Abstract
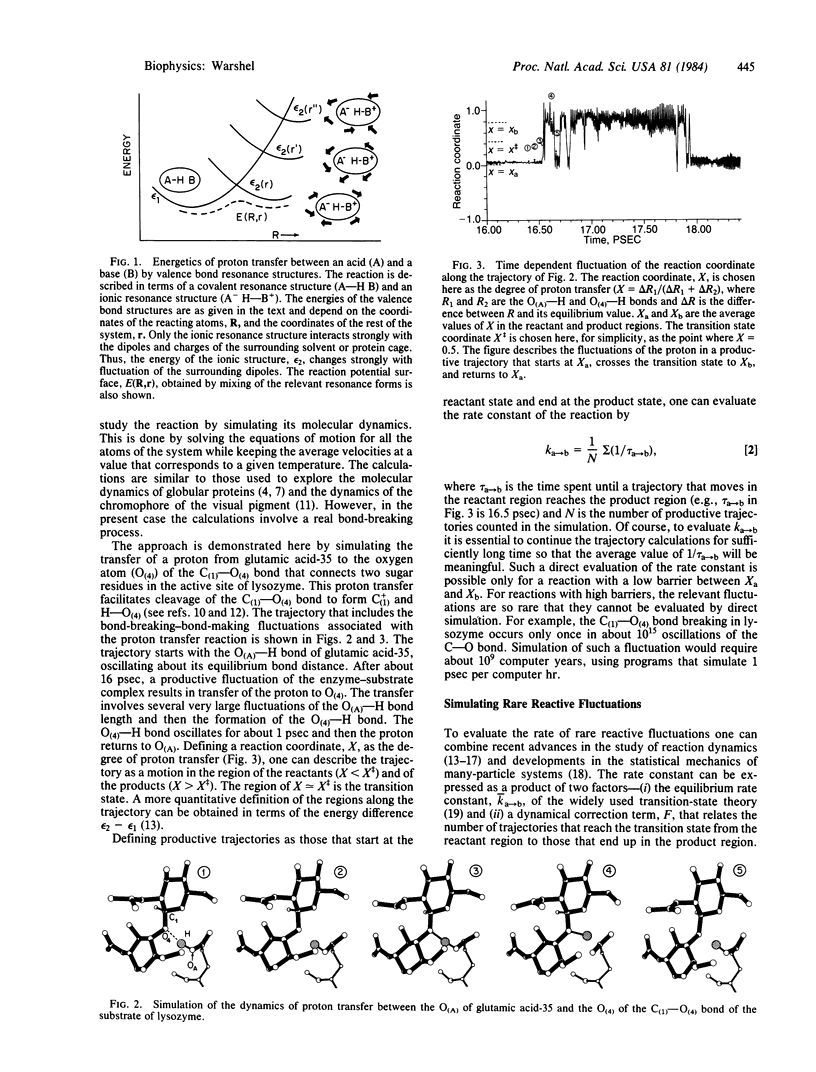
The detailed molecular dynamics of an actual bond-breaking event in a fluctuating enzyme substrate complex is simulated. The method developed allows one to explore what type of fluctuations are involved in enzymatic reactions and to evaluate entropic contributions to enzyme catalysis. The fluctuations of the enzyme electrostatic potential are found to be a key dynamical factor in reactions that involve a large change in the polarity of the reacting bonds.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Careri G., Fasella P., Gratton E. Enzyme dynamics: the statistical physics approach. Annu Rev Biophys Bioeng. 1979;8:69–97. doi: 10.1146/annurev.bb.08.060179.000441. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Gavish B., Werber M. M. Viscosity-dependent structural fluctuations in enzyme catalysis. Biochemistry. 1979 Apr 3;18(7):1269–1275. doi: 10.1021/bi00574a023. [DOI] [PubMed] [Google Scholar]
- Karplus M., McCammon J. A. The internal dynamics of globular proteins. CRC Crit Rev Biochem. 1981;9(4):293–349. doi: 10.3109/10409238109105437. [DOI] [PubMed] [Google Scholar]
- Levitt M. Molecular dynamics of hydrogen bonds in bovine pancreatic trypsin inhibitor protein. Nature. 1981 Nov 26;294(5839):379–380. doi: 10.1038/294379a0. [DOI] [PubMed] [Google Scholar]
- McCammon J. A., Karplus M. Dynamics of activated processes in globular proteins. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3585–3589. doi: 10.1073/pnas.76.8.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. C. The three-dimensional structure of an enzyme molecule. Sci Am. 1966 Nov;215(5):78–90. doi: 10.1038/scientificamerican1166-78. [DOI] [PubMed] [Google Scholar]
- Warshel A. Bicycle-pedal model for the first step in the vision process. Nature. 1976 Apr 22;260(5553):679–683. doi: 10.1038/260679a0. [DOI] [PubMed] [Google Scholar]
- Warshel A. Calculations of enzymatic reactions: calculations of pKa, proton transfer reactions, and general acid catalysis reactions in enzymes. Biochemistry. 1981 May 26;20(11):3167–3177. doi: 10.1021/bi00514a028. [DOI] [PubMed] [Google Scholar]
- Warshel A., Levitt M. Theoretical studies of enzymic reactions: dielectric, electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme. J Mol Biol. 1976 May 15;103(2):227–249. doi: 10.1016/0022-2836(76)90311-9. [DOI] [PubMed] [Google Scholar]



