A 22-day-old male neonate was referred to our emergency department from a community hospital after realization that an acute acetaminophen overdose had occurred, following routine circumcision.
The patient had been born at 40 weeks’ gestation after an uneventful delivery to a gravida 1 mother. The pregnancy had been complicated by pregnancy-induced hypertension, treated with labetalol. Prior to the overdose, the baby had been well.
On the day of presentation, the patient had been given about 800 mg (200 mg/kg) of acetaminophen by his parents before circumcision. The parents had been instructed, by their physician, to give him 40 mg of acetaminophen before bringing him to the hospital for the procedure. The patient’s weight was 4.1 kg; thus, this was an intended dose of 10 mg/kg. The bottle of acetaminophen showed a concentration of 80 mg/mL, which was misinterpreted by the parents, in that they believed that the bottle contained 80 mg of acetaminophen in total. The child was given 10 mL, or about half of the bottle, with the intent of giving him 40 mg. The child underwent his circumcision, and, following the procedure, the physician instructed the parents to give him another dose of acetaminophen if he seemed uncomfortable. At that point, the mother commented that “it seemed like a lot of medicine,” and the error was discovered.
The acetaminophen blood concentration drawn four hours after the overdose was substantially elevated at 1243 (upper end of therapeutic range 66–199) μmol/L. Initial liver indices were normal. Total bilirubin level was 20.4 (normal 3–17) μmol/L, glucose level was 5.7 (normal 4–8) mmol/L and albumin level was 33 (normal 32–45) g/L. Complete blood count, electrolytes and renal function tests were normal. Blood gas, international normalized ratio and partial thromboplastin time tests were not conducted.
The regional poison control centre was consulted. Given that the patient had received more than the toxic dose of 150 mg/kg and because the four-hour blood concentration level of acetaminophen was in the probable toxicity range on the Rumack–Matthew nomogram, treatment with N-acetylcysteine was recommended. Activated charcoal was not given.
Treatment was started within eight hours of the overdose. A standard intravenous N-acetylcysteine protocol was started with 150 mg/kg of N-acetylcysteine mixed in 12 mL of 5% dextrose (3 mL/kg) and infused over 60 minutes, followed by 50 mg/kg of N-acetylcysteine mixed in 40 mL of 5% dextrose (10 mL/kg) and infused over 4 hours, then 100 mg/kg of N-acetylcysteine in 80 mL of 5% dextrose (20 mL/kg) infused over 16 hours. The patient was monitored on the general pediatrics floor and continued to breastfeed well.
Liver indices were repeated 25 hours after the ingestion (18 h after the infusion of N-acetylcysteine had started) and remained normal. At that point, no acetaminophen was detectable in the patient’s blood serum. The N-acetylcysteine infusion was stopped after completion of the 21-hour protocol. The patient was discharged home after 48 hours, to follow up with his community physician. The patient remained clinically well and did not show evidence of long-term consequences of the accidental overdose.
Discussion
Acetaminophen is a commonly used antipyretic and analgesic in children that can have severe consequences when overdose occurs. Acetaminophen overdose is a major cause of acute liver failure and is the most common identifiable cause of acute liver failure in children.1 Repeated supratherapeutic dosing, accidental overdose due to error and intentional ingestion can all result in acute liver failure and even death.
Hepatic toxicity
The toxicity of acetaminophen overdose has long been recognized.2 Potentially toxic doses are those that are greater than 150 mg/kg/dose in children and greater than 7–10 g/dose in adults. With the advent of many combination analgesic medications, the potential for unintentional overdose has increased.3 In the United States, two concentrations of liquid formulations of acetaminophen for infants are now available, further increasing the risk of incorrect dosing.4
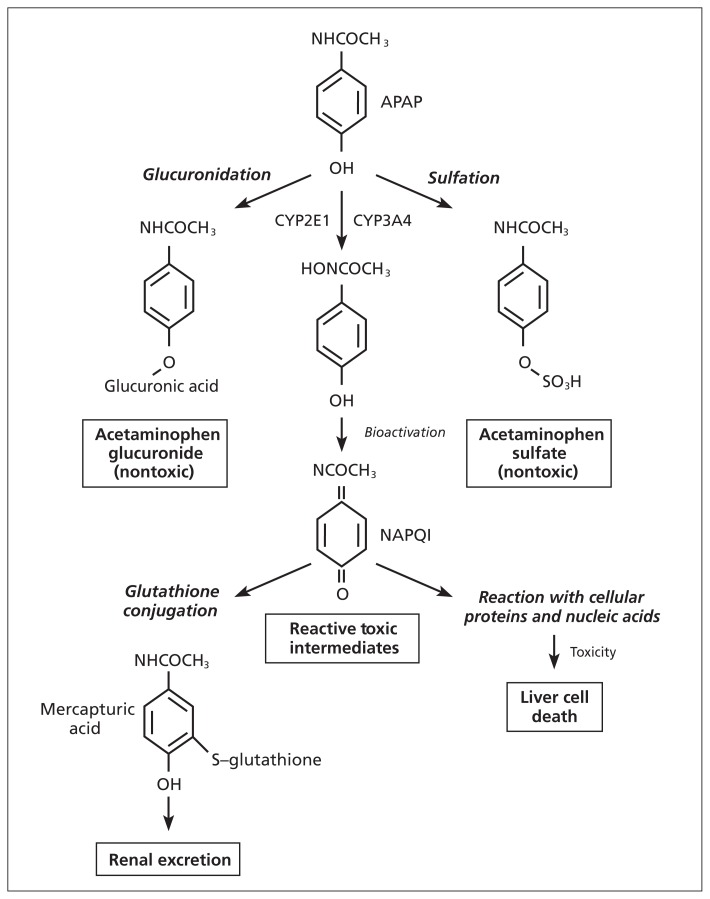
Acetaminophen hepatotoxicity is caused by the formation of a toxic metabolite, N-acetyl-p-benzoquinoneimine (NAPQI). When acetaminophen is used in therapeutic doses, most of the drug is metabolized via glucuronidation and sulfation; a very small amount of acetaminophen is metabolized to NAPQI by the hepatic enzyme cytochrome P450 2E1 (CYP2E1).5 N-acetyl-p-benzoquinoneimine is then conjugated by glutathione to form the benign metabolite, mercapturic acid, which is excreted in the urine. The potential for hepatotoxicity develops when large doses of acetaminophen saturate the typical conjugation pathways and overwhelm available glutathione stores, leading to reduced clearance of the toxic metabolite. Accumulation of the toxic metabolite can then exert untoward effects on key cellular structures and functions (Figure 1).
Figure 1:
Acetaminophen metabolism. N-acetyl-p-aminophenol (APAP) is the active component of acetaminophen and is metabolized by 3 pathways: glucuronidation, sulfation and glutathione conjugation. Glucuronidation and sulfation produce nontoxic metabolites for excretion. N-acetyl-p-benzoquinoneimine (NAPQI) is a toxic intermediate produced by cytochrome P450 2E1 (CYP2E1; the main metabolizing agent) and cytochrome P450 3A4 (CYP3A4) metabolism. NAPQI is then conjugated by glutathione to form a nontoxic metabolite for excretion.
Developmental differences in drug metabolism
Hepatic drug metabolism is classically thought to occur via two distinct routes: phase I and phase II metabolism.6 Phase I reactions are frequently mediated by cytochrome P450 enzymes, such as CYP2E1 in the case of acetaminophen. Phase II reactions involve conjugation pathways such as glucuronidation, sulfation and glutathione conjugation.
Both phases are important in acetaminophen metabolism, with their relative importance varying with age. In neonates and infants, rates of glucuronidation are less than in adults, with a compensatory increase in rates of sulfation.6 There are considerable differences in the maturation of CYP enzyme function depending on the isoenzyme species of interest. For example, NAPQI, the toxic metabolite, is primarily the product of CYP2E1 metabolism, and there is evidence that younger children have a relative immaturity in CYP2E1 metabolism, which would substantially reduce the production of this toxic metabolite in infants.7 After birth, CYP2E1 protein expression and activity increases, reaching adult levels by about 1 year of age.7
The ability of the liver to metabolize acetaminophen changes with age owing to differences in activity of these key metabolic pathways. Although the mechanism of acetaminophen toxicity is well recognized, the clinical implications of age-related differences and the ontogeny of hepatic pathways may not be commonly appreciated.
Treatment options and controversies
Hepatotoxicity related to an acute acetaminophen overdose generally has a good prognosis, particularly with appropriate treatment. When treatment is started within 8 hours of an acute ingestion, hepatotoxicity is as low as 10%.8 Based on observational data from the Pediatric Acute Liver Failure study group, recovery occurred in 94% of instances of acetaminophen overdose in children when treated appropriately.1
Treatment options include trying to limit the absorption of acetaminophen and reducing the accumulation of the toxic metabolite, NAPQI. Activated charcoal can be used to try to reduce the absorption of acetaminophen if the patient presents within one hour of the ingestion.9 Otherwise, the treatment of choice is use of a specific antidote, N-acetylcysteine, which reduces the hepatotoxic effects of acetaminophen overdose by replenishing glutathione stores, thereby enhancing production of the nontoxic metabolites. Ideally, treatment with N-acetylcysteine should be started within 8–10 hours of an acute ingestion. However, beneficial effects can be appreciated even if therapy is started up to 24 hours after the ingestion.9
N-acetylcysteine can be given orally or by intravenous infusion, and controversy exists about the preferred route of administration. There are no randomized controlled trials that compare the 2 modes of therapy; both are deemed effective.8 In Canada, intravenous N-acetylcysteine therapy is essentially the only route used, whereas in the US, both intravenous and oral therapy are available. Intravenous N-acetylcysteine has only recently become available in the US.
Intravenous N-acetylcysteine is associated with fewer of the adverse effects seen with the oral preparation, such as nausea, vomiting, abdominal pain, diarrhea and rash, but it carries a higher risk of anaphylactoid reactions.9 When patients present relatively late after ingestion, oral N-acetylcysteine protocols provide a higher dose given over a longer period, which may be an advantage.2,10 However, the gastrointestinal adverse effects associated with oral therapy are a substantial disadvantage, notably if this results in a longer time for drug absorption and hence a longer time to reach therapeutic effect.2 Since the introduction and adoption of routine use of N-acetylcysteine therapy, the mortality rate in acetaminophen overdose has declined from 3% to 0.7%.11
Prevention of overdose and medication errors in children
This report highlights the issue of accurate dosing in pediatrics. In this situation, well-educated parents miscalculated the dose of acetaminophen for their newborn. Correct dosing in pediatrics is challenged by weight-based dosing and the conversion of a weight-based dose to a volume (i.e., milligrams to millilitres), as many children rely on liquid preparations.
Medication error is a serious problem in the care of children.12 Based on a report from US poison control centres and the American Academy of Pediatrics, 11% of children under the age of 6 years who are exposed to pharmaceuticals experience a medication error (incorrect medication, incorrect dose or incorrect route of administration).13 This report analyzed 238 instances of serious medication error in children under six years of age reported to poison control centres across the US between 2000 and 2004. Incorrect dosing topped the list of errors and was more common among children less than 1 year of age or when less than 1 mL of the medication was to be given. Of the 238 instances, 162 occurred in the home. Acetaminophen overdose was the most common single agent responsible for a serious medication error (e.g., life-threatening, resulting in substantial morbidity or mortality). There were a total of 24 deaths reported and, of these, one-third were due to acetaminophen overdose. Comparable Canadian data have not yet been compiled.
Errors associated with medication administration represent an important opportunity for preventive health care, as these are avoidable events. There is a large body of literature on the prevention of medication errors within health care institutions, but there is a paucity of evidence to guide us in how to reduce medication errors in the outpatient setting.
Intuitively, strategies that improve parent education and promote parent–physician communication should be effective. However, research has not yet identified any single solution. In one study, parent–physician communication on its own did not seem to help.14 Lemer and colleagues did not find a correlation between reduced error rates and provision of advice by a health care provider.14 Variables that were associated with higher error rates were children less than five years of age and children taking two or more medications. A major limitation of this study was that it relied on parent recall to assess whether advice was given.14 The use of parent recall in research has the advantage of being realistic by assessing whether the parents perceived that they were given advice, but the findings more likely speak to the need for better ways to provide education to parents. In this study, most advice was given by verbal communication with very little written communication.14 The effectiveness of written information, including personalized dosages and diagrams, is an area to be explored.
Potential for systems prevention and improved safety
Although physicians and pharmacists should continue to educate parents and caregivers regarding the medications prescribed, one-to-one communication cannot be the sole approach to reducing errors in medication administration. Error reduction on a large scale requires systems-based interventions and prevention.
Hepatic toxicity related to acetaminophen has received increasing attention from regulatory agencies. Both the US Food and Drug Administration and Health Canada have issued advisories to increase public awareness of the potential for hepatotoxicity with use of acetaminophen. There is a heightened awareness of medication safety in pediatrics, and new efforts are being put toward reducing adverse events. Regulations banning cough and cold medications for use in children under the age of 6 years is one such example. In 2009, Health Canada revised the labelling standards for acetaminophen-containing products to enforce stronger warnings on packaging about the risk of overdose.15 Revisions also mandate the inclusion of weight-based dosing charts with each product.15
Although regulatory bodies have begun taking steps to improve labelling and raise awareness of potential harm, there remains substantial room for practical and point-of-care interventions.
In an expert panel review of deaths caused by cough and cold medications, supratherapeutic doses were identified in most of the fatal cases.16 One factor that was thought to contribute to overdoses was the lack of an appropriate device to administer the medication.16,17 Spoons are often used by parents, but are inaccurate,17 as are liquid droppers. From a systems-based perspective, improving packaging to facilitate calculation and delivery of an appropriate dose may be a practical intervention.17 It may also be useful to have children’s acetaminophen behind the counter so that parents are counselled by a pharmacist before purchasing the medication. This counselling could include the provision of written information specifying the child’s weight (based on the parent’s report), dose and volume to be given.
Conclusion
Acetaminophen is a commonly used medication that can have serious adverse effects in the context of overdose. Although a specific antidote, N-acetylcysteine, is available and effective, controversy exists about the best route of administration.
The widespread use and availability of acetaminophen make the potential for overdose a population health concern and warrants a systems-based approach to preventing adverse outcomes. Although some efforts have been made to raise awareness of hepatotoxicity related to acetaminophen use, concern with acetaminophen’s availability as an over-the-counter medication remains.
Fortunately, our patient had a positive outcome and did not have adverse effects of the overdose of acetaminophen. It is important to note that there are developmental differences in hepatic metabolism that may affect the hepatotoxicity seen in infants and young children. The clinical implications of these differences warrant further investigation.
Key points
Acetaminophen overdose is a leading cause of acute liver failure in adults and a major cause of acute liver failure in children.
Developmental differences in hepatic metabolism exist that influence the risk of hepatotoxicity following acetaminophen overdose among infants compared with adults.
Infants and children are particularly susceptible to acute acetaminophen overdose because of dosing errors.
Strategies to prevent potentially fatal overdoses need to target both patient-based and systems-based interventions.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: All authors contributed to the conception and design, and drafting and revising of the manuscript. All authors approved the final version submitted for publication.
References
- 1.Squires RH, Jr, Shneider BL, Bucuvalas J, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr 2006;148:652–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackford MG, Felter T, Gothard MD, et al. Assessment of the clinical use of intravenous and oral N-acetylcysteine in the treatment of acute acetaminophen poisoning in children: a retrospective review. Clin Ther 2011;33:1322–30 [DOI] [PubMed] [Google Scholar]
- 3.Fontana RJ. “Unintentional” acetaminophen overdose on the rise: Who is responsible? Dr. Robert J. Fontana is interviewed by Paul C. Adams. Can J Gastrointerol 2006;20:319–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Food and Drug Administration Addition of another concentration of liquid acetaminophen marketed for infants. Rockville (MD): The Administration; 2011. Available: www.fda.gov/Drugs/DrugSafety/ucm284741.htm (accessed 2012 Feb. 8). [Google Scholar]
- 5.Zhao L, Pickering G. Paracetamol metabolism and related genetic differences. Drug Metab Rev 2011;43:41–52 [DOI] [PubMed] [Google Scholar]
- 6.Williams RT. Comparative patterns of drug metabolism. Fed Proc 1967;26:1029–39 [PubMed] [Google Scholar]
- 7.Hines RN. The ontogeny of drug metabolizing enzymes and implications for adverse drug events. Pharmacol Ther 2008;118: 250–67 [DOI] [PubMed] [Google Scholar]
- 8.Algren DA. Review of N-acetylcysteine for the treatment of acetaminophen (paracetamol) toxicity in pediatrics. Second Meeting of the Subcommittee of the Expert Committee on the Selection and Use of Essential Medicines; 2008. Sep-Oct; Geneva (Switzerland). Geneva: The World Health Organization; 2008. p. 1–18 Available: www.who.int/selection_medicines/committees/subcommittee/2/acetylcysteine_rev.pdf (accessed 2012 Feb. 15). [Google Scholar]
- 9.Chun LJ, Tong MJ, Busuttil RW, et al. Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol 2009;43: 342–9 [DOI] [PubMed] [Google Scholar]
- 10.Prescott L. Oral or intravenous N-acetylcysteine for acetaminophen poisoning? Ann Emerg Med 2005;45:409–13 [DOI] [PubMed] [Google Scholar]
- 11.Brok J, Buckley N, Gluud C. Interventions for paracetamol (acetaminophen) overdoses [review]. Cochrane Database Syst Rev 2002;(3):CD003328. [DOI] [PubMed] [Google Scholar]
- 12.Stucky ER; American Academy of Pediatrics Committee on Drugs, American Academy of Pediatrics Committee on Hospital Care Prevention of medication errors in the pediatric inpatient setting. Pediatrics 2003;112:431–6 [DOI] [PubMed] [Google Scholar]
- 13.Tzimenatos L, Bond GR; Pediatric Therapeutic Error Study Group Severe injury or death in young children from therapeutic errors: a summary of 238 cases from the American Association of Poison Control Centers. Clin Toxicol (Phila) 2009;47:348–54 [DOI] [PubMed] [Google Scholar]
- 14.Lemer C, Bates DW, Yoon C, et al. The role of advice in medication administration errors in the pediatric ambulatory setting. J Patient Saf 2009;5:168–75 [DOI] [PubMed] [Google Scholar]
- 15.Health Canada Guidance Document Acetaminophen labelling standards. Ottawa (ON): Health Canada; 2009. Available: www.hc-sc.gc.ca/dhp-mps/prodpharma/applic-demande/guide-ld/label_stand_guide_ld-eng.php (accessed 2012 Jan. 30). [Google Scholar]
- 16.Dart RC, Paul IM, Bond GR, et al. Pediatric fatalities associated with over the counter (non-prescription) cough and cold remedies. Ann Emerg Med 2009;53:411–7 [DOI] [PubMed] [Google Scholar]
- 17.The Acetaminophen Hepatotoxicity Working Group, Centre for Drug Evaluation and Research, Food and Drug Administration Recommendations for FDA interventions to decrease the occurrence of acetaminophen hepatotoxicity. Rockville (MD): United States Food and Drug Administration; 2008. Available: www.fda.gov/ohrms/dockets/ac/09/briefing/2009-4429b1-02-FDA.pdf (accessed 2012 Mar. 20). [Google Scholar]