Abstract
Objective
Educational attainment is inversely associated with SBP level in young adulthood. This association has not been studied in an older cohort, and confounding and mediating factors are not well known.
Methods
The authors hypothesized that higher education is associated with lower levels of SBP independent of many risk factors for hypertension. This prospective observational study included a sample of 764 older community-living participants in the Maintenance of Balance, Independent Living, Intellect and Zest in the Elderly (MOBILIZE) Boston Study.
Results
Compared to participants with more than college education, regression analyses showed those with a high school education or less had a SBP value 6.33 mmHg higher [95% confidence interval (CI): 2.55–10.10], and those who had a college education had a SBP value 4.01 mmHg higher (95% CI: 0.77–7.25) independent of many hypothesized confounders and mediators.
Discussion
Results of a path analysis confirmed that higher level of education was associated with lower SBP even after adjustment for hypothesized mediators. Although slightly attenuated by multivariable adjustment for hypertension risk factors, the significant inverse association between educational attainment and SBP was not entirely mediated by these risk factors. These findings indicate that education is inversely associated with SBP in a diverse cohort of community-living older adults, independent of many known or suspected risk factors.
Conclusion
This study is the first to report the association between education and SBP in an older sample, representing a population at the highest risk for hypertension-related morbidity and mortality.
Keywords: cardiovascular disease, education, hypertension, older adults, SBP
INTRODUCTION
Cardiovascular disease is endemic worldwide and its burden is substantial [1,2]. Approximately, 54% of stroke, 47% of ischemic heart disease, 25% of other cardiovascular disease and nearly 14% of all deaths are attributable to elevated blood pressure [2]. With the rapid aging of the world population over the next two decades, the cardiovascular morbidity associated with hypertension is likely to increase. This study focused on SBP rather than DBP for a number of reasons. Systolic hypertension is the most common form of hypertension among elderly people, present in approximately two-thirds of hypertensive individuals over 60 years of age [3]. Moreover, the disease burden of hypertension is more attributable to SBP than DBP [4], and SBP may be more responsive than DBP to changes in modifiable risk factors [5,6].
Many risk factors for hypertension have been identified [7,8], although a thorough understanding of the causes of hypertension and pathways through which some risk factors leads to hypertension remain unclear. Socioeconomic factors such as education and income have been shown to be inversely associated with blood pressure, although the mechanisms involved are not adequately understood [9–12]. The association between SBP and level of education has been studied cross-sectionally and longitudinally in younger and middle-aged populations [6,9–12] but not in an older population in whom education is relatively stable, hypertension is more prevalent and hypertensive morbidity and mortality are most common [1,3].
The objective of this study was to examine the association between level of education and SBP in a sample of older (mean age 78 years) community-living adults. Additionally, we evaluated specific factors that could explain the association between education attainment and SBP. We hypothesized that higher levels of education are significantly associated with lower levels of SBP independent of many risk factors for hypertension, and that this association might be attenuated by potential confounders or mediators.
METHODS
Study sample
Study samples were participants in the Maintenance of Balance, Independent Living, Intellect and Zest in the Elderly (MOBILIZE) Boston Study (MBS). The MBS is a prospective observational study of novel risk factors for falls among a large diverse sample of older individuals living in the greater Boston area who were enrolled during 2005–2007. The recruitment strategy targeted older persons living within a 5-mile radius of the Hebrew SeniorLife using probability sampling from town lists and census information. A comparison of the demographics of persons on the town lists with those in the US Census 2000 showed that the town lists have a comparable distribution by age and sex in the population aged 70 and older.
Eligibility and recruitment
Eligibility criteria included age of 70 years or older, ability to speak and understand English, ability to walk across a room, visual ability to read written material, no more than minimal cognitive impairment [Mini-Mental State Examination (MMSE) [13] score ≥18] and the expectation that participants will be living in the area for at least 3 years. Spouses or companions living with a participant also were allowed to join the study if they were eligible. A total of 118 (15%) participants lived with another study participant. Older adults were recruited through door-to-door visits and subsequently contacted via telephone by research staff to confirm eligibility and schedule a baseline assessment, which included a 3-h in-home interview and a 3-h in-clinic examination no more than 4 weeks later during which blood pressure was measured. A total of 816 participants had a baseline home visit, and 765 participants had a baseline clinical examination. Of these, 764 had information about years of education completed and were included in this study. Details of the study design have been previously published [14,15]. The Institutional Review Board of Hebrew SeniorLife approved the MBS, as well as this specific study.
SBP
Baseline SBP was calculated using a Baum (W.A. Baum Co., Copiague, New York, USA) calibrated sphygmomano-meter. The clinical examiners (nurses) were trained and certified in a standardized postural blood pressure protocol. They were trained in techniques to minimize measurement error and bias including use of correct cuff size and cuff positioning on a bare arm, using a pillow under the arm during supine measures if needed to bring the arm to heart level and recognition of auscultatory gaps. A dual-headed stethoscope was used for training and certification to identify and minimize expectation bias and terminal digit preference. The standardized postural blood pressure protocol included assessment for comfort prior to the start of the protocol including asking the participant if he/she needed to use the bathroom and assessing the comfort of room temperature. The participant was asked to rest supine for at least 5 min in a quiet room, to refrain from talking during the measurement procedure and to avoid crossing legs while resting supine.
Education
Baseline education level was self-reported as the highest completed grade or year of college. General Education Development, trade, vocational and technical school, representing only 1% of the sample, were considered equivalent to a 12th grade education. Education was analyzed both as a continuous variable and as a categorical variable using three a priori ordinal groups: high school or less (less than 12 years; lowest group), college (13–16 years; middle group) and graduate school (≥17 years; highest group) [10]. Indicator variables were created using graduate school education as the referent.
Covariates
Potential confounders, mediators and adjustment variables were identified based on a literature review and clinical experience. We considered age, sex and race as potential confounders of the association of education and SBP; Trails-B adjusted, MMSE score, alcohol use, income and self-rated health as mediators; and marital status and gait speed as adjustment variables.
Demographic variables included age, sex, race (white versus non-white), marital status (married or living with a partner versus never married, widowed, divorced or separated) and current household income (less than US$ 5000, 5000–$9999, 10 000–14 999, 15 000–24,99, 25 000– 34 999, 35 000–44 999, ≥45 000). Categories for household income were response options in the study questionnaire.
Health-related variables included DBP, self-reported history of diabetes, stroke, kidney disease, self-rated health, antihypertensive medication use (described below), medication adherence (described below), BMI (kg/m2), supine heart rate counted by a trained researcher using the dominant arm, minor depression based on the CESD-R [16], current smoking status, past-month frequency of alcohol intake, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol. Antihypertensive medication use was a binary variable that was coded positive if any medications were taken for blood pressure control. Medication adherence was defined by a sum of four questions about medication use and adherence; higher numbers indicate better adherence.
Physical function and cognitive variables included gait speed measured using a four meter walk; the Physical Activity Scale for the Elderly, a summary measure of physical activity level in the previous 7 days [17]; MMSE and the Trail-Making Test (TMT)-B [18,19]. The TMT part A is a timed neuropsychological test that measures simple visual search speed and planning, and part B measures those abilities in addition to executive functions such as task switching, planning and attention flexibility. Trails A score were subtracted from trails B to represent executive functions adjusted for psychomotor speed [18,19].
Statistical analyses
Means and percentages were used to describe participant characteristics for continuous and categorical variables, respectively. One-way analysis of variance and χ2-tests were used to test for statistical differences between education groups. The primary dependent variable in the study was SBP and the primary independent variables were two binary indicators for education representing high school or less and college. Graduate-level education served as the reference group. Bivariable linear regressions were used to examine the association between all predictors and SBP. We excluded DBP from regression analyses because it was too highly correlated with SBP and not associated with education. Education was also included as a continuous variable, although only the education group variables were included in the multivariate model. Significant predictors were included together in a multivariable model. Regression assumptions, including multivariable linearity between the outcome and predictors, distribution of residual errors and collinearity were evaluated using graphical displays. To test for differences in the association of education and SBP by sex, age and race, interaction between education and these variables was examined in regression models.
To model associations between education and SBP in a multivariable framework with multiple dependent variables, a path model was estimated to model hypothesized confounding and mediating relationships [20]. Education was treated as a continuously distributed variable to simplify the presentation of results.
The MBS included a follow-up assessment (median: 511 days) after the baseline assessment. In a sensitivity analysis to accommodate measurement error, the regression models described above with a random intercept model of SBP on predictors with a random slope for measurement occasion were re-estimated [21]. In another sensitivity analysis, correlations between SBP among household members were accounted for by including random effects for person and household.
Another sensitivity analysis was performed to assess the potential impact of unmeasured confounding due to parental and childhood socioeconomic status (SES) by estimating the amount of unmeasured confounding required to explain the observed difference in SBP by educational attainment [22]. The authors assumed a dichotomous unmeasured confounder, a binary indicator for low parental and childhood SES, was present with a different prevalence between the high school educated (exposed) and highest education group (unexposed) within the strata of measured confounders.
Analyses were performed using SAS (v9.2; SAS Institute Inc, Cary, North Carolina, USA) and Stata software (v12.1; Stata Corporation, College Station, Texas, USA). Path analyses were performed using Mplus software (v6.11; Muthén & Muthén, Los Angeles, California, USA). No variable was missing in more than 9% of the sample. To account for the small amount of missing data, we used iterative chained equation methods with 11 random draws per missing observation. [23] An α level of 0.05 was used to determine statistical significance and two-sided P values used.
RESULTS
Participants with greater education were younger, more likely to be white, men, married, had a higher income, had lower SBP, had a lower prevalence of diabetes or stroke, had better self-reported health, smoked less, drank alcohol more frequently, had a faster gait speed and had better cognitive and executive function (Table 1). Other covariates in Table 1 did not differ significantly by level of education. The overall average SBP (mmHg) was 130.4±18.2 SD (median 128) and the average level of education was 14.2±3.1 years (median 14). The majority (69.5%) of participants were on antihypertensive treatment, which is similar to other samples of older adults. [24]
TABLE 1.
Descriptive characteristics of the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly Boston study participants
| Full sample N=764 | High school (≤12 years) N=261 | College (13–16 years) N=266 | Graduate school (≥17 years) N=237 | P for group differences | |
|---|---|---|---|---|---|
| Demographic | |||||
| Age, M (SD) | 78.1 (5.4) | 78.7 (5.6) | 78.5 (5.5) | 77.2 (5.1) | 0.01 |
| Race (white), N (%) | 593 (77.7) | 161 (61.7) | 222 (83.8) | 210 (88.6) | <0.001 |
| Sex (male), N (%) | 275 (36.0) | 74 (28.4) | 86 (32.3) | 115 (48.5) | <0.001 |
| Married, N (%) | 325 (42.8) | 81 (31.2) | 109 (41.3) | 135 (57.2) | <0.001 |
| Household income, N (%) | <0.001 | ||||
| <US$ 5000 | 18 (2.6) | 11 (4.7) | 6 (2.5) | 1 (0.5) | |
| US$ 5000–9999 | 69 (10.0) | 38 (16.3) | 18 (7.4) | 13 (6.0) | |
| US$ 10 000–14 999 | 82 (11.8) | 53 (22.7) | 21 (8.7) | 8 (3.7) | |
| US$ 15 000–24,99 | 114 (16.4) | 59 (25.3) | 37 (15.3) | 18 (8.3) | |
| US$ 25 000–34 999 | 75 (10.8) | 27 (11.6) | 34 (14.0) | 14 (6.4) | |
| US$ 35 000–44 999 | 77 (11.1) | 21 (9.0) | 34 (14.0) | 22 (10.1) | |
| ≥US$ 45 000 | 258 (37.2) | 24 (10.3) | 92 (38.0) | 142 (65.1) | |
| Health-related | |||||
| SBP (mmHg), M (SD) | 130.4 (18.2) | 134.2 (20.2) | 130.7 (19.0) | 125.7 (13.5) | <0.001 |
| DBP (mmHg), M (SD) | 70.1 (8.7) | 70.0 (9.2) | 70.4 (8.8) | 69.9 (8.1) | 0.76 |
| Diabetes, N (%) | 140 (18.6) | 74 (28.8) | 43 (16.3) | 23 (9.9) | <0.001 |
| Stroke, N (%) | 76 (10.0) | 33 (12.7) | 28 (10.6) | 15 (6.4) | 0.02 |
| Kidney disease, N (%) | 44 (5.8) | 16 (6.1) | 17 (6.6) | 11 (4.7) | 0.50 |
| Self-rated health, M (SD) | 2.5 (1.0) | 2.1 (0.9) | 2.6 (0.9) | 2.7 (0.9) | <0.001 |
| Hypertension medication, N (%) | 531 (69.5) | 193 (73.9) | 180 (67.7) | 158 (66.7) | 0.07 |
| Medication adherence, M (SD) | 0.7 (0.9) | 0.8 (1.0) | 0.6 (0.8) | 0.7 (0.9) | 0.25 |
| BMI (kg/m2), M (SD) | 27.3 (5.1) | 27.8 (5.6) | 27.3 (5.0) | 26.9 (4.8) | 0.14 |
| Heart rate (pulse; beats/min), M (SD) | 66.1 (8.5) | 66.7 (9.3) | 66.0 (8.1) | 65.6 (8.0) | 0.34 |
| CESD-R minor depression, N (%) | 51 (6.7) | 19 (7.3) | 21 (7.9) | 11 (4.6) | 0.25 |
| Current smoker, N (%) | 37 (4.8) | 19 (7.3) | 16 (6.0) | 2 (0.8) | <0.001 |
| Alcohol drinking frequency, M (SD) | 2.1 (2.1) | 1.2 (1.8) | 2.1 (2.2) | 2.9 (2.2) | <0.001 |
| High-density lipoprotein cholesterol, M (SD) | 50.6 (15.9) | 48.3 (15.3) | 52.2 (16.4) | 51.3 (15.8) | 0.021 |
| Low-density lipoprotein cholesterol, M (SD) | 107.8 (33.6) | 107.6 (36.5) | 107.2 (32.2) | 108.6 (32.1) | 0.91 |
| Physical and cognitive function | |||||
| Gait speed (m/s), M (SD) | 0.9 (0.3) | 0.9 (0.2) | 1.0 (0.2) | 1.0 (0.3) | <0.001 |
| Physical activity (PASE), M (SD) | 107.5 (71.0) | 100.6 (62.8) | 107.6 (73.8) | 114.9 (75.6) | 0.08 |
| Trails B adjusted, M (SD) | 89.2 (63.9) | 123.4 (69.5) | 81.5 (58.5) | 64.6 (48.1) | <0.001 |
| Mini-Mental State Examination, M (SD) | 27.1 (2.6) | 25.6 (3.0) | 27.5 (2.1) | 28.2 (2.0) | <0.001 |
CESD-R, Center for Epidemiologic Studies Depression Scale-Revised; M, mean; N, sample size; PASE, Physical Activity Scale for the Elderly (higher numbers indicate more activity); SD, standard deviation.
Table 2 presents the coefficients and 95% confidence intervals (CIs) from bivariable linear regressions. The SBP of participants in the lowest education group was 8.46 mmHg higher on average than participants in the highest education group (95% CI: 5.31–11.60). The SBP of participants in the middle education group was on average 5.09 mmHg higher than participants in the highest education group (95% CI: 1.96–8.22). When education was examined as a continuously distributed variable, each year of education was associated with a significant decrease (−0.97 mmHg) in mean SBP (95% CI: −1.38 to −0.56). Older age, lower income, worse self-reported health, kidney disease and higher LDL cholesterol were associated with higher average SBP (Table 2). Lower executive function (trails B adjusted) and cognitive status ability (MMSE), slower gait speed and less frequent alcohol use were associated with higher mean SBP. Whites, men and married participants had lower SBP on average.
TABLE 2.
Separate (unadjusted) linear regressions of SBP on predictors (Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly Boston Study, n = 764)
| β | (95% CI) | |
|---|---|---|
| Education (categorical) | ||
| High school (≤12 years) | 8.46* | (5.31–11.60) |
| College | 5.09* | (1.96–8.22) |
| Graduate | REF | – |
| Education (years) | −0.97* | (−1.38 to −0.56) |
| Demographic | ||
| Age | 0.48* | (0.25–0.72) |
| Race (white) | −4.87* | (−7.94 to −1.80) |
| Sex (male) | −2.74* | (−5.42 to −0.06) |
| Married | −3.38* | (−5.98 to −0.78) |
| Household income | −1.10* | (−1.79 to −0.41) |
| Health-related | ||
| Diabetes | 2.46 | (−0.85 to 5.76) |
| Stroke | 2.14 | (−2.17 to 6.45) |
| Kidney disease | 6.97* | (1.62 to 12.32) |
| Self-rated health | −2.08* | (−3.39 to −0.76) |
| Hypertension medication | 1.74 | (−1.06 to 4.55) |
| Medication adherence | −0.69 | (−2.09 to 0.71) |
| BMI | 0.08 | (−0.18 to 0.33) |
| Heart rate (pulse) | 0.00 | (−0.15 to 0.15) |
| CESD-R minor depression | −1.99 | (−7.16 to 3.18) |
| Current smoker | 4.45 | (−1.56 to 10.45) |
| Alcohol drinking frequency | −0.59* | (−1.19 to 0.00) |
| High-density lipoprotein cholesterol | 0.03 | (−0.05 to 0.11) |
| Low-density lipoprotein cholesterol | 0.06* | (0.03–0.10) |
| Physical and cognitive function | ||
| Gait speed (m/s) | −6.96* | (−11.94 to −1.97) |
| Physical activity (PASE) | 0.00 | (−0.02 to 0.01) |
| Trails B Adjusted | 0.04* | (0.02–0.06) |
| Mini-Mental State Examination | −0.91* | (−1.40 to −0.40) |
CESD-R, Center for Epidemiologic Studies Depression Scale-Revised; CI, confidence interval; PASE, Physical Activity Scale for the Elderly (higher numbers indicate more activity). Regression coefficients are interpretable as the average difference in the outcome per unit difference in the predictor.
P<0.05
Table 3 presents the coefficients and 95% CI from multivariable (adjusted) linear regressions of SBP on predictors that were statistically significant in bivariable models. After adjusting for age, race, sex, marital status, household income, kidney disease, self-rated health, alcohol drinking frequency, LDL cholesterol, gait speed, executive function and cognitive ability, level of education remained inversely associated with SBP. Participants in the lowest education group had a 6.41 mmHg higher SBP compared to the participants in the highest education group (95% CI: 2.67–10.15). Participants in the middle education group had 4.04 mmHg higher SBP compared to participants in the highest education group (95% CI: 0.82–7.26). The association between education and SBP was not modified by sex, age or race. Additionally, we ran all analyses among participants who were not on antihypertensive treatment. Although the association was somewhat attenuated, there was still a significant association [e.g. ≤high school (referent: graduate) β=7.1 95% CI (1.5–9.4)] in the subsample.
TABLE 3.
Multivariable (adjusted) linear regression of SBP on predictors (Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly Boston Study, n = 764)
| β | (95% CI) | |
|---|---|---|
| Education | ||
| High school (≤12 years) | 6.41* | (2.67–10.15) |
| College | 4.04* | (0.82–7.26) |
| Graduate | REF | – |
| Demographic | ||
| Age | 0.47* | (0.21–0.73) |
| Race (White) | −3.52* | (−7.04 to −0.01) |
| Sex (Male) | −1.49 | (−4.43 to 1.46) |
| Married | −1.36 | (−4.35 to 1.64) |
| Household income | 0.49 | (−0.43 to 1.42) |
| Health-related | ||
| Kidney disease | 6.77* | (1.17–12.36) |
| Self-rated health | −1.29 | (−2.76 to 0.19) |
| Alcohol drinking frequency | 0.19 | (−0.50 to 0.89) |
| Low-density lipoprotein cholesterol | 0.06* | (0.02–0.10) |
| Physical and cognitive function | ||
| Gait speed (m/s) | 1.65 | (−4.32 to 7.62) |
| Trails B adjusted | 0.01 | (−0.02 to 0.03) |
| Mini-Mental State Examination | −0.07 | (−0.71 to 0.57) |
CI, confidence interval.
P<0.05.
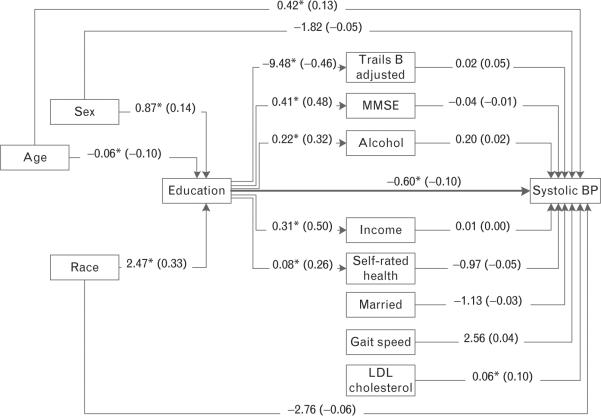
Results of path analyses that modeled potential confounding and mediating effects of the variables in Table 3 are showed in Fig. 1. Findings are consistent with results from regression analysis. Education had a direct inverse effect on SBP (β=−0.60 mmHg per year of education, 95% CI: −1.15 to −0.06). Older age and LDL cholesterol were the only other significant predictors of SBP. Male sex, younger age and white race were associated with higher educational attainment. Although higher education was associated with better TMT-B adjusted, higher MMSE, greater alcohol use, higher income and better self-rated health, the sum of the indirect associations of education and SBP through these mediators was not significant (nonstandardized indirect effect =−0.19 mmHg per year of education, 95% CI: −0.59 to 0.20; not shown in Fig. 1). The sum of direct and indirect effects of education on SBP, the total effect, was statistically significant (nonstandardized total effect=−0.80 mmHg per year of education, 5% CI: −1.28 to −0.32; between not shown in Fig. 1). Relationships education and marital status, gait speed and LDL cholesterol were not specified because direct associations between these variables were not hypothesized.
FIGURE 1.
Path diagram estimating direct and indirect effects from education to SBP (Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly Boston Study). Regression coefficients are interpretable as the average difference in the outcome per unit difference in the predictor. The direct effect between education and SBP [β = −0.60 mmHg per year of education, 95% confidence interval (CI): −1.15 to −0.06] is shown in the Figure. Indirect effects are associations between education and blood pressure (BP) that occur through mediating variables and were not statistically significant (nonstandardized indirect effect= −0.19 mmHg per year of education, 95% CI: −0.59 to 0.20; not shown in Figure). The sum of direct and indirect effects is the total effect (nonstandardized total effect= −0.80 mmHg per year of education, 95% CI: −1.28 to −0.32; not shown in Figure). Values shown on lines are raw (standardized) regression coefficients. *P < 0.05. BP, blood pressure; HTN, hypertension; LDL, Low-density lipoprotein cholesterol; MMSE, Mini-Mental State Examination.
In a sensitivity analysis, SBP measurement error was examined for by taking into account a second SBP measurement using random effects models with a random intercept for participant. This approach yielded similar results to the main analyses presented (high school versus graduate school: β=3.73 mmHg, 95% CI: 1.48–5.98; college versus graduate school: β=3.19 mmHg, 95% CI: 0.30–6.10) because although the average correlation between SBP within a person was 0.46 (intraclass correlation), the average number of observations per group was small (1.8). To accommodate correlations among members of the same household, another random effects model on baseline data with a random intercept for household was estimated. The average correlation between SBP among participants sharing a household was 0, and relationships between education and SBP were mostly unchanged (high school versus graduate school: β=8.52 mmHg, 95% CI: 5.36–11.70; college versus graduate school: β=5.02 mmHg, 95% CI: 1.86–8.17).
In sensitivity analysis, controlling for varying degrees of unmeasured confounding due to parental and childhood SES, the corrected estimated differences in SBP by education group were attenuated toward the null. However, the amount of unmeasured confounding necessary to explain away the entire difference in SBP between low and high education groups was implausible. When the effect of low parental and childhood SES (versus high) on SBP was 6 mmHg, as previously reported, [25] low SES had to be 40% more prevalent in low education group than in high education group in order to explain away all the observed effect of low education on SBP. This result suggests robustness of our findings against unmeasured confounding (results available upon request).
DISCUSSION
Study results indicate that level of education is inversely associated with SBP in a diverse cohort of community-living older adults, independent of numerous risk factors for hypertension. Multivariable regression and path analysis results show that risk factors attenuated the association between educational attainment and SBP but did not nullify the significant association. This study replicates finding from similar studies involving younger participants and additionally offers new findings of importance. First, this study is the first to report the association between education and SBP in an older sample, representing a population at the highest risk for hypertension-related morbidity and mortality. Second, in cross-sectional studies involving younger adults, it is difficult to identify the temporality of the relationship between education and SBP. However, in the current study most participants were in their seventh decade, suggesting a distant effect of education on SBP, as education was likely completed decades before the SBP assessment. Thus, level of education could have influenced SBP.
A third important finding of the present study is because older individuals are at the highest risk of hypertension-related morbidity and mortality, it is possible to apply findings from studies involving older individuals at risk for hypertension-related cardiovascular disease. For example, higher SBP is associated with an elevated risk of heart disease. Published multivariable-adjusted proportional-hazards regression analyses using Framingham Heart Study participants aged 60 years and older indicate that a 10 mmHg increase in SBP is associated with a 17% increased risk of developing coronary heart disease (CHD) [26]. Applying this information to our findings with education and SBP, MBS participants with less than or equal to a high school education would have approximately a 11% increased risk of developing CHD relative to those with higher than a college education, independent of many known risk factors. The difference between a college degree and graduate school represents approximately a 7% increase in CHD risk.
The findings of this study of older adults are consistent with published cross-sectional and longitudinal studies of younger adults. Using a nationally representative sample of 14 000 young adults (mean age 29 years), Brummett et al. [6] assessed the association of SES and SBP and examined the role of potential mediators. They reported that higher income and education were significantly associated with lower SBP in the age-adjusted, sex-adjusted and medication-adjusted analyses but the education association statistically lost significance after further adjustment for BMI, waist circumference and heart rate. In contrast, the present study and another [27] reported income and education were associated with SBP or hypertension in unadjusted analyses, but only education was associated with SBP or hypertension after multivariable adjustment. Differences in findings may be partially explained by age differences of study participants and meanings of income and education in these samples. In younger populations, income can fluctuate over time and is thus susceptible to misclassification. In contrast, income in an older population, wherein persons may have accumulated wealth and are earning social security and pension, may have a different relationship with health-related behaviors than in a younger population that is actively working [27]. Moreover, education is likely more static in an older population relative to a younger population, making it a more influential predictor of behaviors than other known risk factors associated with hypertension.
Loucks et al. [10] examined the association between SBP and education level among 3890 participants of the Framingham Offspring Study (mean age 37 years). Using multiple longitudinal assessments, multivariable-adjusted mixed linear models revealed that education level was inversely associated with SBP. However, this study did not adjust for income, which has been shown to be associated with education and SBP in the current study as well as other studies [6,27].
Conen et al. [27] prospectively examined the association between education and blood pressure progression and incident hypertension among 27 207 female health professionals (mean age 54 years). After multivariable adjustment, lower educational level was significantly associated with increased risk of blood pressure progression and incident hypertension.
Diez Roux et al. [9] studied 8555 participants (mean age 53 years) longitudinally over 9 years. Proportional hazards regressions revealed that lower education level was associated with a higher likelihood of being hypertensive after adjustment for age and sex. These associations were only marginally significant when further adjusted for baseline blood pressure.
Strand and Tverdal [11] studied the effects of educational inequalities in cardiovascular risk factors longitudinally in 48 422 Norwegian residents (age range 35–49 years). Based on sex-stratified mixed effects linear age-adjusted models, they found that higher education level was associated with lower SBP.
Among 2913 participants aged 18–30 years, Yan et al. [12] found that those with less than a high school education had an average 15-year mean increase in SBP of 8.2 mmHg, whereas participants with greater than a college degree education had an average 15-year mean increase in SBP of only 0.7 mmHg. Although this longitudinal difference was not adjusted for potential confounders, it was statistically significant.
A limitation of this study is that its cross-sectional design limits causal inferences. However, given the average age (78 years) of the participants, it is very likely that their level of education was completed many decades before the SBP assessment, suggesting that education was attained long before the SBP assessment. Another limitation is that we were unable to account for some factors that might influence the association between education and SBP, such as family history of hypertension, genetic profiles, diet, waist circumference, stress and occupation. An additional limitation is that persons with cognitive impairment (MMSE <18) were excluded from the study. Older adults living with dementia tend to have lower blood pressures [28,29] and less education [30], which would weaken associations found in our study. Thus, our findings may not generalize to more cognitively impaired populations. Finally, MBS participants were older, community-living and predominately white; accordingly the findings may not generalize to individuals of different races or residences.
Other possible mechanisms should be considered in future research. Family history of hypertension may partially account for the association between education and SBP. Genetic heritability of intelligence is about 50% [31]. Parental blood pressure was reported as a strong determinant of the natural history of blood pressure in their offspring from childhood into young adulthood [32]. Genetic profiles may also influence the association between education and SBP [33]. Diet is known to be associated with hypertension and education. A diet rich in fruits, vegetables and low-fat dairy products, and reduced saturated and total fat intake, was associated with reduced blood pressure [34]. Sodium intake was reported to decrease as educational level increased [35]. Intake of energy from fat and dietary cholesterol has been shown to decrease [36], and intake of fruits and vegetables increase by increasing level of education [36,37]. Lower educational attainment has been associated with stressful jobs involving high demands and low job control, which have been associated with hypertension [38,39].
The findings of this study strongly suggest that educational attainment is inversely associated with SBP in a community-living cohort of older individuals, independent of many risk factors for hypertension. The results are consistent with previous studies of younger individuals and suggest that a considerable proportion of CHD may be attributable to the influence of low education on hyper-tension. Given the relatively low amount of total variance in SBP that is explained by education, other factors not examined in this study are likely involved in this association. Future studies should examine genetic, nutritional and other factors that might further help to explain the complex association between educational attainment and SBP in elderly people.
ACKNOWLEDGEMENTS
We thank the participants, staff and investigators of the MOBILIZE Boston Study who made this study possible.
This work was supported by National Institute on Aging Program Project Grants P01AG004390 and P01AG031720, and a Merit Award R37AG025037. A.L.G. was supported by a National Institutes of Health Translational Research in Aging fellowship (T32AG023480-07). L.A.L. holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife.
Abbreviations
- CESD-R
Center for Epidemiologic Studies Depression Scale-revised
- CHD
coronary heart disease
- CI
confidence interval
- MBS
MOBILIZE Boston Study
- MMSE
Mini-Mental State Examination
- MOBILIZE
Maintenance of Balance Independent Living Intellect and Zest in the Elderly
- PASE
Physical Activity Scale for the Elderly
- SES
socioeconomic status
- TMT
trail-making test
Footnotes
Conflicts of interest There are no conflicts of interest.
REFERENCES
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 3.Izzo JL, Jr, Levy D, Black HR. Clinical advisory statement. Importance of systolic blood pressure in older Americans. Hypertension. 2000;35:1021–1024. doi: 10.1161/01.hyp.35.5.1021. [DOI] [PubMed] [Google Scholar]
- 4.Williams B, Lindholm LH, Sever P. Systolic pressure is all that matters. Lancet. 2008;371:2219–2221. doi: 10.1016/S0140-6736(08)60804-1. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med. 2010;170:126–135. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brummett BH, Babyak MA, Siegler IC, Shanahan M, Harris KM, Elder GH, et al. Systolic blood pressure, socioeconomic status, and biobehavioral risk factors in a nationally representative US young adult sample. Hypertension. 2011;58:161–166. doi: 10.1161/HYPERTENSIONAHA.111.171272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kannel WB. Risk factors in hypertension. J Cardiovasc Pharmacol. 1989;13(Suppl 1):S4–10. doi: 10.1097/00005344-198900131-00003. [DOI] [PubMed] [Google Scholar]
- 8.Whelton PK. Epidemiology of hypertension. Lancet. 1994;344:101–106. doi: 10.1016/s0140-6736(94)91285-8. [DOI] [PubMed] [Google Scholar]
- 9.Diez Roux AV, Chambless L, Merkin SS, Arnett D, Eigenbrodt M, Nieto FJ, et al. Socioeconomic disadvantage and change in blood pressure associated with aging. Circulation. 2002;106:703–710. doi: 10.1161/01.cir.0000025402.84600.cd. [DOI] [PubMed] [Google Scholar]
- 10.Loucks EB, Abrahamowicz M, Xiao Y, Lynch JW. Associations of education with 30 year life course blood pressure trajectories: Framingham Offspring Study. BMC Public Health. 2011;11:139. doi: 10.1186/1471-2458-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strand BH, Tverdal A. Trends in educational inequalities in cardiovascular risk factors: a longitudinal study among 48 000 middle-aged Norwegian men and women. Eur J Epidemiol. 2006;21:731–739. doi: 10.1007/s10654-006-9046-5. [DOI] [PubMed] [Google Scholar]
- 12.Yan LL, Liu K, Daviglus ML, Colangelo LA, Kiefe CI, Sidney S, et al. Education, 15-year risk factor progression, and coronary artery calcium in young adulthood and early middle age: the Coronary Artery Risk Development in Young Adults study. JAMA. 2006;295:1793–1800. doi: 10.1001/jama.295.15.1793. [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Leveille SG, Kiel DP, Jones RN, Roman A, Hannan MT, Sorond FA, et al. The MOBILIZE Boston Study: design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC Geriatr. 2008;8:16. doi: 10.1186/1471-2318-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samelson EJ, Kelsey JL, Kiel DP, Roman AM, Cupples LA, Freeman MB, et al. Issues in conducting epidemiologic research among elders: lessons from the MOBILIZE Boston Study. Am J Epidemiol. 2008;168:1444–1451. doi: 10.1093/aje/kwn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eaton W, Muntaner C, Smith C, Tien A, Ybarra M, editors. Center for epidemiologic studies depression scale: review and revision (CESD and CESD-R) Lawence Erlbaum Association, Inc.; Mahwah, NJ: 2004. [Google Scholar]
- 17.Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 18.Gordon NG. The trail making test in neuropsychological diagnosis. J Clin Psychol. 1972;28:167–169. doi: 10.1002/1097-4679(197204)28:2<167::aid-jclp2270280212>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Reitan R. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 20.Wright S. The method of path coefficients. Ann Math Stat. 1934;5:161–215. [Google Scholar]
- 21.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 22.Vanderweele TJ, Arah OA. Bias formulas for sensitivity analysis of unmeasured confounding for general outcomes, treatments, and confounders. Epidemiology. 2011;22:42–52. doi: 10.1097/EDE.0b013e3181f74493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin D. Multiple imputation for nonresponse in surveys. Wiley & Sons; New York: 1987. [Google Scholar]
- 24.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005;294:466–472. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- 25.Kivimaki M, Smith GD, Elovainio M, Pulkki L, Keltikangas-Jarvinen L, Talttonen L, et al. Socioeconomic circumstances in childhood and blood pressure in adulthood: the cardiovascular risk in young Finns study. Ann Epidemiol. 2006;16:737–742. doi: 10.1016/j.annepidem.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. doi: 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- 27.Conen D, Glynn RJ, Ridker PM, Buring JE, Albert MA. Socioeconomic status, blood pressure progression, and incident hypertension in a prospective cohort of female health professionals. Eur Heart J. 2009;30:1378–1384. doi: 10.1093/eurheartj/ehp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 29.Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 30.Stern Y, Tang MX, Denaro J, Mayeux R. Increased risk of mortality in Alzheimer's disease patients with more advanced educational and occupational attainment. Ann Neurol. 1995;37:590–595. doi: 10.1002/ana.410370508. [DOI] [PubMed] [Google Scholar]
- 31.Plomin R, Spinath FM. Intelligence: genetics, genes, and genomics. J Pers Soc Psychol. 2004;86:112–129. doi: 10.1037/0022-3514.86.1.112. [DOI] [PubMed] [Google Scholar]
- 32.van den Elzen AP, de Ridder MA, Grobbee DE, Hofman A, Witteman JC, Uiterwaal CS. Families and the natural history of blood pressure. A 27-year follow-up study. Am J Hypertens. 2004;17:936–940. doi: 10.1016/j.amjhyper.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Levy D, DeStefano AL, Larson MG, O'Donnell CJ, Lifton RP, Gavras H, et al. Evidence for a gene influencing blood pressure on chromosome 17: genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the Framingham Heart Study. Hypertension. 2000;36:477–483. doi: 10.1161/01.hyp.36.4.477. [DOI] [PubMed] [Google Scholar]
- 34.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 35.Tian HG, Hu G, Dong QN, Yang XL, Nan Y, Pietinen P, et al. Dietary sodium and potassium, socioeconomic status and blood pressure in a Chinese population. Appetite. 1996;26:235–246. doi: 10.1006/appe.1996.0018. [DOI] [PubMed] [Google Scholar]
- 36.Popkin BM, Zizza C, Siega-Riz AM. Who is leading the change?. U.S. dietary quality comparison between 1965 and 1996. Am J Prev Med. 2003;25:1–8. doi: 10.1016/s0749-3797(03)00099-0. [DOI] [PubMed] [Google Scholar]
- 37.Kant AK, Graubard BI. Secular trends in the association of socioeconomic position with self-reported dietary attributes and biomarkers in the US population: National Health and Nutrition Examination Survey (NHANES) 1971–1975 to NHANES 1999–2002. Public Health Nutr. 2007;10:158–167. doi: 10.1017/S1368980007246749. [DOI] [PubMed] [Google Scholar]
- 38.Schnall PL, Pieper C, Schwartz JE, Karasek RA, Schlussel Y, Devereux RB, et al. The relationship between `job strain,' workplace diastolic blood pressure, and left ventricular mass index. Results of a case-control study. JAMA. 1990;263:1929–1935. [PubMed] [Google Scholar]
- 39.Steptoe A, Willemsen G. The influence of low job control on ambulatory blood pressure and perceived stress over the working day in men and women from the Whitehall II cohort. J Hypertens. 2004;22:915–920. doi: 10.1097/00004872-200405000-00012. [DOI] [PubMed] [Google Scholar]