Abstract
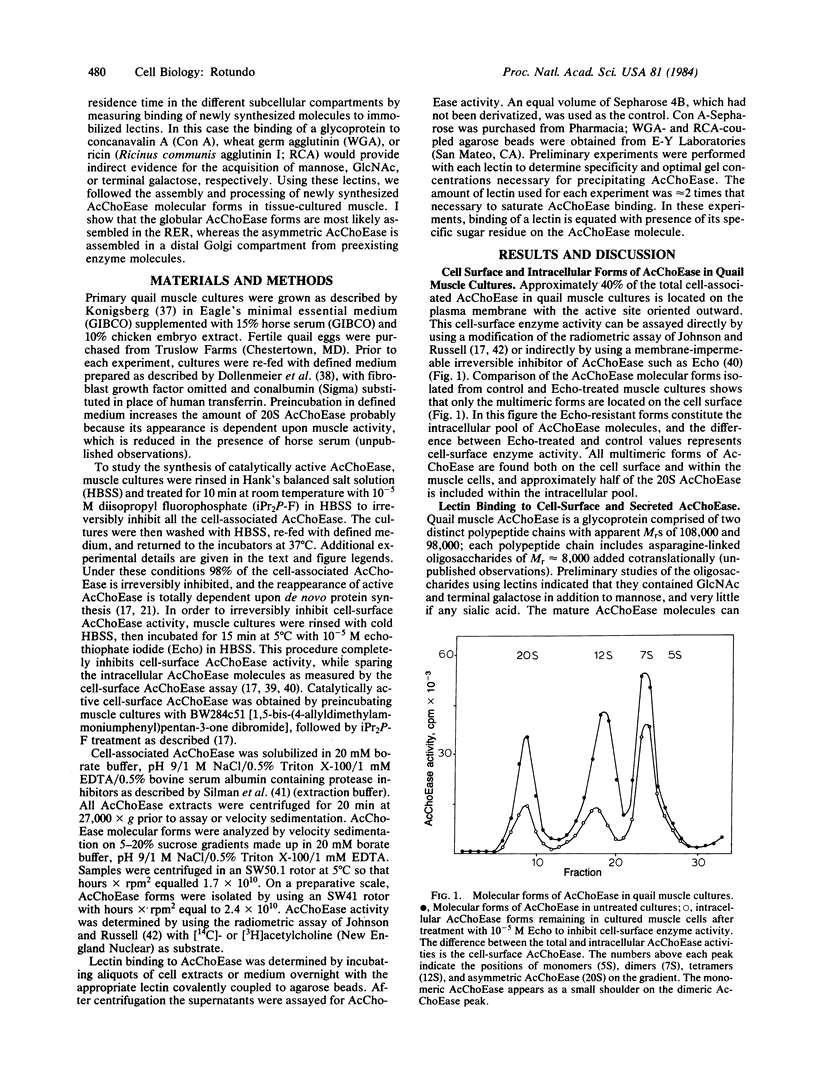
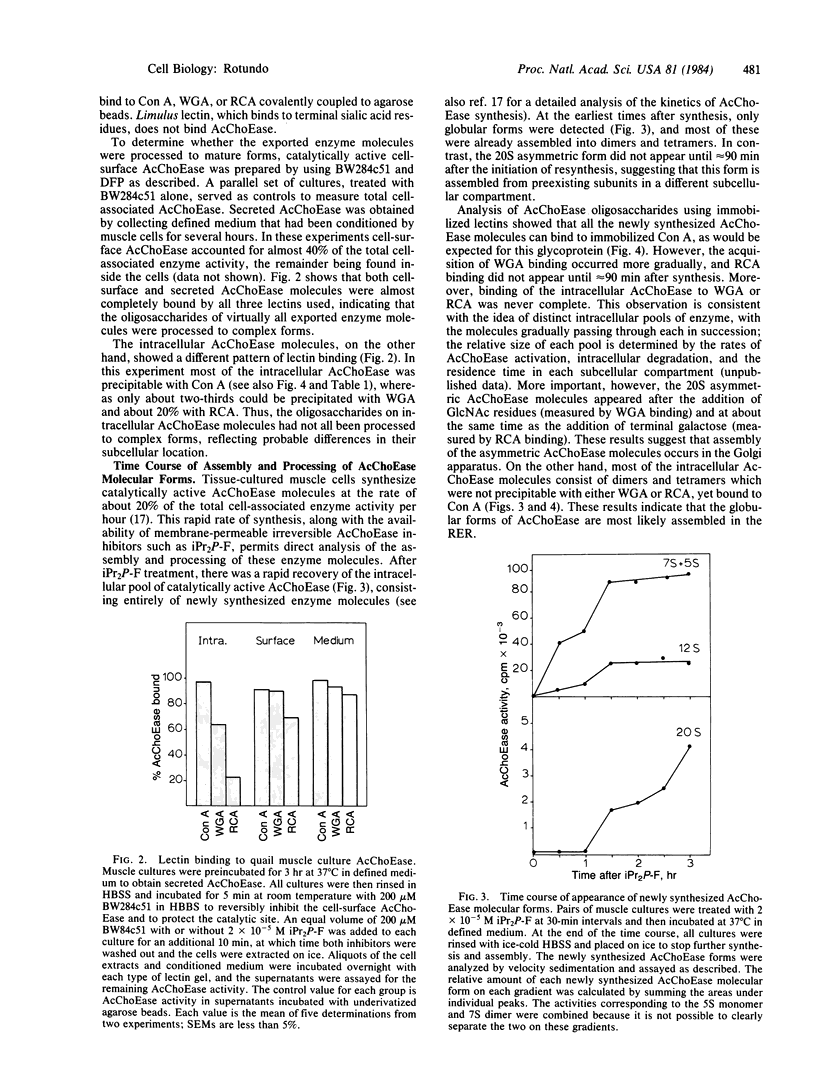
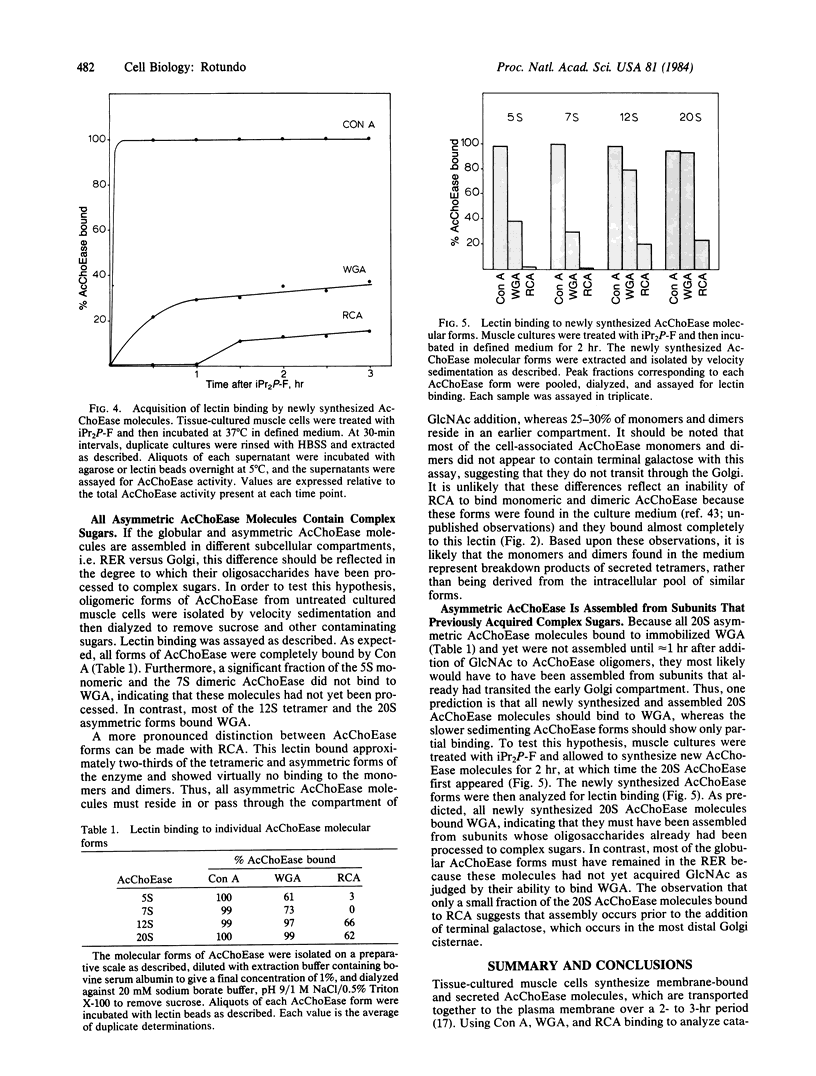
The synthesis, assembly, and processing of the multiple molecular forms of acetylcholinesterase (AcChoEase; acetylcholine acetylhydrolase, EC 3.1.1.7) in quail muscle cultures was studied by using lectins to distinguish enzyme molecules residing in different subcellular compartments. Special emphasis was given to the assembly of asymmetric AcChoEase molecules because these appear to be the predominant, if not unique, forms of AcChoEase at the vertebrate neuromuscular junction. All cell surface and secreted AcChoEase forms bind to immobilized wheat germ agglutinin, ricin, and concanavalin A, indicating that they have complex oligosaccharides. After treatment of muscle cells with a membrane-permeable irreversible AcChoEase inhibitor, there is a rapid reappearance of the globular monomeric, dimeric, and tetrameric AcChoEase forms. However, the collagen-tailed asymmetric form does not appear until about 90 min after treatment. Analysis of the AcChoEase oligosaccharides with lectins indicates maturation to complex forms over a 90-min period. A large fraction of the intracellular globular AcChoEase molecules bind only to concanavalin A, indicating that they are assembled in the rough endoplasmic reticulum. In contrast, all intracellular asymmetric AcChoEase binds to wheat germ agglutinin, and a significant fraction binds to ricin, indicating that this unique AcChoEase form is assembled from subunits that have previously acquired complex sugars. I conclude that assembly of asymmetric AcChoEase, hence acquisition of information specifying basal lamina localization, occurs in the Golgi apparatus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anglister L., Silman I. Molecular structure of elongated forms of electric eel acetylcholinesterase. J Mol Biol. 1978 Nov 5;125(3):293–311. doi: 10.1016/0022-2836(78)90404-7. [DOI] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bon S., Cartaud J., Massoulié J. The dependence of acetylcholinesterase aggregation at low ionic strength upon a polyanionic component. Eur J Biochem. 1978 Apr;85(1):1–14. doi: 10.1111/j.1432-1033.1978.tb12207.x. [DOI] [PubMed] [Google Scholar]
- Brockman S. K., Przybylski R. J., Younkin S. G. Cellular localization of the molecular forms of acetylcholinesterase in cultured embryonic rat myotubes. J Neurosci. 1982 Dec;2(12):1775–1785. doi: 10.1523/JNEUROSCI.02-12-01775.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger J. E., Randall W. R., Nieberg P. S., Patterson G. T., McNamee M. G., Wilson B. W. Regulation of acetylcholinesterase forms in quail and chicken muscle cultures. Dev Neurosci. 1982;5(5-6):474–483. doi: 10.1159/000112708. [DOI] [PubMed] [Google Scholar]
- Dollenmeier P., Turner D. C., Eppenberger H. M. Proliferation and differentiation of chick skeletal muscle cells cultured in a chemically defined medium. Exp Cell Res. 1981 Sep;135(1):47–61. doi: 10.1016/0014-4827(81)90298-6. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Brands R., Burke B., Louvard D., Warren G. Viral membrane proteins acquire galactose in trans Golgi cisternae during intracellular transport. J Cell Biol. 1982 Dec;95(3):781–792. doi: 10.1083/jcb.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z. W., Kelly R. B. Enzymatic detachment of endplate acetylcholinesterase from muscle. Nat New Biol. 1971 Jul 14;232(28):62–63. doi: 10.1038/newbio232062a0. [DOI] [PubMed] [Google Scholar]
- Hall Z. W. Multiple forms of acetylcholinesterase and their distribution in endplate and non-endplate regions of rat diaphragm muscle. J Neurobiol. 1973;4(4):343–361. doi: 10.1002/neu.480040404. [DOI] [PubMed] [Google Scholar]
- Johnson C. D., Russell R. L. A rapid, simple radiometric assay for cholinesterase, suitable for multiple determinations. Anal Biochem. 1975 Mar;64(1):229–238. doi: 10.1016/0003-2697(75)90423-6. [DOI] [PubMed] [Google Scholar]
- Koenig J., Vigny M. Neural induction of the 16S acetylcholinesterase in muscle cell cultures. Nature. 1978 Jan 5;271(5640):75–77. doi: 10.1038/271075a0. [DOI] [PubMed] [Google Scholar]
- Konigsberg I. R. Skeletal myoblasts in culture. Methods Enzymol. 1979;58:511–527. doi: 10.1016/s0076-6879(79)58166-x. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Heinemann S., Taylor P. Structural characterization of the asymmetric (17 + 13) S forms of acetylcholinesterase from Torpedo. I. Analysis of subunit composition. J Biol Chem. 1982 Oct 25;257(20):12282–12291. [PubMed] [Google Scholar]
- Lwebuga-Mukasa J. S., Lappi S., Taylor P. Molecular forms of acetylcholinesterase from Torpedo californica: their relationship to synaptic membranes. Biochemistry. 1976 Apr 6;15(7):1425–1434. doi: 10.1021/bi00652a012. [DOI] [PubMed] [Google Scholar]
- Lømo T., Slater C. R. Control of junctional acetylcholinesterase by neural and muscular influences in the rat. J Physiol. 1980 Jun;303:191–202. doi: 10.1113/jphysiol.1980.sp013280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoulié J., Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annu Rev Neurosci. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- McMahan U. J., Sanes J. R., Marshall L. M. Cholinesterase is associated with the basal lamina at the neuromuscular junction. Nature. 1978 Jan 12;271(5641):172–174. doi: 10.1038/271172a0. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Rieger F., Koenig J., Vigny M. Spontaneous contractile activity and the presence of the 16 S form of acetylcholinesterase in rat muscle cells in culture: reversible suppressive action of tetrodotoxin. Dev Biol. 1980 May;76(2):358–365. doi: 10.1016/0012-1606(80)90385-1. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L., Richardson J. M. Structure of 18S and 14S acetylcholinesterase. Identification of collagen-like subunits that are linked by disulfide bonds to catalytic subunits. Biochemistry. 1977 Aug 9;16(16):3550–3558. doi: 10.1021/bi00635a008. [DOI] [PubMed] [Google Scholar]
- Roth J., Berger E. G. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol. 1982 Apr;93(1):223–229. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E. The golgi apparatus: two organelles in tandem. Science. 1981 Sep 11;213(4513):1212–1219. doi: 10.1126/science.7268428. [DOI] [PubMed] [Google Scholar]
- Rotundo R. L., Fambrough D. M. Secretion of acetylcholinesterase: relation to acetylcholine receptor metabolism. Cell. 1980 Nov;22(2 Pt 2):595–602. doi: 10.1016/0092-8674(80)90369-4. [DOI] [PubMed] [Google Scholar]
- Rotundo R. L., Fambrough D. M. Synthesis, transport and fate of acetylcholinesterase in cultured chick embryos muscle cells. Cell. 1980 Nov;22(2 Pt 2):583–594. doi: 10.1016/0092-8674(80)90368-2. [DOI] [PubMed] [Google Scholar]
- Rubin L. L., Schuetze S. M., Fischbach G. D. Accumulation of acetylcholinesterase at newly formed nerve--muscle synapases. Dev Biol. 1979 Mar;69(1):46–58. doi: 10.1016/0012-1606(79)90273-2. [DOI] [PubMed] [Google Scholar]
- Rubin L. L., Schuetze S. M., Weill C. L., Fischbach G. D. Regulation of acetylcholinesterase appearance at neuromuscular junctions in vitro. Nature. 1980 Jan 17;283(5744):264–267. doi: 10.1038/283264a0. [DOI] [PubMed] [Google Scholar]
- Silman I., di Giamberardino L., Lyles L., Couraud J. Y., Barnard E. A. Parallel regulation of acetylcholinesterase and pseudocholinesterase in normal, denervated and dystrophic chicken skeletal muscle. Nature. 1979 Jul 12;280(5718):160–162. doi: 10.1038/280160a0. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M. The Golgi complex: crossroads for vesicular traffic. Int Rev Exp Pathol. 1980;22:227–251. [PubMed] [Google Scholar]
- Torres J. C., Behrens M. I., Inestrosa N. C. Neural 16S acetylcholinesterase is solubilized by heparin. Biochem J. 1983 Oct 1;215(1):201–204. doi: 10.1042/bj2150201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg C. B., Hall Z. W. Junctional form of acetylcholinesterase restored at nerve-free endplates. Dev Biol. 1979 Feb;68(2):631–635. doi: 10.1016/0012-1606(79)90233-1. [DOI] [PubMed] [Google Scholar]
- Wilson B. W., Nieberg P. S. Recovery of acetylcholinesterase forms in quail muscle cultures after intoxication with diisopropylfluorophosphate. Biochem Pharmacol. 1983 Mar 1;32(5):911–918. doi: 10.1016/0006-2952(83)90596-8. [DOI] [PubMed] [Google Scholar]
- Wilson B. W., Nieberg P. S., Walker C. R., Linkhart T. A., Fry D. M. Production and release of acetylcholinesterase by cultured chick embryo muscle. Dev Biol. 1973 Aug;33(2):285–299. doi: 10.1016/0012-1606(73)90138-3. [DOI] [PubMed] [Google Scholar]
- Wilson B. W., Walker C. R. Regulation of newly synthesized acetylcholinesterase in muscle cultures treated with diisopropylfluorophosphate. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3194–3198. doi: 10.1073/pnas.71.8.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]



