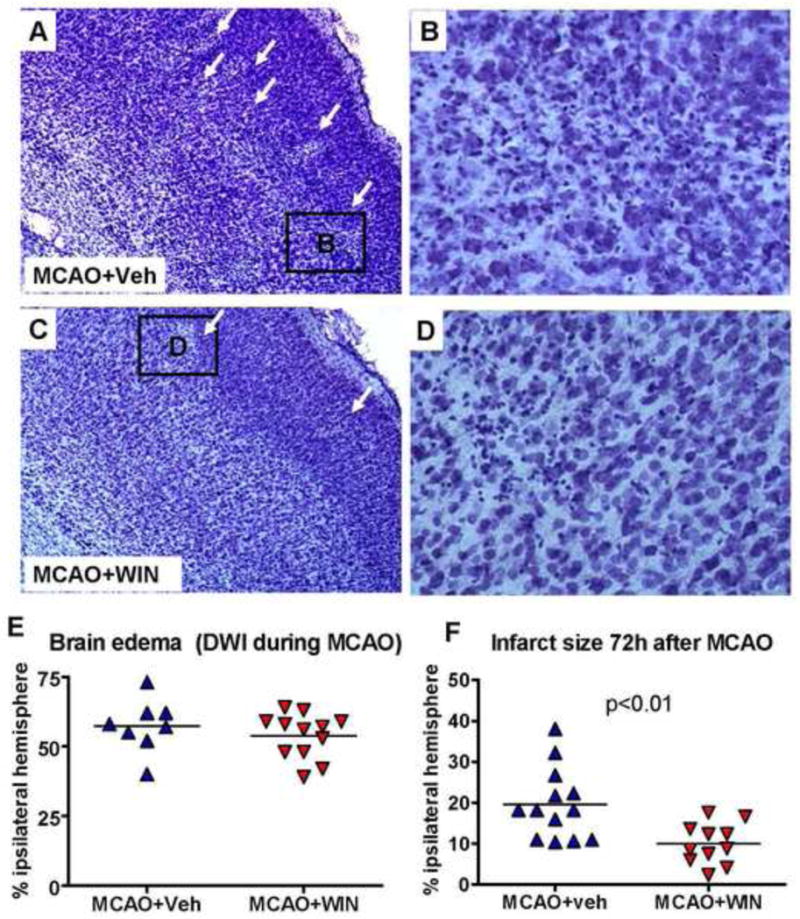
A–D: Nissl-stained sections showing a reduced severity of injury in the ischemic hemisphere (white arrows) of WIN-treated animals 72 hours after neonatal stroke (magnification 4x in A and C, 20x in B and D). E: Volumetric analysis of brain edema based on DWI performed during MCAO (n=8–12 per group). F: Volumetric analysis of brain injury (Cavalieri probe) from Nissl-stained serial sections. n=11–13 per group; Student’s t-test p<0.01.
1.6. Quantitative RT-PCR
For the analysis of gene expression by qRT-PCR animals were sacrificed by decapitation, the brains were dissected and tissue from the ischemic hemisphere and contralateral cortex was flash-frozen. Total RNA was isolated from the brain samples using RNeasy MinElute Kit (Quiagen). Quantitative reverse transcriptase-polymerase chain reaction was performed as previously described (Sobrado et al., 2009). Specific primers for rat genes were designed using Primer Express software (Applied Biosystems), and were as follows: CB2R (forward 5′AAAGCACACCAACATGTAGCCAGC; reverse 5′ ACCAGCATATGAGCAGAACAGCCA), CB1R (forward 5′ GCTGCAATCTGTTTGCTCGGACAT; reverse 5′ CACAATGAACAGCAGCAGCACACT), TNFα (forward 5′ GACCCTCACACTCAGATCATCTTCT; reverse 5′-TGCTACGACGTGGGCTACG); IL-1β (forward 5′ GACCTGTTCTTTGAGGCTGACA 3′; reverse 5′ CTCATCTGGACAGCCCAAGTC 3′); CCR2 (forward 5′ GACCGAGTGAGCTCAACATTT; reverse 5′ AACCCAACTGAGACTTCTTGC); CX3CR1 (forward 5′ CTACACAAGCGAGGGAGAGG; reverse 5′ TGGTCCGTATTTCTTGCACA); beta-actin (forward 5′-TGAGCGCAAGTACTCTGTGTGGAT; reverse 5′-TAGAAGCATTTGCGGTGCACGATG). Data were expressed as fold mRNA increase over naive animals treated with WIN (1mg/kg) or vehicle.
1.7. Western blot
Flash-frozen tissue samples from ipsilateral and contralateral brain hemispheres were processed for western blot as previously described (Fernández-López et al., 2006). Blots were incubated with anti-Iba1 (Wako, 1:500) overnight at 4°C and secondary anti-rabbit (Santa Cruz, 1:2000) 1 hour at RT.
1.8. Cytokine and chemokine measurements
Cytokine and chemokine concentrations were simultaneously quantified in the same samples from injured and contralateral brain tissue using a LINCOplex™ rat cytokine kit (LINCO Research) using Luminex100 reader (Luminex) and StatLIA® software (Brendan Scientific) with a 5-parameter logistic curve-fitting.
1.9. Statistical analysis
Data are expressed as mean ± SD. Comparisons between animal groups were performed using unpaired Student t test. p<0.05 was considered statistically significant.
2. RESULTS
2.1. WIN reduces infarct size 72 hours after neonatal stroke
The extent of injury was determined 72 hours after 90 minutes MCAO in P7 rats by stereological evaluation of infarct volume (Cavalieri probe) in Nissl-stained brain serial sections. Rats treated with WIN had a significantly smaller infarct volume than rats that received vehicle (11.04±6.02 % Vs. 19.37±9.01 % of injured hemisphere in MCAO+WIN and MCAO+veh, respectively) (figure 1A, 1B vs 1C, 1D; figure 1F). The volumes of tissue with abnormal DWI during MCAO, i.e., tissue at risk, were similar in both groups (figure 1E), indicating that WIN is protective during the reperfusion phase after neonatal stroke. The protective effect was not due to drug-induced hypothermia as rectal temperature was not affected by WIN administration (34.8±0.7 °C vs 34.6±0.3 °C on day 0; 35.5±0.8 °C vs 35.7±0.3 °C on day 1; 34.9±1.2 °C vs 35.6±1.1 °C on day 2 in vehicle- vs WIN-treated groups, respectively).
2.2. WIN limits microglial accumulation in the ischemic-reperfused cortex
To investigate whether anti-inflammatory mechanisms are involved in the protective effect of WIN, we analyzed expression of the CB receptors and density of Iba1+ cells in the injured brain. While mRNA expression of CB1R (mostly expressed in neurons) remained unchanged 24 hours after reperfusion (figure 3B), mRNA expression of CB2R, which we determined at the same time point, was strongly induced (figure 3A). Consistent with our previous observations (Denker et al., 2007), a marked increase in the expression of the microglial cell marker Iba1 occurred in the injured hemisphere of the brain at 24 hours after MCAO (n=3, figure 3C). Accumulation of activated microglia (ameboid Iba1+ cells) persisted in the ischemic core and even more in the ischemic penumbra at 72 hours after reperfusion (figure 2A–2C, 2G, 2H). Treatment with WIN partially but significantly reduced the overall expression of Iba1 (figure 3C) and accumulation of Iba1+ cells in the ischemic core and penumbra (figure 2D–2F, 2G, 2H).
Figure 3. mRNA expression of CBRs and chemokine receptors and protein expression of Iba1 24 hours after neonatal MCAO.
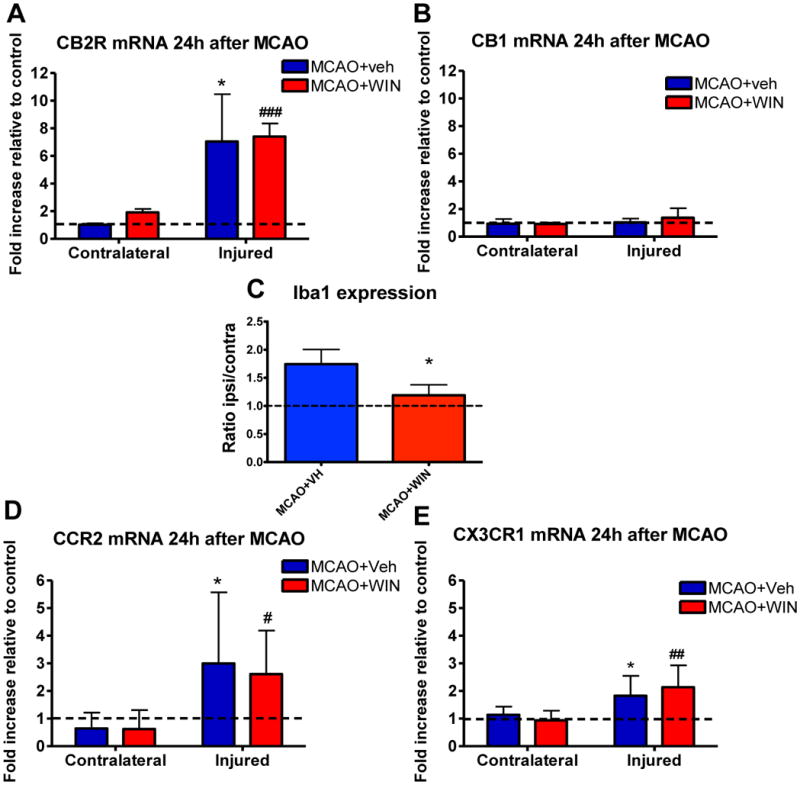
MCAO in P7 rats induced the expression of CB2R (A), but not of CB1R (B) 24 hours after neonatal stroke. Expression of CBRs was unaffected by WIN (A and B, n=5–7). WIN led to a decreased protein expression of Iba1 in the injured brain at 24 hours (C). MCAO in P7 rats induced the expression of CCR2 and CX3CR1, that was unaffected by WIN (D and E, n=5–7). Data are expressed as fold increase expression vs the corresponding naives (Veh or WIN) or the contralateral hemisphere; (*) Student’s t-test p<0.05 vs contralateral MCAO+Veh; (#, ##, ###) Student’s t-test p<0.05, p<0.01, p<0.001, respectively, vs contralateral MCAO+WIN.
Figure 2. WIN reduces the accumulation of Iba1+ microglia/macrophages in the injured cortex after neonatal MCAO.
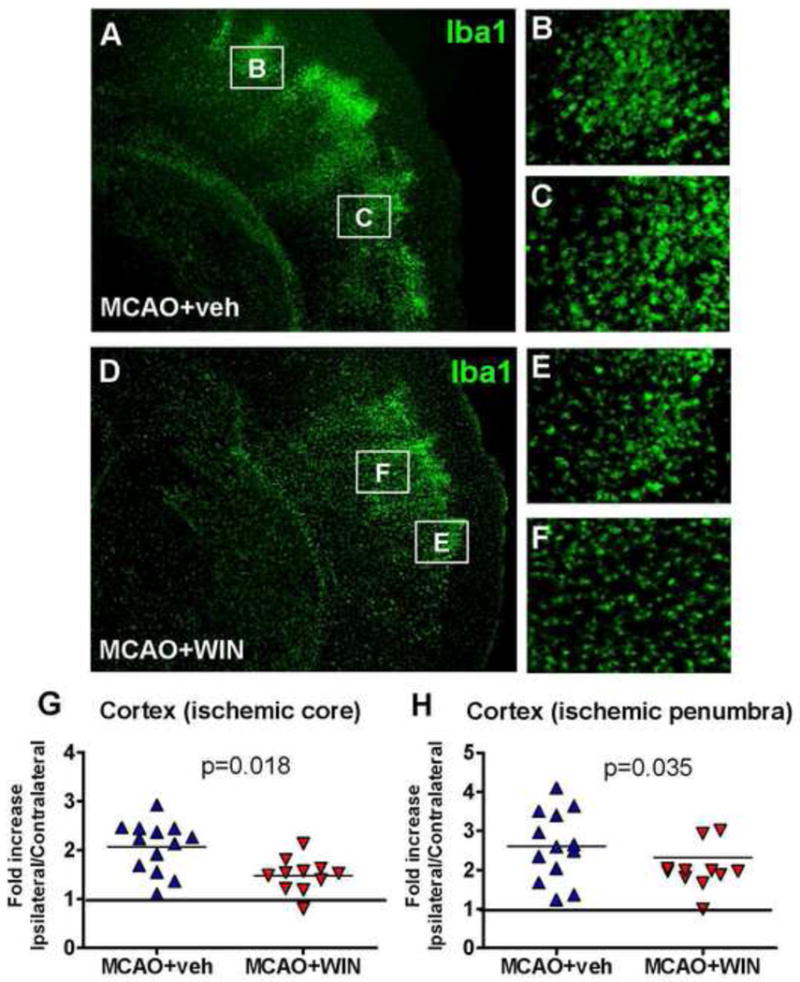
A–F: Immunofluorescence showing a reduced accumulation of Iba1+ cells in the ischemic penumbra (B, E) and ischemic core (C, F) in WIN-treated animals 72 hours after neonatal stroke (magnification 2.5x in A and D, 20x in B, C, E and F). G, H: Analysis of cortical Iba1 density in the ischemic core (G) and the ischemic penumbra (H) 72 hours after neonatal stroke. Data are expressed as fold increase vs the corresponding areas in the contralateral hemishere. n=11–13 per group; Student’s t-test p<0.05.
Considering that accumulation of Iba1+ cells can occur via multiple mechanisms, including microglial proliferation and chemotaxis, we determined whether WIN affects proliferation of microglia and expression of CX3CR1 and CCR2, two major receptors involved in chemotactic activity of these cells (Imai et al., 1997; Kuziel et al., 1997). At 72 hours we observed that, as expected, proliferation of microglia during the last 6 hours prior to sacrifice (when BrdU was administered) was markedly higher after stroke in the injured hemisphere compared to the matching contralateral areas (figure 4A–4D). Interestingly, WIN administration resulted in reduced proliferation of microglia in the ischemic core (figure 4D), indicating that WIN inhibits proliferation of these cells in the injured brain after neonatal MCAO. mRNA expression of both CX3CR1 and CCR2 was significantly increased in the ischemic hemisphere at 24 hours after neonatal MCAO (figure 3D, 3E). Although CX3CR1 and CCR2 can be expressed by both microglia and monocytes, the Iba1+ population is comprised of microglial cells, but not invading monocytes, in acutely injured neonatal brain (Denker et al., 2007), indicating that these chemotactic receptors are expressed on microglia. However, WIN did not significantly affect CX3CR1 or CCR2 expression (figures 3D–3E), suggesting that the reduced density of Iba1+ cells observed after MCAO is unlikely due to the effects on CCR2- or CX3CR1-mediated chemotaxis, but rather due to effects on the proliferation of microglia.
Figure 4. WIN reduces microglial cell proliferation in the injured cortex 72 hours after neonatal MCAO.
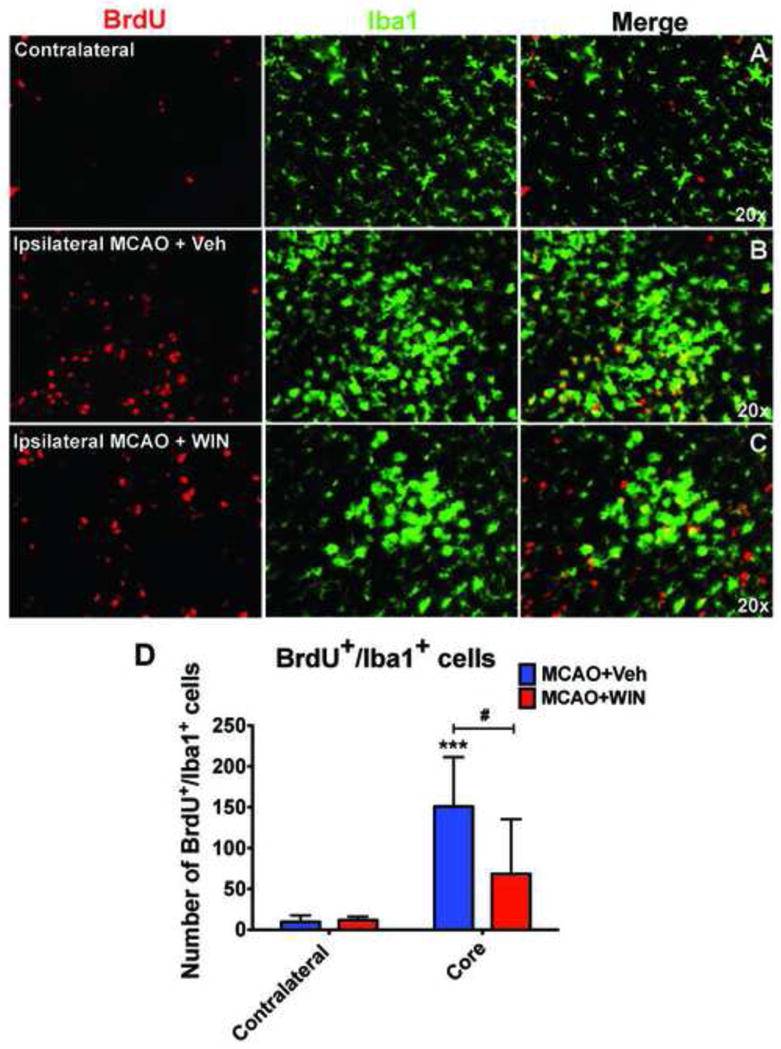
Proliferation of Iba1+ cells is increased in the injured cortex 72 hours after MCAO (A vs B). WIN led to a significant reduction in the number of proliferating Iba1+ cells in the ischemic cortex (C vs B, D). n=6–9 per group; (***) Student’s t-test p<0.001, respectively, vs contralateral MCAO+Veh; (#) Student’s t-test p<0.05 vs core MCAO+Veh.
2.3. WIN modulates expression of cytokines and chemokines in the neonatal brain after stroke
Next we explored the effect of WIN on accumulation of inflammatory mediators after neonatal stroke. We analyzed mRNA and/or protein expression of several cytokines and chemokines that are known to activate and regulate function of microglia in the ischemic brain. mRNA expression of the cytokines TNFα and IL-1β was significantly increased by the MCAO at 24 hours (figure 5A, 5B). However, protein expression of only TNFα but not IL-1β was significantly increased at this time point (figure 5C, 5D). WIN did not affect mRNA and/or protein expression of TNFα (figure 5B, 5D), but further increased mRNA expression of IL-1β (figure 5A). MIP-1α and MCP-1 mRNA were below detectable levels in both injured and uninjured brain regions but protein expression of these chemokines was significantly induced in injured brain regions (figure 5E, 5F). The plasma levels of these chemokines were similar to those observed in control animals at the same time point (data not shown). Protein expression of MIP-1α tended to be lower (n.s.) in animals treated with WIN (figure 5E). Expression of other cytokines and chemokines, which were also induced in injured brain regions, including IL-18 and GRO/KC, was not affected by the treatment (data not shown).
Figure 5. mRNA and/or protein expression of pro-inflammatory mediators 24 hours after neonatal MCAO.
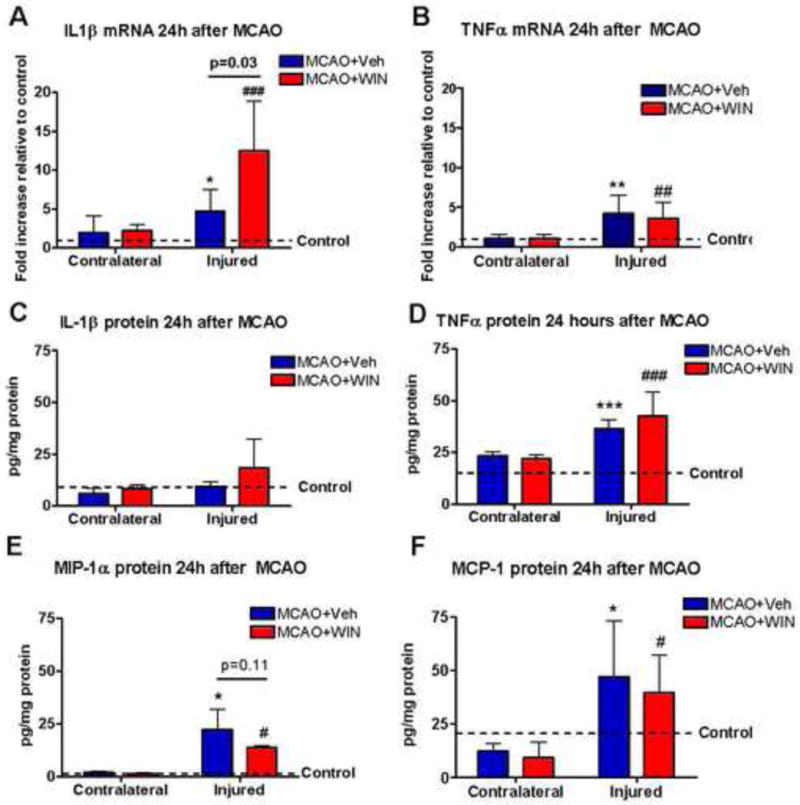
mRNA expression of IL-1β (A) and TNF (B) was induced in the ischemic brain 24 hours after MCAO. Protein expression of TNFα (D), MIP1α (E) and MCP-1 (F), but not of IL-1β (C) was also increased after MCAO. WIN increased the mRNA expression of IL-1β (A) and partially reduced the protein expression of MIP1α (E). mRNA data are expressed as fold increase expression vs the corresponding naives (Veh or WIN); protein data are expressed as concentration per mg of total protein; n=5–7 per group. (*, **, ***) Student’s t-test p<0.05, p<0.01, p<0.001, respectively, vs contralateral MCAO+veh; (#, ##, ###) Student’s t-test p<0.05, p<0.01, p<0.001, respectively, vs contralateral MCAO+WIN.
3. DISCUSSION
We show for the first time that the mixed CB1R and CB2R agonist WIN significantly reduces brain injury during the reperfusion phase after neonatal focal stroke. We also demonstrate that reduction of injury size produced by WIN is associated with decline in proliferation and accumulation of activated microglia in the injured cortex.
It is well known that expression of CB2R is up-regulated in microglia and peripheral monocytes in response to inflammatory stimuli (Maresz et al., 2005; Mukhopadhyay et al., 2006). We observed that CB2R mRNA expression is induced in the injured regions 24 hours after transient MCAO in neonatal rats. This observation is in agreement with previous reports showing increased CB2R mRNA expression at the same (or later, at 72 hours) time points after brain ischemia-reperfusion in the adult (Zhang et al., 2008; Ashton et al., 2007), and at 48 hours after neonatal hypoxia-ischemia (Fernández-López et al., 2010). In contrast to that for CB2R, the expression of CB1R remained unchanged at 24 hours in our study, consistent with the notion that CB1R mRNA expression may be induced only transiently before, as was reported after MCAO in the adult (Mukhopadhyay et al., 2006). Others, however, reported an increased CBR1 protein expression up to 72 hours in the ischemic penumbra (Jin et al., 2000).
MCAO induced TNFα, IL-1β or MCP-1 expression 24 hours after reperfusion, and WIN administration did not decrease mRNA or protein expression of these inflammatory mediators. While seems surprising, our data is consistent with results of a study in which administration of a selective CB2R agonist after transient focal ischemia in adult mice resulted in neuroprotection at 72 hours without significantly affecting the early increase in expression of TNFα, IL-1β or MCP-1 (Murikinati et al., 2010). Given that we evaluated changes of cytokine production only at one time point, we cannot rule out a possibility that cytokine production could have been influenced at earlier times. Also, based on our recent findings that these chemokines and cytokines are expressed by several cell types in the injured neonatal brain, including activated endothelium and astrocytes, and that production of these mediators continues even when microglia are depleted (Faustino et al., 2011), these cytokines can be increased at different time points in different cell types. Interestingly, IL-1β mRNA expression was further increased in our study by WIN. The effects of this cytokine in injured neonatal brain are rather complex and the mechanisms that ultimately lead to increased IL-1β mRNA following WIN administration are yet to be understood. While IL-1β may contribute to injury after neonatal hypoxia-ischemia (Liu et al., 1999), genetic deletion of IL-1β or IL-1α, alone or in combination (IL-1αβ knockout), did not protect one week after hypoxia-ischemia (Hedtjarn et al., 2005) whereas administration of IL-1ra protected (Girard et al., 2008). Stimulation of neonatal mice with TLR2- or TLR4- specific ligands, Pam3CSK4 or LPS, respectively, showed that IL-1β increases in the brain are stimulus/receptor-dependent (Stridh et al., 2011). Also, protection achieved by minocycline in our model was not associated with reduction of the brain IL-1β levels increased by MCAO (Fox et al., 2005), suggesting that IL-1β might either not be injurious after acute injury, or its effects may depend on the cell type producing this cytokine, as cell-type specific effects shown for TNFα (Lambertsen et al., 2009). The accumulated Iba1+ macrophage population may be diverse by nature and consist of activated microglia and infiltrated monocytes. Furthermore, each of the subpopulations—brain macrophages and invaded macrophages—may be heterogeneous along the M1/M2 continuum, thereby differentially affecting injury. Protection by selective activation of CB2R after stroke in the adult was not only associated with the reduction of microglial activation but also with reduced expression of the adhesion molecules (Zhang et al., 2007), leukocyte/endothelial interactions and neutrophil recruitment (Murikinati et al., 2010; Zhang et al., 2007; Zhang et al., 2009). WIN is also known to reduce adhesion of macrophages to atherosclerotic blood vessels (Zhao et al., 2010) and expression of vascular and intercellular adhesion molecules under chronic neuroinflammatory conditions in experimental multiple sclerosis (Mestre et al., 2009). However, infiltration of peripheral monocytes (Denker et al., 2007) and neutrophils (unpublished) is minimal in our animal model during the first 24 hours after reperfusion, the time when we observed decreased expression of Iba1 in the injured brain of WIN-treated animals. Our data also show that decreased accumulation of Iba1+ cells at 72 hours is coupled to decreased proliferation of microglial cells in WIN-treated animals, further suggesting WIN effects on microglia. Although it is conceivable that at later time WIN affects injury mediated by invading monocytes as well, cumulatively, our findings show that during the subacute injury phase WIN acts on microglia rather than on peripheral and invading monocytes. Therefore, our data strongly suggest that WIN exerts its protection largely through the effects on the microglial population. Further studies are needed to explore the potential of cannabinergic agonists to provide long-term protection after neonatal stroke.
HIGHLIGHTS.
WIN 55,212-2 protects the neonatal brain against focal ischemia.
Expression of CB2R, but not of CB1R, is induced after neonatal focal ischemia.
WIN 55,212-2 prevents microglia/macrophage accumulation after neonatal stroke.
WIN 55,212-2 does not affect expression of chemotactic receptors in microglia.
WIN does not affect expression of cytokines/chemokines after neonatal stroke
Acknowledgments
This work was supported by grants from NIH/RO1 NS055915 and NIH/ROI NS44025 (ZSV), Spanish Ministry of Science and Innovation SAF2009-08145 (MAM), SAF2008-03122 (IL) and Spanish Ministry of Health RENEVAS RD06/0026/0005 (IL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ashton JC, Rahman RM, Nair SM, Sutherland BA, Glass M, Appleton I. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci Lett. 2007;412:114–117. doi: 10.1016/j.neulet.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 2.Baker D, Jackson SJ, Pryce G. Cannabinoid control of neuroinflammation related to multiple sclerosis. Br J Pharmacol. 2007;152:649–654. doi: 10.1038/sj.bjp.0707458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chabrier S, Husson B, Dinomais M, Landrieu P, Nguyen The Tich New insights (and new interrogations) in perinatal arterial ischemic stroke. Thromb Res. 2011;127:13–22. doi: 10.1016/j.thromres.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Denker SP, Ji S, Dingman A, Lee SY, Derugin N, Wendland MF, Vexler ZS. Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. J Neurochem. 2007;100:893–904. doi: 10.1111/j.1471-4159.2006.04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derugin N, Dingman A, Wendland MF, Fox C, Bollen A, Vexler ZS. Magnetic resonance imaging as a surrogate measure for histological sub-chronic endpoint in a neonatal rat stroke model. Brain Res. 2005;1066:49–56. doi: 10.1016/j.brainres.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 6.Derugin N, Wendland M, Muramatsu K, Roberts TP, Gregory G, Ferriero DM, Vexler ZS. Evolution of brain injury after transient middle cerebral artery occlusion in neonatal rats. Stroke. 2000;31:1752–1761. doi: 10.1161/01.str.31.7.1752. [DOI] [PubMed] [Google Scholar]
- 7.Docagne F, Muneton V, Clemente D, Ali C, Loria F, Correa F, Hernangomez M, Mestre L, Vivien D, Guaza C. Excitotoxicity in a chronic model of multiple sclerosis: Neuroprotective effects of cannabinoids through CB1 and CB2 receptor activation. Mol Cell Neurosci. 2007;34:551–561. doi: 10.1016/j.mcn.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Faustino J, Wang X, Johnson C, Klibanov A, Derugin N, Wendland M, Vexler Z. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci. 2011 doi: 10.1523/JNEUROSCI.2102-11.2011. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Lopez D, Martinez-Org, Nunez E, Romero J, Lorenzo P, Moro MA, Lizasoain I. Characterization of the neuroprotective effect of the cannabinoid agonist WIN-55212 in an in vitro model of hypoxic-ischemic brain damage in newborn rats. Pediatr Res. 2006;60:169–173. doi: 10.1203/01.pdr.0000228839.00122.6c. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Lopez D, Pazos MR, Tolon RM, Moro MA, Romero J, Lizasoain I, Martinez-Org The cannabinoid agonist WIN55212 reduces brain damage in an in vivo model of hypoxic-ischemic encephalopathy in newborn rats. Pediatr Res. 2007;62:255–260. doi: 10.1203/PDR.0b013e318123fbb8. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Lopez D, Pradillo JM, Garcia-Yebenes I, Martinez-Org, Moro MA, Lizasoain I. The cannabinoid WIN55212-2 promotes neural repair after neonatal hypoxia-ischemia. Stroke. 2010;41:2956–2964. doi: 10.1161/STROKEAHA.110.599357. [DOI] [PubMed] [Google Scholar]
- 12.Fox C, Dingman A, Derugin N, Wendland MF, Manabat C, Ji S, Ferriero DM, Vexler ZS. Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2005;25:1138–1149. doi: 10.1038/sj.jcbfm.9600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girard S, Kadhim H, Larouche A, Roy M, Gobeil F, Sebire G. Pro-inflammatory disequilibrium of the IL-1 beta/IL-1ra ratio in an experimental model of perinatal brain damages induced by lipopolysaccharide and hypoxia-ischemia. Cytokine. 2008;43:54–62. doi: 10.1016/j.cyto.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez FF, McQuillen P, Mu D, Chang Y, Wendland M, Vexler Z, Ferriero DM. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev Neurosci. 2007;29:321–330. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- 15.Hedtjarn M, Mallard C, Iwakura Y, Hagberg H. Combined deficiency of IL-1beta18, but not IL-1alphabeta, reduces susceptibility to hypoxia-ischemia in the immature brain. Dev Neurosci. 2005;27:143–148. doi: 10.1159/000085986. [DOI] [PubMed] [Google Scholar]
- 16.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 17.Jin KL, Mao XO, Goldsmith PC, Greenberg DA. CB1 cannabinoid receptor induction in experimental stroke. Ann Neurol. 2000;48:257–261. [PubMed] [Google Scholar]
- 18.Kim SH, Won SJ, Mao XO, Jin K, Greenberg DA. Molecular mechanisms of cannabinoid protection from neuronal excitotoxicity. Mol Pharmacol. 2006;69:691–696. doi: 10.1124/mol.105.016428. [DOI] [PubMed] [Google Scholar]
- 19.Kirton A, deVeber G. Advances in perinatal ischemic stroke. Pediatr Neurol. 2009;40:205–214. doi: 10.1016/j.pediatrneurol.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci U S A. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, Nielsen HH, Haugaard LS, Wirenfeldt M, Nielsen M, Dagnaes-Hansen F, Bluethmann H, Faergeman NJ, Meldgaard M, Deierborg T, Finsen B. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29:1319–1330. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu XH, Kwon D, Schielke GP, Yang GY, Silverstein FS, Barks JD. Mice deficient in interleukin-1 converting enzyme are resistant to neonatal hypoxic-ischemic brain damage. J Cereb Blood Flow Metab. 1999;19:1099–1108. doi: 10.1097/00004647-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Manabat C, Han BH, Wendland M, Derugin N, Fox CK, Choi J, Holtzman DM, Ferriero DM, Vexler ZS. Reperfusion differentially induces caspase-3 activation in ischemic core and penumbra after stroke in immature brain. Stroke. 2003;34:207–213. doi: 10.1161/01.STR.0000047101.87575.3C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchalant Y, Rosi S, Wenk GL. Anti-inflammatory property of the cannabinoid agonist WIN-55,212-2 in a rodent model of chronic brain inflammation. Neuroscience. 2007;144(4):1516–22. doi: 10.1016/j.neuroscience.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- 26.Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der SM, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di MV, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Org, Fernandez-Frutos B, Gonzalez R, Romero E, Uriguen L, Romero J, Viveros MP. Neuroprotection by the cannabinoid agonist WIN-55212 in an in vivo newborn rat model of acute severe asphyxia. Brain Res Mol Brain Res. 2003;114:132–139. doi: 10.1016/s0169-328x(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Org, Fernandez-Lopez D, Lizasoain I, Romero J. The seek of neuroprotection: introducing cannabinoids. Recent Patents CNS Drug Discov. 2007;2:131–139. doi: 10.2174/157488907780832724. [DOI] [PubMed] [Google Scholar]
- 29.Mechoulam R, Spatz M, Shohami E. Endocannabinoids and neuroprotection. Sci STKE 2002. 2002:RE5. doi: 10.1126/stke.2002.129.re5. [DOI] [PubMed] [Google Scholar]
- 30.Mestre L, Docagne F, Correa F, Loria F, Hernangomez M, Borrell J, Guaza C. A cannabinoid agonist interferes with the progression of a chronic model of multiple sclerosis by downregulating adhesion molecules. Mol Cell Neurosci. 2009;40:258–266. doi: 10.1016/j.mcn.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Mukhopadhyay S, Das S, Williams EA, Moore D, Jones JD, Zahm DS, Ndengele MM, Lechner AJ, Howlett AC. Lipopolysaccharide and cyclic AMP regulation of CB(2) cannabinoid receptor levels in rat brain and mouse RAW 264.7 macrophages. J Neuroimmunol. 2006;181:82–92. doi: 10.1016/j.jneuroim.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Murikinati S, Juttler E, Keinert T, Ridder DA, Muhammad S, Waibler Z, Ledent C, Zimmer A, Kalinke U, Schwaninger M. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J. 2010;24:788–798. doi: 10.1096/fj.09-141275. [DOI] [PubMed] [Google Scholar]
- 33.Nagayama T, Sinor AD, Simon RP, Chen J, Graham SH, Jin K, Greenberg DA. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J Neurosci. 1999;19:2987–2995. doi: 10.1523/JNEUROSCI.19-08-02987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes (Lond) 2006;30(Suppl 1):S13–S18. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- 35.Sagredo O, Garcia-Arencibia M, de Lago E, Finetti S, Decio A, Fernandez-Ruiz J. Cannabinoids and neuroprotection in basal ganglia disorders. Mol Neurobiol. 2007;36:82–91. doi: 10.1007/s12035-007-0004-3. [DOI] [PubMed] [Google Scholar]
- 36.Sarne Y, Asaf F, Fisbein M, Gafni M, Keren O. The dual neuroprotective-neurotoxic profile of cannabinoid drugs. Br J Pharmacol. 2011;163(7):1391–401. doi: 10.1111/j.1476-5381.2011.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shohami E, Gallily R, Mechoulam R, Bass R, Ben Hur T. Cytokine production in the brain following closed head injury: dexanabinol (HU-211) is a novel TNF-alpha inhibitor and an effective neuroprotectant. J Neuroimmunol. 1997;72:169–177. doi: 10.1016/s0165-5728(96)00181-6. [DOI] [PubMed] [Google Scholar]
- 38.Sobrado M, Pereira MP, Ballesteros I, Hurtado O, Fernandez-Lopez D, Pradillo JM, Caso JR, Vivancos J, Nombela F, Serena J, Lizasoain I, Moro MA. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci. 2009;29:3875–3884. doi: 10.1523/JNEUROSCI.5529-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stridh L, Smith PL, Naylor AS, Wang X, Mallard C. Regulation of Toll-like receptor 1 and -2 in neonatal mice brains after hypoxia-ischemia. J Neuroinflammation. 2011;8:45. doi: 10.1186/1742-2094-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vexler ZS, Yenari MA. Does inflammation afftect the developing brain differently than the adult brain? Dev Neurosci. 2009;31(5):378–93. doi: 10.1159/000232556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M, Adler MW, Abood ME, Ganea D, Jallo J, Tuma RF. CB2 receptor activation attenuates microcirculatory dysfunction during cerebral ischemic/reperfusion injury. Microvasc Res. 2009;78:86–94. doi: 10.1016/j.mvr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang M, Martin BR, Adler MW, Razdan RK, Ganea D, Tuma RF. Modulation of the balance between cannabinoid CB(1) and CB(2) receptor activation during cerebral ischemic/reperfusion injury. Neuroscience. 2008;152:753–760. doi: 10.1016/j.neuroscience.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang M, Martin BR, Adler MW, Razdan RK, Jallo JI, Tuma RF. Cannabinoid CB(2) receptor activation decreases cerebral infarction in a mouse focal ischemia/reperfusion model. J Cereb Blood Flow Metab. 2007;27:1387–1396. doi: 10.1038/sj.jcbfm.9600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y, Yuan Z, Liu Y, Xue J, Tian Y, Liu W, Zhang W, Shen Y, Xu W, Liang X, Chen T. Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. J Cardiovasc Pharmacol. 2010;55:292–298. doi: 10.1097/FJC.0b013e3181d2644d. [DOI] [PubMed] [Google Scholar]


