Abstract
In organisms that provision young between fertilization and birth, mothers and their developing embryos are expected to be in conflict over embryonic growth. In mammalian embryos, the expression of Insulin-like growth factor II (IGF2) plays a key role in maternal-fetal interactions and is thought to be a focus of maternal-fetal conflict. Recent studies have suggested that IGF2 is also a focus of maternal-fetal conflict in placental fish in the family Poeciliidae. However, whether the expression of IGF2 influences offspring size, the trait over which mothers and embryos are likely to be in conflict, has not been assessed in a poeciliid. We tested whether embryonic IGF2 expression varied among four populations of a placental poeciliid that display large and consistent differences in offspring size at birth. We found that IGF2 expression varied significantly among embryonic stages with expression being 50% higher in early stage embryos than late stage embryos. There were no significant differences among populations in IGF2 expression; small differences in expression between population pairs with different offspring sizes were comparable in magnitude to those between population pairs with the same offspring sizes. Our results indicate that variation in IGF2 transcript abundance does not contribute to differences in offspring size among H. formosa populations.
Introduction
In organisms that provision young after fertilization parents and offspring are likely to be in conflict over the level of parental investment with the optimal level of parental investment being higher from the offspring’s perspective than from the parent’s [1]. This conflict is predicted to be an important influence on the evolution of parent-offspring interactions in species with post-natal parental care as well as species such as placental mammals in which mothers provision embryos between fertilization and birth [1], [2], [3], [4]. In placental species, maternal-fetal conflict is hypothesized to influence the evolution of reproductive isolation, epigenetic phenomena such as genomic imprinting, and possibly the evolution of placentation itself from less elaborate forms of viviparity [2], [3], [5], [6].
In species with post-natal parental care, parent-offspring conflict involves behavioral interactions between parents and their dependent young [1], [7], [8]. In contrast, conflict between mothers and their developing embryos is likely to involve the expression of growth enhancing and growth suppressing genes by embryos, as well as the response of mothers to the expression of these genes. Perhaps the most dramatic example of maternal-fetal conflict over maternal investment involves the expression of Insulin-like growth factor II (IGF2) and the Insulin-like growth factor II receptor (IGF2r) in mouse embryos [6], [9]. IGF2 is a potent growth factor while IGF2r inhibits prenatal growth by degrading IGF2. These two genes are oppositely imprinted in mice with the paternally inherited copy of the growth-enhancing gene (IGF2) and the maternally inherited copy of the growth-suppressing gene (IGF2r) being expressed in developing embryos [10], [11]. The pattern of imprinting and the phenotypic effects of these two genes are consistent with their involvement in intragenomic conflict over prenatal maternal investment [6].
While most studies of maternal-fetal conflict have focused on mammals, maternal-fetal conflict may also be an important force in placental fish in the family Poeciliidae [12], [13], [14]. With the exception of a single species, all poeciliid fish give birth to fully developed, independent young [15], [16]. However, there is considerable variation among species in both the presence and degree of post-fertilization maternal investment [17]. Some species do not provide developing embryos with nutrients beyond those provided in the egg (lecithotrophs). Other species provision developing embryos via a placenta composed of the maternal ovarian follicle and the embryo’s pericardial sac (matrotrophs). In addition to variation in the presence and degree of matrotrophy, there is variation among populations of some species in the level of matrotrophy [14], [18], [19]. Finally, the mating systems of many poeciliids are characterized by high levels of multiple paternity which is expected to increase the magnitude of maternal-fetal conflict [20].
Two previous studies have examined the role that IGF2 plays in maternal-fetal conflict in poeciliids. In the first study, Lawton et al. [21] tested whether IGF2 is imprinted in two matrotrophic poeciliids (Heterandria formosa and Poeciliopsis prolifica). In both species, IGF2 was biallelically expressed, suggesting that maternal-fetal conflict has not driven the evolution of genomic imprinting in poeciliids. In the second study, O’Neill et al. [22] found that IGF2 expression in H. formosa is localized in the embryonic contribution to the placenta (the pericardial sac) and that IGF2 has evolved under strong positive selection in poeciliids. The evidence for positive selection was especially strong in lineages that have evolved placentas recently. These observations are consistent with the hypothesis that IGF2 has been a focus of maternal-fetal conflict in poeciliid fish.
Studies of the expression and evolution of IGF2 in poeciliids and its growth enhancing function in mammalian embryos suggest that altering the expression of this gene may allow poeciliid embryos to influence their own growth. While there is ample evidence that IGF2 expression influences offspring size in mammals [11], no studies have investigated whether embryonic IGF2 expression influences offspring size at birth in poeciliids. Here we address this gap by testing whether there is an association between embryonic IGF2 expression and offspring size at birth in four populations of a matrotrophic poeciliid that exhibit large and consistent differences in size at birth.
Methods
Ethics statement: All work was approved by the Florida State University Institutional Animal Care and Use Committee (protocol number 9321) and carried out in strict accordance with national guidelines. Females were euthanized with an overdose of anesthetic (MS222).
Heterandria formosa is a highly matrotrophic poeciliid fish distributed along the southeastern coastal plain of the United States. In addition to being highly matrotrophic, H. formosa females simultaneously provision several broods of developing embryos, a phenomenon referred to as superfetation. The combination of matrotrophy and superfetation in this species increases the potential for maternal-fetal conflict and sibling competition for maternally supplied resources [14], [23]. In addition, previous work has demonstrated that variation among populations in offspring size at birth is due to a combination of maternal and direct effects of offspring genotype on offspring size [14], [19].
We quantified IGF2 expression in embryos from four H. formosa populations located in North Florida: Moore Lake (ML), Trout Pond (TP), Wacissa River (WR), and Wakulla Springs (WS). Offspring from Wacissa River and Wakulla Springs are, on average 40% larger than offspring from Moore Lake and Trout Pond. These differences are present under field and laboratory conditions and have been the focus of study for over 20 years [14], [19], [24], [25], [26], [27]. In two previous studies involving these populations, we tested whether differences in offspring size at birth are due to differences in the size of mature ova or differences in post-fertilization maternal investment. These studies show that differences between populations in offspring size at birth are reflected in the size of early stage embryos and are due to differences in post-fertilization maternal provisioning not differences in the size of mature ova [14], [19].
We collected pregnant females from each population during the first week of May 2011. Females were euthanized with an overdose of anesthetic (MS222) and dissected in the field to remove the ovary containing developing embryos. The ovary of each female was preserved in RNAlater (Qiagen, Valencia, CA) and placed on ice until samples were retuned to the lab and stored at -20 C. We dissected each preserved ovary to remove developing embryos. Embryos were placed into a developmental stage following Travis et al. [26]. We retained embryos from stages 2-5 (early-eyed, mid-eyed, late-eyed, and very late-eyed embryos) and placed broods from each stage of each female in a new tube containing RNAlater.
We quantified the expression of IGF2 in embryos relative to a control gene, Elongation factor 1 α (EF1 α) using quantitative real time RTPCR (QPCR). This gene is frequently used as a control gene in QPCR studies (e.g. [28]) and preliminary work indicated that its expression varied little among embryonic stages and populations. Whole embryos were homogenized using QIAshredders (Qiagen, Valencia, CA) and RNA was extracted from whole embryo homogenates using RNeasy mini kits (Qiagen, Valencia, CA). Total RNA was reverse transcribed into cDNA using Super Script III, First Stand Synthesis Supermix (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
IGF2 and EF1α primer sequences were taken from [21], [28]. QPCR reactions contained 5 µl SYBR® Green PCR master mix, 1 µl of 2.5 µM forward primer, 1 µl of 2.5 µM reverse primer, and 3 µl cDNA. Three technical replicates of each cDNA sample were performed and control reactions with no cDNA template were included on each plate to determine the level of background contamination. A 5-step serial dilution standard curve was generated for each gene using pooled cDNA from embryos from each developmental stage. Quantification of gene expression was performed with an ABI Prism 7900 sequence detector (Applied Biosystems, Foster City, CA). We estimated the efficiency of the PCR reaction for each gene (E) using the slope of CT on log quantity from the dilution series. Relative IGF2 expression (RE) was calculated as:
In total, we measured IGF2 expression from 203 embryos from 34 females. While the total numbers of embryos were comparable among ML, TP, WR, and WS (46, 51, 48, and 58 embryos respectively), the numbers of female parents were more variable (6, 10, 8, and 10 respectively). We used between 7 and 22 embryos at each combination of population and stage except stage 2 at ML, for which we obtained only 2 embryos.
To analyze the expression data, we performed a two-way ANOVA, considering stage and population as fixed effects and pooling variance attributable to the identity of individual mothers (which was not statistically significant) into the residual variation. We report these results; however, we performed several alternative analyses with different statistical assumptions that yielded nearly identical results. We performed all analyses on log-transformed relative expression levels so that the assumptions of linear analyses would be met and used Type III SS to assess statistical significance.
Results
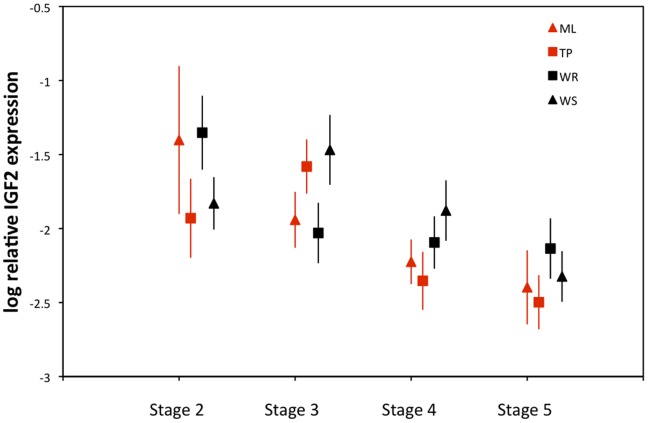
Levels of IGF2 expression were highly variable, even among embryos of the same female at the same stage. Nonetheless, there was a regular decrease in the average levels of IGF2 expression from the earliest to the latest embryonic stages (Fig. 1); on the logarithmic scale, average expression in stage-5 embryos was about 50% lower than that for stage-2 embryos. The variation among developmental stages was significant (two-way ANOVA, F3, 195 = 10.44, P<0.0001); post-hoc pairwise comparisons using Tukey’s method found that the averages fell into two groups, one including stages 2 and 3 and another including stages 4 and 5. These results are consistent with those of Lawton et al. [21] who found IGF2 expression to peak in early and mid-eyed H. formosa embryos.
Figure 1. Log relative IGF2 expression (means ± SE) in stage 2, 3, 4 and 5 H. formosa embryos from Moore Lake (ML, red triangles), Trout Pond (TP, red squares), Wacissa River (WR, black squares), and Wakulla Springs (WS, black triangles).
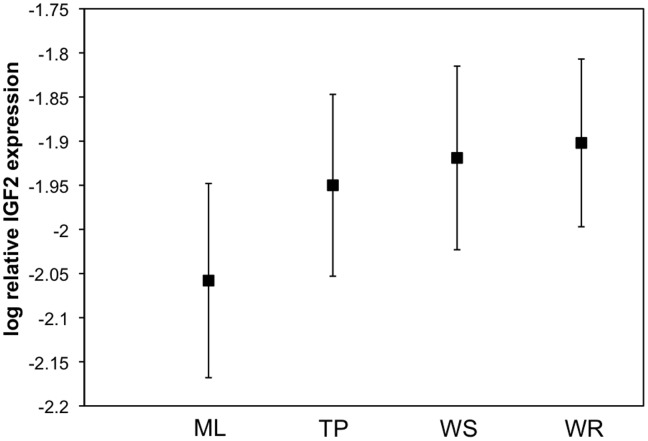
Despite the fact that offspring size varies dramatically among these populations, there was no evidence for variation among their average levels of IGF2 expression (Fig. 2). On the logarithmic scale, there was only an 8% difference in relative IGF2 expression between the populations with the lowest and highest average expression levels (ML and WS respectively). There were no significant differences among populations and less than 1% of the total variance in IGF2 expression was attributable to population (Two-way ANOVA; F3, 195 = 0.43, P = 0.73).
Figure 2. Log relative IGF2 expression (least squares mean ± SE) in H. formosa embryos from Moore Lake (ML), Trout Pond (TP), Wacissa River (WR), and Wakulla Springs (WS).
Values are averaged across developmental stages.
Discussion
IGF2 is a major axis of maternal-fetal conflict in mammals and is hypothesized to be a focus of maternal-fetal conflict in poeciliid fish. The evidence that maternal-fetal conflict in mammals involves IGF2 is especially compelling for two reasons. First, IGF2 expression has a clear influence on offspring size at birth, the trait over which mothers and embryos are expected to be in conflict [11]. Second, IGF2 is imprinted with the paternal copy expressed in developing embryos. This pattern of expression is consistent with the kinship theory of genomic imprinting [6]. The evidence for maternal-fetal conflict over IGF2 expression in poeciliids is based mainly on the observation that it is expressed in the poeciliid placenta and has evolved under strong positive selection, especially in matrotrophic lineages [22]. However, IGF2 is not imprinted in placental poeciliids [21] and our results indicate that divergence in offspring size at birth does not involve divergence in IGF2 expression.
The absence of an association between IGF2 expression and offspring size in our study is surprising, considering that IGF2 expression has been shown to differ between fast and slow growing strains of some fish species [29]. This suggests that the expression of other growth factors (e.g. IGF1) may be an important determinant of offspring size in H. formosa. Alternatively, offspring size may be influenced by the interaction between IGF2 expression and the expression of other genes. For example, O’Neill et al. [22] found that the two IGF2 sites with the strongest signature of positive selection in matrotrophic poeciliids were adjacent to the type 1 IGF receptor. It is possible that the interaction between the expression of IGF2 and the IGF1 receptor in H. formosa embryos influences offspring size at birth.
One might argue that our results are not definitive because even very small differences in IGF2 expression, smaller than those we had the statistical power to detect, could induce substantial differences in offspring size. Indeed, studies of mice suggest that small changes in IGF2 expression may influence offspring size. For example, a targeted mutation in IGF2 resulting in a 10% decline in IGF2 expression was associated with a 60% decline in offspring size in mice [9], [11]. It is unlikely that this is the case in our data. The difference in IGF2 expression between pairs of populations characterized by small and large offspring was between 8% (Moore Lake and Wakulla Springs) and about 1.6% (Trout Pond and Wacissa River). This range is comparable to the differences seen between pairs with similarly sized offspring, 6% between Moore Lake and Trout Pond and 0.9% between Wacissa River and Wakulla Springs (Figure 2). This pattern argues that divergence in offspring size among these populations does not involve the regulation of this gene.
The rapid evolution of IGF2 in poeciliid fish is suggests that this gene plays a major role in the evolution of placentation. However, if IGF2 expression is a focal point of maternal-fetal conflict in placental poeciliids, our observations suggest this conflict is not manifested at the level of IGF2 mRNA abundance in H. formosa. It is possible that post-transcriptional processing of IGF2 or interactions between IGF2 and other genes determine the outcome of maternal-fetal interactions in poeciliids. The development of genomic resources for placental poeciliid fish [30] may allow broader transcriptomic comparisons among these populations, which in turn could identify the genes important in maternal-fetal conflict and the elaboration of matrotrophy.
Acknowledgments
We thank K. McGhee and two anonymous reviewers for comments on the manuscript. R. Fuller and A. Bell kindly provided lab space to M. Schrader. T. Newman provided invaluable help with the QPCR reactions
Funding Statement
This work was funded by a grant by the US National Science Foundation (DEB 08-22547). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Trivers RL (1974) Parent-offspring conflict. American Zoologist 14: 249–264. [Google Scholar]
- 2. Zeh DW, Zeh JA (2000) Reproductive mode and speciation: the viviparity-driven conflict hypothesis. Bioessays 22: 938–946. [DOI] [PubMed] [Google Scholar]
- 3. Zeh JA, Zeh DW (2008) Viviparity-driven Conflict More to Speciation than Meets the Fly. Year in Evolutionary Biology 2008 1133: 126–148. [DOI] [PubMed] [Google Scholar]
- 4. Parker GA, Royle NJ, Hartley IR (2002) Intrafamilial conflict and parental investment: a synthesis. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 357: 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crespi B, Semeniuk C (2004) Parent-offspring conflict in the evolution of vertebrate reproductive mode. American Naturalist 163: 635–653. [DOI] [PubMed] [Google Scholar]
- 6. Haig D (2000) The kinship theory of genomic imprinting. Annual Review of Ecology and Systematics 31: 9–32. [Google Scholar]
- 7. Godfray HCJ (1991) Signaling of need by offspring to their parents. Nature 352: 328–330. [Google Scholar]
- 8. Smith HG, Montgomerie R (1991) Nestling American robins compete with siblings by begging. Behavioral Ecology and Sociobiology 29: 307–312. [Google Scholar]
- 9. Vrana PB (2007) Genomic imprinting as a mechanism of reproductive isolation in mammals. Journal of Mammalogy 88: 5–23. [Google Scholar]
- 10. Barlow DP, Stoger R, Herrmann BG, Saito K, Schweifer N (1991) The mouse insulin-like growth-factor type-2 receptor is imprinted and closely linked to the time locus. Nature 349: 84–87. [DOI] [PubMed] [Google Scholar]
- 11. Dechiara TM, Efstratiadis A, Robertson EJ (1990) A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor-II gene disrupted by targeting. Nature 345: 78–80. [DOI] [PubMed] [Google Scholar]
- 12. Pollux BJA, Pires MN, Banet AI, Reznick DN (2009) Evolution of Placentas in the Fish Family Poeciliidae: An Empirical Study of Macroevolution. Annual Review of Ecology Evolution and Systematics 40: 271–289. [Google Scholar]
- 13. Schrader M, Travis J (2008) Testing the Viviparity-Driven-Conflict Hypothesis: Parent-Offspring Conflict and the Evolution of Reproductive Isolation in a Poeciliid Fish. American Naturalist 172: 806–817. [DOI] [PubMed] [Google Scholar]
- 14. Schrader M, Travis J (2009) Do embryos influence maternal investment? Evaluating maternal-fetal coadaptation and the potential for parent-offspring conflict in placental fish. Evolution 63: 2805–2815. [DOI] [PubMed] [Google Scholar]
- 15.Pires MN, Banet AI, Pollux BJA, Reznick DN (2011) Variation and evolution of reproductive strategies; Evans JP, Pilastro A, Schlupp I, editors. 28–37 p.
- 16.Reznick DN, Miles DB (1989) Review of life history patterns in poeciliid fishes. 125–148 p.
- 17. Reznick DN, Mateos M, Springer MS (2002) Independent origins and rapid evolution of the placenta in the fish genus Poeciliopsis. Science 298: 1018–1020. [DOI] [PubMed] [Google Scholar]
- 18. Pires MN, McBride KE, Reznick DN (2007) Interpopulation variation in life-history traits of Poeciliopsis prolifica: Implications for the study of placental evolution. Journal of Experimental Zoology Part a-Ecological Genetics and Physiology 307A: 113–125. [DOI] [PubMed] [Google Scholar]
- 19.Schrader M, Travis J (2005) Population differences in pre- and post-fertilization offspring provisioning in the Least Killifish, Heterandria formosa. Copeia: 649–656.
- 20. Schrader M, Travis J, Fuller RC (2011) Do density-driven mating system differences explain reproductive incompatibilities between populations of a placental fish? Molecular Ecology 20: 4140–4151. [DOI] [PubMed] [Google Scholar]
- 21. Lawton BR, Sevigny L, Obergfell C, Reznick D, O’Neill RJ, et al. (2005) Allelic expression of IGF2 in live-bearing, matrotrophic fishes. Development Genes and Evolution 215: 207–212. [DOI] [PubMed] [Google Scholar]
- 22. O’Neill MJ, Lawton BR, Mateos M, Carone DM, Ferreri GC, et al. (2007) Ancient and continuing Darwinian selection on insulin-like growth factor II in placental fishes. Proceedings of the National Academy of Sciences of the United States of America 104: 12404–12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schrader M, Travis J (2012) Variation in offspring size with birth order in placental fish: a role for asymmetric sibling competition? Evolution 66: 272–279. [DOI] [PubMed] [Google Scholar]
- 24. Leips J, Travis J (1999) The comparative expression of life-history traits and its relationship to the numerical dynamics of four populations of the least killifish. Journal of Animal Ecology 68: 595–616. [Google Scholar]
- 25. Leips J, Travis J, Rodd FH (2000) Genetic influences on experimental population dynamics of the least killifish. Ecological Monographs 70: 289–309. [Google Scholar]
- 26. Travis J, Farr JA, Henrich S, Cheong RT (1987) Testing theories of clutch overlap with the reproductive ecology of Heterandria formosa . Ecology 68: 611–623. [Google Scholar]
- 27.Schrader M, Travis J (2012) - Assessing the roles of population density and predation risk in the evolution of offspring size in populations of a placental fish. Ecology and Evolution in press. [DOI] [PMC free article] [PubMed]
- 28. Fuller RC, Claricoates KM (2011) Rapid light-induced shifts in opsin expression: finding new opsins, discerning mechanisms of change, and implications for visual sensitivity. Molecular Ecology 20: 3321–3335. [DOI] [PubMed] [Google Scholar]
- 29. Peterson BC, Waldbieser GC, Bilodeau L (2004) IGF-I and IGF-II mRNA expression in slow and fast growing families of USDA 103 channel catfish (Ictalurus punctatus). Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology 139: 317–323. [DOI] [PubMed] [Google Scholar]
- 30. Panhuis TM, Broitman-Maduro G, Uhrig J, Maduro M, Reznick DN (2011) Analysis of Expressed Sequence Tags from the Placenta of the Live-Bearing Fish Poeciliopsis (Poeciliidae). Journal of Heredity 102: 352–361. [DOI] [PubMed] [Google Scholar]