Abstract
Background:
Patients with type 2 diabetes have a 40% increased risk of bladder cancer. Thiazolidinediones, especially pioglitazone, may increase the risk. We conducted a systematic review and meta-analysis to evaluate the risk of bladder cancer among adults with type 2 diabetes taking thiazolidinediones.
Methods:
We searched key biomedical databases (including MEDLINE, Embase and Scopus) and sources of grey literature from inception through March 2012 for published and unpublished studies, without language restrictions. We included randomized controlled trials (RCTs), cohort studies and case–control studies that reported incident bladder cancer among people with type 2 diabetes who ever (v. never) were exposed to pioglitazone (main outcome), rosiglitazone or any thiazolidinedione.
Results:
Of the 1787 studies identified, we selected 4 RCTs, 5 cohort studies and 1 case–control study. The total number of patients was 2 657 365, of whom 3643 had newly diagnosed bladder cancer, for an overall incidence of 53.1 per 100 000 person-years. The one RCT that reported on pioglitazone use found no significant association with bladder cancer (risk ratio [RR] 2.36, 95% confidence interval [CI] 0.91–6.13). The cohort studies of thiazolidinediones (pooled RR 1.15, 95% CI 1.04–1.26; I2 = 0%) and of pioglitazone specifically (pooled RR 1.22, 95% CI 1.07–1.39; I2 = 0%) showed significant associations with bladder cancer. No significant association with bladder cancer was observed in the two RCTs that evaluated rosiglitazone use (pooled RR 0.87, 95% CI 0.34–2.23; I2 = 0%).
Interpretation:
The limited evidence available supports the hypothesis that thiazolidinediones, particularly pioglitazone, are associated with an increased risk of bladder cancer among adults with type 2 diabetes.
People with type 2 diabetes are at increased risk of several types of cancer, including a 40% increased risk of bladder cancer, compared with those without diabetes.1,2 The strong association with bladder cancer is hypothesized to be a result of hyperinsulinemia, whereby elevated insulin levels in type 2 diabetes stimulate insulin receptors on neoplastic cells, promoting cancer growth and division.1,3–5 Additional risk factors for bladder cancer include increased age, male sex, smoking, occupational and environmental exposures and urinary tract disease.6 Exogenous insulin and other glucose-lowering medications such as sulfonylureas, metformin and thiazolidinediones, may further modify the risk of bladder cancer.1
Data from the placebo-controlled PROactive trial of pioglitazone (PROspective pioglitAzone Clinical Trial in macroVascular Events) suggested a higher incidence of bladder cancer among pioglitazone users than among controls.7 Subsequent randomized controlled trials (RCTs) and observational studies have reported conflicting results for pioglitazone, with various studies reporting a significant increase,8,9 a nonsignificant increase10 and even a decreased risk11 of bladder cancer.
To test the hypothesis that pioglitazone use is associated with an increased risk of bladder cancer, we conducted a systematic review and meta-analysis of RCTs and observational studies reporting bladder cancer among adults with type 2 diabetes taking pioglitazone. To clarify the possibility of a drug-class effect, we also examined data for all thiazolidinediones and for rosiglitazone alone.
Methods
The protocol for this study was developed in advance to outline our search strategy, criteria for study selection, procedures for data abstraction and assessment of bias, and methods for data analysis.
Literature search
We conducted a comprehensive search of the following key electronic biomedical databases from inception through March 2012: MEDLINE, Embase, the Cochrane Library (Cochrane Database of Systematic Reviews, DARE [Database of Abstracts of Reviews of Effects], Health Technology Assessment Database, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, Economic Evaluations Database), Science Citation Index Expanded, Conference Proceedings Citation Index – Science, PubMed (search terms combined with cancer subset, limited to adults), Toxnet and Scopus. No study-design filters or language restrictions were applied. The search strategy was broad to capture all potentially suitable studies and was created with the assistance of a librarian experienced in systematic reviews. A sample search is provided in Appendix 1 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.112102/-/DC1).
We also searched the proceedings of five international conferences of major diabetes and diabetes-related organizations (International Society of Pharmacoepidemiology, American Diabetes Association, Canadian Diabetes Association, European Association for the Study of Diabetes and Canadian Association of Population Therapeutics) from 2008 onward; Google Scholar; registries of clinical trials (ClinicalTrials.gov and International Clinical Trials Registry Platform); and databases of international drug safety surveillance agencies (US Food and Drug Administration, Health Canada and European Medicines Agency). In addition, we manually searched reference lists of relevant studies and contacted experts in the field.
A checklist was used to assess whether studies met our inclusion criteria for population (adults with type 2 diabetes), exposure (ever used a thiazolidinedione), comparison group (never used a thiazolidinedione), outcome (incident bladder cancer, even if it was not a main outcome) and study design (RCT, cohort study or case–control study, including case/noncase study). We excluded duplicate reports from the same study, studies involving patients with type 1 diabetes only and descriptive observational studies.
Data collection
Two trained reviewers (I.N.C. and S.L.B.) independently conducted the study selection and abstraction of data. Where necessary, they contacted authors of included studies for additional information. The reviewers resolved any disagreements by discussion or in consultation with another coauthor (J.A.J.).
To assess the risk of bias in the included studies, the two reviewers used the Cochrane risk-of-bias tool12 for the RCTs. For the cohort and case–control studies, they used a modified version of the Newcastle–Ottawa Scale,13 with a score of 5 or less (out of 8) indicating a high risk of bias.
Data synthesis
We tabulated pertinent descriptive data from the included studies. In a random-effects model, we pooled adjusted risk estimates using inverse variance calculations for the observational studies, and unadjusted risk estimates using Mantel–Haenszel calculations for the RCTs.14 We quantified statistical heterogeneity using the I2 statistic. As a criterion for data pooling, we considered a maximum heterogeneity of no more than 75%. We defined heterogeneity as “low” (≤ 25%), “moderate” (> 25%–50%) or “high” (> 50%–75%) and explored possible sources of heterogeneity if the I2 value was greater than 25%.
In our primary analysis, we examined exposure to pioglitazone and stratified our results by study design. In secondary analyses, we considered exposure to rosiglitazone and to any thiazolidinedione. Ascertainment of exposures to all thiazolidinediones was independent of other existing therapies or exposures. We planned subgroup analyses among patients receiving monotherapy with pioglitazone or rosiglitazone but had insufficient reports to conduct such analyses. We were unable to assess publication bias through construction of funnel plots because of a limited number of reports.15
All analyses were conducted using RevMan version 5.1 (Nordic Cochrane Centre, Copenhagen, Denmark).
Results
Study selection
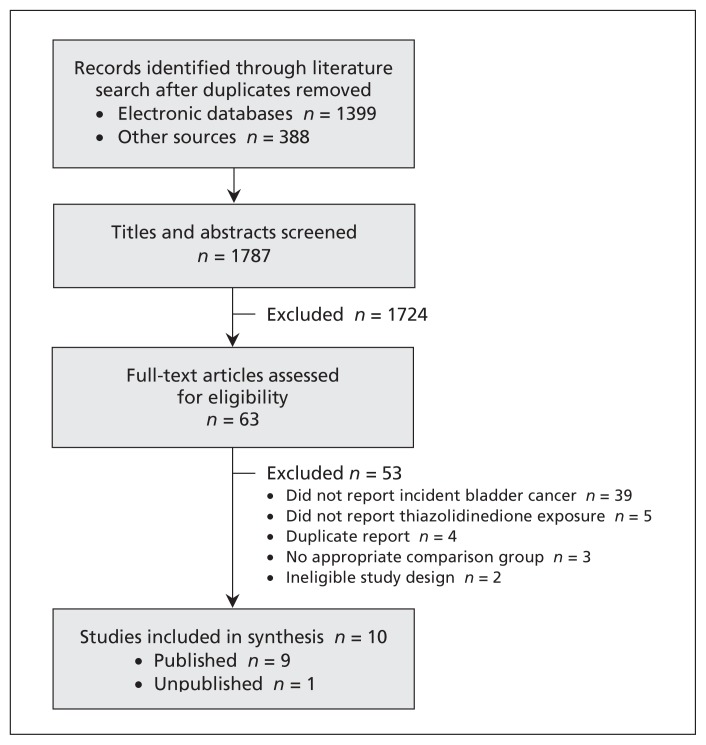
Our literature search returned 1787 results once duplicates were removed. After screening of the titles and abstracts, 63 articles (62 in English, 1 in French) were deemed relevant and the full-text versions obtained for further evaluation. Studies were excluded because they did not report incident bladder cancer (39) or thiazolidinedione exposure (5), were duplicate reports of the same study (4), did not have an appropriate comparison group (3) or had an ineligible study design (2) (Figure 1). Citations of the excluded studies are provided in Appendix 2 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.112102/-/DC1).
Figure 1:
Selection of studies.
Study characteristics
Overall, we analyzed data from 10 studies (4 RCTs and 6 observational studies), involving a total of 2 657 365 patients (Table 1).7,9–11,16–21 One of the observational studies used a case/noncase design9 and was included as a case–control study. No other case–control studies met our inclusion criteria.
Table 1:
Characteristics of studies included in the meta-analysis of the risk of bladder cancer associated with thiazolidinedione use
| Study | Medication studied | No. of patients | Study period | Mean length of follow-up, yr | Covariates | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Exposed group | Comparison group | Exposed group | Comparison group | ||||
| RCT | |||||||
|
| |||||||
| Dormandy et al., 2005 (PROactive study, multicentre)7 | Pioglitazone | No TZD use | 2 605 | 2 633 | 2001–2004 | 2.9 | NA |
|
| |||||||
| Kahn et al., 2006 (ADOPT, multicentre)16 | Rosiglitazone (monotherapy) | No TZD use (metformin or glibenclamide monotherapy) | 1 456 | 2 895 | 2000–2006 | 3.4 | NA |
|
| |||||||
| Home et al., 2009 (RECORD, multicentre)17 | Rosiglitazone (+ sulfonylurea or metformin) | No TZD use (sulfonylurea and metformin) | 2 220 | 2 227 | 2001–2008 | 5.5 | NA |
|
| |||||||
| Sanofi-Aventis 2009 (multicentre, United States)21 | TZD (unspecified) (+ insulin glargine and sulfonylurea or metformin) | No TZD use (insulin glargine, metformin and sulfonylurea) | 256 | 130 | 2006–2008 | NR§ | NA |
|
| |||||||
| Cohort | |||||||
|
| |||||||
| Oliveria et al., 2008 (United States)18* | TZD (unspecified) | No TZD use | NR | NR | 2000–2004 | 3.9 | Yes¶ |
|
| |||||||
| Lewis et al., 2011 (Kaiser Permanente, California, United States)10 | Pioglitazone | No pioglitazone use | 30 173 | 162 926 | 1997–2008 | 3.3 (exposed group); 6.2 (comparison group) | Yes** |
|
| |||||||
| Tseng, 2011 (National Health Insurance, Taiwan)11 | Pioglitazone and/or rosiglitazone | No TZD use | 1 028 | 112 520 | 2003–2005 | 3.0 | Yes†† |
|
| |||||||
| Tseng, 2012 (National Health Insurance, Taiwan)20 | Pioglitazone | No pioglitazone use | 2 545 | 52 383 | 2006–2009 | NR | Yes‡‡ |
|
| |||||||
| Neumann et al., 2012 (National Health Insurance, France)19† | Pioglitazone | No pioglitazone use | 155 535 | 1 335 525 | 2006–2009 | 2.4 (pioglitazone exposed group) | Yes§§ |
|
| |||||||
| Rosiglitazone | No rosiglitazone use | 153 334 | 1 337 726 | ||||
|
| |||||||
| Case–control | |||||||
|
| |||||||
| Piccinni et al., 2011 (FDA Adverse Event Reporting System, United States)9‡ | Pioglitazone | No pioglitazone use | 37 841 | 561 244 | 2004–2009 | NA | NR |
Note: ADOPT = A Diabetes Outcome Progression Trial, FDA = US Food and Drug Administration, NA = not applicable, NR = not reported, PROactive trial = PROspective pioglitAzone Clinical Trial in macroVascular Events, RCT = randomized controlled trial, RECORD = Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycemia in Diabetes, TZD = thiazolidinedione.
The total number of patients in this study was 191 223.
The total number of patients in this study was 1 491 060.
Case/noncase study design.
The duration of the trial was 12 weeks, and 10% of patients were lost to follow-up.
Age, sex, risk factors for bladder cancer (pelvic radiation, schistosomiasis). concentration, smoking status, history of bladder conditions (urinary tract
Age, sex, race/ethnicity, incident diabetes at baseline, baseline hemoglobin A1C conditions, urolithiasis, incontinence and “other bladder or urethral conditions”), comorbidities (congestive heart failure, renal function, other cancer before baseline), use of other glucose-lowering therapies (metformin, sulfonylureas, other TZDs, other oral glucose-lowering drugs, insulin), income.
Age, sex, duration of diabetes, comorbidities (urinary tract disease, nephropathy, hypertension, chronic obstructive pulmonary disease, stroke, ischemic heart disease, peripheral arterial disease, eye disease, dyslipidemia), use of glucose-lowering agents (metformin, sulfonylureas, acarabose, TZDs, insulin), other medications (statins, fibrates, angiotensin-converting-enzyme inhibitors and/or angiotensin-receptor blockers, calcium-channel blockers), living region, occupation.
Same covariates as in Tseng 2011 study, as well as heart failure, use of meglinitide, use of rosiglitazone (instead of “TZDs”) and other cancers before baseline (excluding bladder cancer).
Age, sex, use of other glucose-lowering agents.
All four RCTs included adults with type 2 diabetes randomly assigned to receive thiazolidinedione treatment. They assessed the following noncancer outcomes: cardiovascular outcomes,17 hemoglobin A1c levels,16,21 and macrovascular morbidity and mortality.7 Two of the trials were open label,17,21 and one was unpublished.21
One publication22 reported unpublished cancer outcomes from two RCTs that we included in our review16,17 and was used to supplement data for these trials. One included cohort study19 was previously published as a government report,8 which we used to supplement data not found in the published study. Two cohort studies were conducted in the same population (Taiwan) but during different periods (2003–200511 and 2006–200920) and with different definitions of exposure (thiazolidinedione use ever v. never,11 and pioglitazone use ever v. never20); we included both studies.
We assigned a high risk of bias to two RCTs because of substantial differential losses to follow-up;16,17 to one unpublished RCT because of insufficient reporting of methods and early termination;21 and to one observational study because of inadequately defined cases and unrepresentative controls.9 No cohort study was assigned a high risk of bias (Tables 2 and 3).
Table 2:
Risk-of-bias assessment of the randomized controlled trials*
| Study | Adequate sequence generation? | Concealment of allocation? | Blinding of particpants, personnel, outcome assessors? | Incomplete health data addressed? | Free of selective reporting? | Free of other bias? | Overall risk of bias |
|---|---|---|---|---|---|---|---|
| Dormandy et al.7 (PROactive study) | Unclear | Yes | Yes | Yes | Unclear | Unclear (industry sponsored) | Unclear |
| Kahn et al.16 (ADOPT) | Unclear | Yes | Unclear | No | Yes | No (very high attrition rate; industry sponsored) | High |
| Home et al.17 (RECORD) | Yes | Yes | Unclear | Yes | Yes | No (differential withdrawls; industry sponsored) | High |
| Sanofi-Aventis trial21 | Unclear | Unclear | No | Yes | Yes | Unclear (industry sponsored) | High |
Note: ADOPT = A Diabetes Outcome Progression Trial, PROactive trial = PROspective pioglitAzone Clinical Trial in macroVascular Events, RECORD = Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycemia in Diabetes.
Risk of bias was assessed with use of the Cochrane risk-of-bias tool.12
Table 3:
Risk-of-bias assessment of the observational studies*
| Study | Representativeness | Ascertainment of exposure | Demonstration that outcome was not present at study start | Comparability of cohorts on basis of design or analysis | Assessment of outcome | Was follow-up long enough for outcome to occur? | Adequacy of follow-up of cohorts | Overall risk of bias | |
|---|---|---|---|---|---|---|---|---|---|
| Exposed cohort | Unexposed cohort | ||||||||
| Cohort | |||||||||
| Oliveria et al.18 | Truly representative† | Same community† | Secure record† | Yes† | Controlled for age and sex but not for smoking or use of other glucose-lowering drugs | Independent assessment and record linkage† | Yes (> 1 yr)† | NR | 6 |
| Lewis et al.10 | Truly representative† | Same community† | Secure record† | Yes† | Controlled for age, sex, smoking (proxy) and use of other glucose-lowering drugs† | Record linkage† | Yes (> 1 yr)† | NR | 7 |
| Tseng11 | Truly representative† | Same community† | Secure record† | Yes† | Controlled for age, sex and use of other glucose- lowering drugs but not for smoking | Record linkage† | Yes (> 1 yr)† | NR | 6 |
| Tseng20 | Truly representative† | Same community† | Secure record† | Yes† | Controlled for age, sex and use of other glucose- lowering drugs but not for smoking | Record linkage† | Yes (> 1 yr)† | NR | 6 |
| Neumann et al.19 | Truly representative† | Same community† | Secure record† | Yes† | Controlled for age, sex and use of other glucose- lowering drugs but not for smoking | Record linkage† | Yes (> 1 yr)† | NR | 6 |
| Is the case definition adequate? | Representativeness of cases | Selection of controls | Definition of controls | Comparability of cases and controls on basis of design or analysis | Ascertainment of exposure | Same method of ascertainment for both groups | Nonresponse rate | ||
| Case–control | |||||||||
| Piccinni et al.9 | No (record linkage) | Consecutive series† | From same community† | Not stated | No adjustment (stratification on sex) | Written report | Yes† | Same for both groups† | 4 |
Note: NR = not reported.
Risk of bias was assessed with use of a modified version of the Newcastle–Ottawa Scale.13 A higher overall score corresponds to a lower risk of bias; a score of 5 or less (out of 8) indicates a high risk of bias.
Item met assessment criterion.
Incidence of bladder cancer
A total of 3643 patients had newly diagnosed bladder cancer, for an overall incidence of 53.1 per 100 000 person-years.
Randomized trials
All of the RCTs presented data on bladder cancer as crude numbers, lacking further demographic information. We combined participants from three trials (the fourth study lacked information on length of follow-up21) to estimate the incidence of bladder cancer per 100 000 person-years: 101.0 among those who used a thiazolidinedione and 65.5 among those who did not. In the PROactive study,7 the incidence per 100 000 person-years was 186.9 among pioglitazone users and 79.3 among controls. Comparatively, the incidence per 100 000 person-years among rosiglitazone users was 102.4 in the ADOPT study (A Diabetes Outcome Progression Trial)16 and 48.6 in the RECORD trial (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycemia in Diabetes);17 the respective rates among controls were 87.4 and 40.7 per 100 000 person-years.
Observational studies
The observational studies reported results according to demographic or clinical features. Three studies reported a higher risk of bladder cancer among men than among women exposed to thiazolidinediones,10,11,19 and one study reported history of bladder disease to predict bladder cancer independent of thiazolidinedione exposure.11
Neumann and colleagues reported the incidence of bladder cancer per 100 000 person-years, standardized to the world population, as 14.6 among men and 2.0 among women.19
Three cohort studies reported the incidence of bladder cancer among pioglitazone users as 81.5,10 49.419 and 104.520 per 100 000 person-years; the rates reported among nonusers were 68.8, 42.8 and 78.9 per 100 000 person-years, respectively. Thiazolidinedione use, reported in two cohort studies, was associated with an incidence of bladder cancer of 32.4411 and 53.418 per 100 000 person-years; the rates among those who never used thiazolidinediones were 65.6 and 50.9 per 100 000 person-years, respectively.
Results of the meta-analyses
Pioglitazone use and bladder cancer
All of the five studies assessing pioglitazone exposure reported an elevated7,10,20 or significantly increased9,19 risk of bladder cancer associated with pioglitazone use ever (v. never). Three studies assessed cumulative pioglitazone exposure.10,19,20 A significant association with bladder cancer was reported in one study after more than 12 months’ exposure19 and in both studies that assessed exposure after more than 24 months.10,19 Three studies explored a dose–response relationship: two reported a cumulative pioglitazone dose of more than 28 000 mg to be significantly (hazard ratio [HR] 1.75, 95% confidence interval [CI] 1.22–2.50)19 and nonsignificantly (HR 1.4, 95% CI 0.96–2.1)10 associated with elevated risks of bladder cancer. The third study looked at exposure to more than 10 500 mg of pioglitazone but observed no association.20
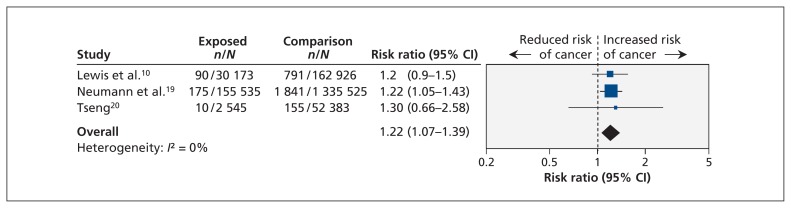
In the RCT, 14 of the 2605 pioglitazone users and 6 of the 2633 controls had newly diagnosed bladder cancer (risk ratio [RR] 2.36, 95% CI 0.91–6.13).7 The case/noncase study reported significantly increased odds of pioglitazone use among patients in whom bladder cancer developed (odds ratio 4.30, 95% CI 2.82–6.52).9 We pooled the results from three cohort studies, representing 1 739 087 patients, of whom 188 253 were pioglitazone users; we found a significantly increased risk of bladder cancer associated with the use of pioglitazone (pooled RR 1.22, 95% CI 1.07–1.39; I2 = 0%) (Figure 2).
Figure 2:
Meta-analysis of the risk of bladder cancer associated with pioglitazone use among adults with type 2 diabetes in cohort studies. A value greater than 1.0 indicates an increased risk of bladder cancer with pioglitazone use. CI = confidence interval.
Rosiglitazone use and bladder cancer
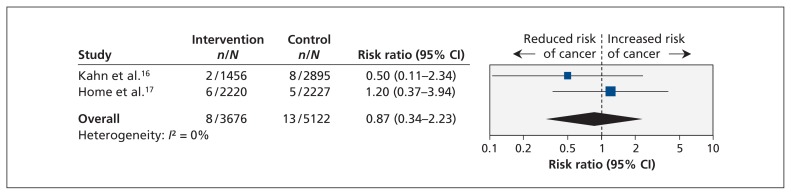
Two RCTs (n = 8798) and one cohort study (n = 1 491 060) reported bladder cancer among rosiglitazone users.16,17,19 The cohort study was designed to assess pioglitazone exposure and bladder cancer incidence and included rosiglitazone exposure as a subgroup.19 Both RCTs compared rosiglitazone with other glucose-lowering treatments, and only one was blinded.17 We observed no association between bladder cancer and rosiglitazone use in the two RCTs (pooled RR 0.87, 95% CI 0.34–2.23; I2 = 0%) (Figure 3) or in the cohort study (HR 1.08, 95% CI 0.92–1.26) (Figure 4).
Figure 3:
Meta-analysis of the risk of bladder cancer associated with rosiglitazone use among patients with type 2 diabetes in randomized controlled trials. A value greater than 1.0 indicates an increased risk of bladder cancer with rosiglitazone use. CI = confidence interval.
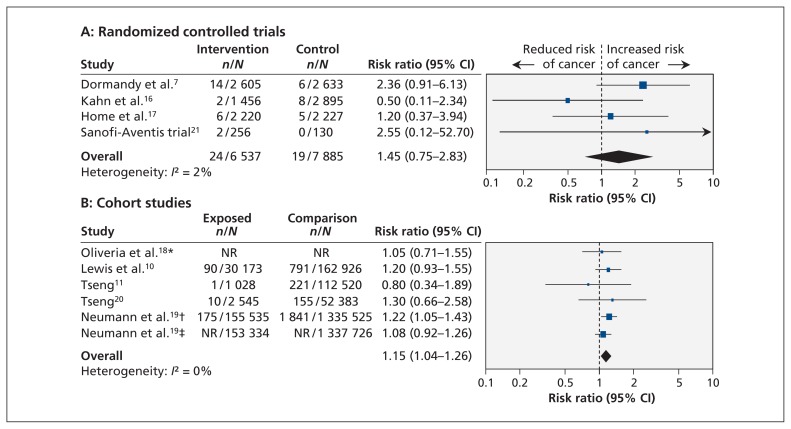
Figure 4:
Meta-analysis of the risk of bladder cancer associated with the use of any thiazolidinedione in randomized controlled trials (A) and cohort studies (B). A value greater than 1.0 indicates an increased risk of bladder cancer with the use of any thiazolidinedione. *A total of 178 cases of bladder cancer occurred in the study population. †Pioglitazone; ‡rosiglitazone. CI = confidence interval, NR = not reported.
Thiazolidinedione use and bladder cancer
In the four RCTs, representing a total of 14 422 participants, the risk of bladder cancer appeared to be elevated with exposure to any thiazolidinedione, but the association was not statistically significant (pooled unadjusted RR 1.45, 95% CI 0.75–2.83; I2 = 2%) (Figure 4, top panel).7,16,17,21 The risk of bladder cancer associated with exposure to any thiazolidinedione was significantly increased in the five cohort studies, representing 2 043 858 patients (pooled adjusted RR 1.15, 95% CI 1.04–1.26; I2 = 0%) (Figure 4, bottom panel).10,11,18–20
Interpretation
In this rigorous systematic review and meta-analysis of randomized and nonrandomized studies, we observed an increased risk of bladder cancer associated with the use of thiazolidinediones. In particular, use of pioglitazone was associated with an increased risk of bladder cancer based on a pooled estimate from three cohort studies involving more than 1.7 million individuals, which was consistent with the risk estimates from the RCTs and the case/noncase study. We observed no association between rosiglitazone use and bladder cancer.
Thiazolidinediones are insulin-receptor sensitizers and exert their effects through activation of the peroxisome proliferator-activated receptor (PPARγ). 23 Although studies suggest PPARγis involved in known tumour-suppression pathways,23,24 mechanisms linking thiazolidinediones with the development or progression of bladder neoplasms have not been fully elucidated. Pre-clinical research involving female rats exposed to rosiglitazone showed a higher incidence of bladder tumours among rats given rosiglitazone than among controls.25 Another study reported a substantially higher incidence of bladder tumours among male rats given pioglitazone than among controls.26 The latter study reported no difference in the occurrence of other cancers.
Concerns over a potential association between pioglitazone use and bladder cancer among humans emerged after publication of the PROactive study, which reported a nonsignificant increased risk of bladder cancer among participants exposed to pioglitazone compared with controls (Figure 4).7 Subsequent review of these cases suggested that the true incidence was lower in both groups.27 Three subsequent observational studies supported the initial findings of the PROactive study and further suggested associations with dose and duration of treatment.9,10,19 In response to these findings, France removed pioglitazone from the market; the European Medicines Agency and Health Canada called for close selection and monitoring of patients using the medication,28,29 and the US Food and Drug Administration issued warnings against the use of pioglitazone in patients with active or previous bladder cancer.30
Limitations
Our study has limitations, most of which are predicated on the lack of primary studies available for synthesis and the different study designs and methods among the included studies. We did not have individual patient data and were therefore unable to exclude the small number of individuals with type 1 diabetes, examine other known risk factors for bladder cancer (especially smoking and occupational exposure) or control for duration of exposure.
Our definition of exposure to agents ever (v. never) captured real-world prescription patterns of glucose-lowering agents (i.e., combination therapy) but may have led to conflicting associations between other agents (e.g., insulin, sulfonylureas, metformin) and bladder cancer;1 this may have biased our estimate in a nondifferential manner.
The high risk of bias among the RCTs is a limitation, but it does not meaningfully change our interpretation of estimates for rare and unexpected events such as bladder cancer. Further, results from the RCTs were similar to those from the observational studies, which were at low risk of bias. In the observational studies, bladder cancer was captured through usual care, where more severe and symptomatic (and thus most easily recognized) cases are more likely to be identified. Consequently, we may have underestimated the true number of patients with bladder cancer, although this is unlikely to affect the relative risk estimate.
Conclusion
Our results suggest an association between pioglitazone use and bladder cancer in adults with type 2 diabetes. Given the limited evidence among rosiglitazone users, it remains unclear if the association with bladder cancer is a class effect of all thiazolidinediones. Evidence surrounding the association between pioglitazone and bladder cancer requires cautious interpretation, because our evidence is based on only three, albeit large and well-conducted, observational studies.10,19,20 Future research is required to improve our understanding and should include large population-based cohort studies involving individuals with type 2 diabetes; include a reference group of individuals without diabetes; have a minimum dose and duration of exposure; and account for important risk factors for bladder cancer (e.g., smoking status and history of bladder disease).31
Although the absolute risk of bladder cancer associated with pioglitazone was small, other evidence-based treatments for type 2 diabetes may be equally effective and do not carry a risk of cancer. This study quantifies the association between pioglitazone use and bladder cancer and may help inform decisions around safer use of pioglitazone in individuals with type 2 diabetes.
Supplementary Material
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Jeffrey Johnson, Isabelle Colmers and Sumit Majumdar conceived and designed the review, and Jeffrey Johnson, Samantha Bowker and Sumit Majumdar provided supervision. Isabelle Colmers and Samantha Bowker identified and acquired reports of trials, abstracted data and assessed risk of bias. Isabelle Colmers conducted the statistical analyses and contacted authors of included studies to obtain additional information. All of the authors contributed to the interpretation of data. Isabelle Colmers drafted the manuscript, and all of the authors critically revised the manuscript. All of the authors approved the final version of the manuscript submitted for publication and are guarantors for the study.
Funding: This work was supported in part by an operating grant from the Canadian Institutes of Health Research (CIHR) (grant no. MOP-82737) and a CIHR Team Grant to the Alliance for Canadian Health Outcomes Research in Diabetes (ACHORD) (grant no. OTG-88588) sponsored by the CIHR Institute of Nutrition, Metabolism and Diabetes. The funding bodies had no role in the study design, the collection and analysis of data, the decision to publish or the preparation of the manuscript.
Isabelle Colmers holds a graduate student scholarship from the Alberta Diabetes Foundation and is a recipient of the ACHORD Trainee Award. Sumit Majumdar holds the Endowed Chair in Patient Health Management (Faculties of Medicine and Dentistry and of Pharmacy and Pharmaceutical Sciences, University of Alberta) and a Health Scholar award from Alberta Innovates — Health Solutions (AIHS). Jeffrey Johnson is a Senior Scholar with AIHS and holds a Canada Research Chair in Diabetes Health Outcomes.
References
- 1.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010;33:1674–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsson SC, Orsini N, Brismar K, et al. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia 2006;49:2819–23 [DOI] [PubMed] [Google Scholar]
- 3.Yoshimura R, Matsuyama M, Segawa Y, et al. Expression of peroxisome proliferator-activated receptors (PPARs) in human urinary bladder carcinoma and growth inhibition by its agonists. Int J Cancer 2003;104:597–602 [DOI] [PubMed] [Google Scholar]
- 4.Johnson JA, Bowker SL. Intensive glycaemic control and cancer risk in type 2 diabetes: a meta-analysis of major trials. Diabetologia 2011;54:25–31 [DOI] [PubMed] [Google Scholar]
- 5.Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer 2011;18:R125–47 [DOI] [PubMed] [Google Scholar]
- 6.Kakehi Y, Hirao Y, Kim W-J, et al. Bladder Cancer Working Group report. Jpn J Clin Oncol 2010;40(Suppl 1):i57–64 [DOI] [PubMed] [Google Scholar]
- 7.Dormandy JA, Charbonnel B, Eckland DJA, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomized controlled trial. Lancet 2005;366:1279–89 [DOI] [PubMed] [Google Scholar]
- 8.Risque de cancer de la vessie chez les personnes diabétiques traitées par pioglitazone en France: une étude de cohorte sur les données du SNIIRAM et du PMSI. Paris (France): Caisse National d’Assurance Maladie; 2011. Available: www.afssaps.fr/var/afssaps_site/storage/original/application/b42a6bf9a1b63c3dbec7388d3914687b.pdf (accessed 2012 June 6). [Google Scholar]
- 9.Piccinni C, Motola D, Marchesini G, et al. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care 2011;34:1369–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011;34: 916–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia 2011;54:2009–15 [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT, Altman DG, Sterne JAC, editors. Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011. Available: www.cochrane-handbook.org (accessed 2012 June 6). [Google Scholar]
- 13.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Available: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 2012 June 6).
- 14.Reeves BC, Deeks JJ, Higgins JPT, et al. Including nonrandomized studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011. Available: www.cochrane-handbook.org (accessed 2012 June 6). [Google Scholar]
- 15.Sterne JAC, Egger M, Moher D, editors. Addressing reporting biases. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011. Available: www.cochrane-handbook.org (accessed 2012 June 6). [Google Scholar]
- 16.Kahn SE, Haffner SM, Heise MA, et al. ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–43 [DOI] [PubMed] [Google Scholar]
- 17.Home PD, Pocock SJ, Beck-Nielsen H, et al. RECORD Study Team Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009;373: 2125–35 [DOI] [PubMed] [Google Scholar]
- 18.Oliveria SA, Koro C, Ulcickas Yood M, et al. Cancer incidence among patients treated with antidiabetic therapy. Diabetes Metab Syndr 2009;2:45–57 [Google Scholar]
- 19.Neumann A, Weill A, Ricordeau P, et al. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia 2012;55:1953–62 Mar. 31 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tseng CH. Pioglitazone and bladder cancer: a population-based study of Taiwanese. Diabetes Care 2012;35:278–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Insulin glargine injection treatment in place of thiazolidinedione (TZD), sulfonylurea, or metformin in triple agent therapy for type 2 diabetes mellitus (T2DM) subjects with unsatisfactory control [trial registration no. NCT00283049]. ClinicalTrials.gov. Available: http://clinicaltrials.gov/ct2/show/NCT00283049 (accessed 2012 June 6).
- 22.Home PD, Kahn SE, Jones NP, et al. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010;53:1838–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannini S, Serio M, Galli A. Pleiotropic effects of thiazolidinediones: taking a look beyond antidiabetic activity. J Endocrinol Invest 2004;27:982–91 [DOI] [PubMed] [Google Scholar]
- 24.Mansure JJ, Nassim R, Kassouf W. Peroxisome proliferator-activated receptor gamma in bladder cancer: a promising therapeutic target. Cancer Biol Ther 2009;8:6–15 [DOI] [PubMed] [Google Scholar]
- 25.Lubet RA, Fischer SM, Steele VE, et al. Rosiglitazone, a PPAR gamma agonist: potent promoter of hydroxybutyl(butyl)nitro-samine-induced urinary bladder cancers. Int J Cancer 2008;123: 2254–9 [DOI] [PubMed] [Google Scholar]
- 26.Actos (pioglitazone hydrochloride): full prescribing information. Deerfield (IL): Takeda Pharmaceuticals America; 2012. Available: http://general.takedapharm.com/content/file/pi.pdf?applicationcode=8a9c4571-a123-4477-91de-b9cafe7d07e3&filetypecode=actospi (accessed 2012 June 6). [Google Scholar]
- 27.Hillaire-Buys D, Faillie JL, Montastruc JL. Pioglitazone and bladder cancer [letter]. Lancet 2011;378:1543–4 [DOI] [PubMed] [Google Scholar]
- 28.European Medicines Agency clarifies opinion on pioglitazone and the risk of bladder cancer [press release]. London (UK): European Medicines Agency; 2011. Available: www.ema.europa.eu/docs/en_GB/document_library/Press_release/2011/10/WC500116936.pdf (accessed 2012 June 6). [Google Scholar]
- 29.Health Canada reviewing diabetes drug pioglitazone (Actos) and potential risk of bladder cancer. Ottawa (ON): Health Canada; 2011. Available: www.hc-sc.gc.ca/ahc-asc/media/advisories-avis/_2011/2011_79-eng.php (accessed 2012 June 6). [Google Scholar]
- 30.FDA Drug Safety Communication Updated drug labels for pioglitazone-containing medicines. Silver Spring (MD): Food and Drug Administration; 2011. Available: www.fda.gov/Drugs/DrugSafety/ucm266555.htm (accessed 2012 June 6). [Google Scholar]
- 31.Johnson JA, Carstensen B, Witte D, et al. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia 2012;55:1607–18 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.