Abstract
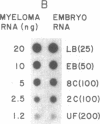
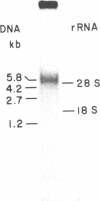
Early mouse embryos express two morphological subtypes of intracisternal A-type particles, one resembling those occurring in mouse tumors (referred to as IAP) and the other apparently specific for early embryos [referred to as IAP(epsilon)]. Using cloned fragments of IAP genes as labeled probes in dot-hybridization experiments, we detected IAP-related RNA sequences in mouse oocytes and preimplantation embryos. IAP RNA is relatively abundant in ovarian oocytes, is reduced in amount to approximately equal to 1/10th in the ovulated egg, and increases approximately equal to 100 times (from approximately equal to 1.3 X 10(3) to approximately equal to 1.5 X 10(5) molecules per embryo) between the one-cell stage and late blastocyst stage. Most of the IAP RNA consists of a single size class of about 5.4 kilobases, and a major fraction of this RNA is polyadenylylated. Quantitative considerations suggest that only a few percent of the IAP RNA in embryos are associated with particles. In two-cell embryos, the number of IAP RNA molecules is less than 1/10th the number of IAP(epsilon) particles, suggesting that IAP(epsilon) is genetically distinct from IAP and presumably represents a family of as yet unidentified retrovirus-like elements.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Biczysko W., Pienkowski M., Solter D., Koprowski H. Virus particles in early mouse embryos. J Natl Cancer Inst. 1973 Sep;51(3):1041–1050. doi: 10.1093/jnci/51.3.1041. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Calarco P. G., Brown E. H. An ultrastructural and cytological study of preimplantation development of the mouse. J Exp Zool. 1969 Jul;171(3):253–283. doi: 10.1002/jez.1401710303. [DOI] [PubMed] [Google Scholar]
- Calarco P. G., Szollosi D. Intracisternal A particles in ova and preimplantation stages of the mouse. Nat New Biol. 1973 May 16;243(124):91–93. [PubMed] [Google Scholar]
- Callahan R., Kuff E. L., Lueders K. K., Birkenmeier E. Genetic relationship between the Mus cervicolor M432 retrovirus and the Mus Musculus intracisternal type A particle. J Virol. 1981 Dec;40(3):901–911. doi: 10.1128/jvi.40.3.901-911.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase D. G., Pikó L. Expression of A- and C-type particles in early mouse embryos. J Natl Cancer Inst. 1973 Dec;51(6):1971–1975. doi: 10.1093/jnci/51.6.1971. [DOI] [PubMed] [Google Scholar]
- Childs G., Maxson R., Kedes L. H. Histone gene expression during sea urchin embryogenesis: isolation and characterization of early and late messenger RNAs of Strongylocentrotus purpuratus by gene-specific hybridization and template activity. Dev Biol. 1979 Nov;73(1):153–173. doi: 10.1016/0012-1606(79)90144-1. [DOI] [PubMed] [Google Scholar]
- Clegg K. B., Pikó L. Poly(A) length, cytoplasmic adenylation and synthesis of poly(A)+ RNA in early mouse embryos. Dev Biol. 1983 Feb;95(2):331–341. doi: 10.1016/0012-1606(83)90034-9. [DOI] [PubMed] [Google Scholar]
- Clegg K. B., Pikó L. Size and specific activity of the UTP pool and overall rates of RNA synthesis in early mouse embryos. Dev Biol. 1977 Jul 1;58(1):76–95. doi: 10.1016/0012-1606(77)90075-6. [DOI] [PubMed] [Google Scholar]
- Cole M. D., Ono M., Huang R. C. Intracisternal A-particle genes: structure of adjacent genes and mapping of the boundaries of the transcriptional unit. J Virol. 1982 Apr;42(1):123–130. doi: 10.1128/jvi.42.1.123-130.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. D., Ono M., Huang R. C. Terminally redundant sequences in cellular intracisternal A-particle genes. J Virol. 1981 May;38(2):680–687. doi: 10.1128/jvi.38.2.680-687.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Calame K., Early P. W., Livant D. L., Joho R., Weissman I. L., Hood L. An immunoglobulin heavy-chain gene is formed by at least two recombinational events. Nature. 1980 Feb 21;283(5749):733–739. doi: 10.1038/283733a0. [DOI] [PubMed] [Google Scholar]
- Eppig J. J. Analysis of mouse oogenesis in vitro. Oocyte isolation and the utilization of exogenous energy sources by growing oocytes. J Exp Zool. 1976 Dec;198(3):375–382. doi: 10.1002/jez.1401980311. [DOI] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Huang T. T., Jr, Calarco P. G. Evidence for the cell surface expression of intracisternal A particle-associated antigens during early mouse development. Dev Biol. 1981 Mar;82(2):388–392. doi: 10.1016/0012-1606(81)90462-0. [DOI] [PubMed] [Google Scholar]
- Huang T. T., Jr, Calarco P. G. Immunologic relatedness of intracisternal A-particles in mouse embryos and neoplastic cell lines. J Natl Cancer Inst. 1982 Apr;68(4):643–649. [PubMed] [Google Scholar]
- Huang T. T., Jr, Calarco P. G. Immunoprecipitation of intracisternal A-particle-associated antigens from preimplantation mouse embryos. J Natl Cancer Inst. 1981 Nov;67(5):1129–1134. [PubMed] [Google Scholar]
- Kelly F., Condamine H. Tumor viruses and early mouse embryos. Biochim Biophys Acta. 1982 Apr 29;651(2-3):105–141. doi: 10.1016/0304-419X(82)90009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Rechavi G., Givol D., Canaani E. Homology between an endogenous viral LTR and sequences inserted in an activated cellular oncogene. Nature. 1983 Apr 7;302(5908):547–548. doi: 10.1038/302547a0. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Smith L., Hawley R., Hozumi N., Shulman M. Intracisternal A-particle genes as movable elements in the mouse genome. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1992–1996. doi: 10.1073/pnas.80.7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Leuders K. K., Ozer H. L., Wivel N. A. Some structural and antigenic properties of intracisternal A particles occurring in mouse tumors (complement fixation-immunodiffusion-neuroblastoma-plasma-cell tumor). Proc Natl Acad Sci U S A. 1972 Jan;69(1):218–222. doi: 10.1073/pnas.69.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Smith L. A., Lueders K. K. Intracisternal A-particle genes in Mus musculus: a conserved family of retrovirus-like elements. Mol Cell Biol. 1981 Mar;1(3):216–227. doi: 10.1128/mcb.1.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Genetic individuality of intracisternal A-particles of Mus musculus. J Virol. 1979 Apr;30(1):225–231. doi: 10.1128/jvi.30.1.225-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Intracisternal A-particle genes: identification in the genome of Mus musculus and comparison of multiple isolates from a mouse gene library. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3571–3575. doi: 10.1073/pnas.77.6.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Sequences associated with intracisternal A particles are reiterated in the mouse genome. Cell. 1977 Dec;12(4):963–972. doi: 10.1016/0092-8674(77)90161-1. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Segal S., Kuff E. L. RNA sequences specifically associated with mouse intracisternal A particles. Cell. 1977 May;11(1):83–94. doi: 10.1016/0092-8674(77)90319-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Ono M., Cole M. D., White A. T., Huang R. C. Sequence organization of cloned intracisternal A particle genes. Cell. 1980 Sep;21(2):465–473. doi: 10.1016/0092-8674(80)90483-3. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Segal S., Lueders K. K., Kuff E. L. RNA associated with murine intracisternal type A particles codes for the main particle protein. J Virol. 1978 Jul;27(1):118–126. doi: 10.1128/jvi.27.1.118-126.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikó L., Clegg K. B. Quantitative changes in total RNA, total poly(A), and ribosomes in early mouse embryos. Dev Biol. 1982 Feb;89(2):362–378. doi: 10.1016/0012-1606(82)90325-6. [DOI] [PubMed] [Google Scholar]
- Shen-Ong G. L., Cole M. D. Differing populations of intracisternal A-particle genes in myeloma tumors and mouse subspecies. J Virol. 1982 May;42(2):411–421. doi: 10.1128/jvi.42.2.411-421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]
- Wilson S. H., Kuff E. L. A novel DNA polymerase activity found in association with intracisternal A-type particles. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1531–1536. doi: 10.1073/pnas.69.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsuyanagi Y., Szöllösi D. Early mouse embryo intracisternal particle: Fourth type of retrovirus-like particle associated with the mouse. J Natl Cancer Inst. 1981 Sep;67(3):677–685. [PubMed] [Google Scholar]