Abstract
BACKGROUND
The medial temporal structures, including the hippocampus and the entorhinal cortex, are critical for the ability to transform daily experience into lasting memories. We tested the hypothesis that deep-brain stimulation of the hippocampus or entorhinal cortex alters memory performance.
METHODS
We implanted intracranial depth electrodes in seven subjects to identify seizure-onset zones for subsequent epilepsy surgery. The subjects completed a spatial learning task during which they learned destinations within virtual environments. During half the learning trials, focal electrical stimulation was given below the threshold that elicits an afterdischarge (i.e., a neuronal discharge that occurs after termination of the stimulus).
RESULTS
Entorhinal stimulation applied while the subjects learned locations of landmarks enhanced their subsequent memory of these locations: the subjects reached these landmarks more quickly and by shorter routes, as compared with locations learned without stimulation. Entorhinal stimulation also resulted in a resetting of the phase of the theta rhythm, as shown on the hippocampal electroencephalogram. Direct hippocampal stimulation was not effective. In this small series, no adverse events associated with the procedure were observed.
CONCLUSIONS
Stimulation of the entorhinal region enhanced memory of spatial information when applied during learning. (Funded by the National Institutes of Health and the Dana Foundation.)
Loss of the ability to remember is one of the most dreaded afflictions of the human condition. Decades of research and clinical observations have established that declarative memory, the ability to remember recently experienced facts and events, depends on the hippocampus and associated structures in the medial temporal lobe, including the entorhinal, perirhinal, and parahippocampal cortexes.1
Deep-brain stimulation has emerged as a technique to treat neurologic and neuropsychiatric disorders, including Parkinson’s disease, dystonia, depression, and obsessive–compulsive disorder.2–5 The nature of the stimulation-induced modification of the neural circuit that results in improvement in patients with these disorders is not completely understood. However, it has been established that the ability of deep-brain stimulation to modify brain functions depends on the application of stimulation at specific sites in the complex neuronal circuitry underlying these functions.2–5
In rodents, electrical stimulation of the perforant pathway, which originates in the entorhinal cortex and projects into the hippocampus, results in long-term potentiation, release of acetylcholine, and resetting of the theta phase, all of which are associated with improved memory.6–9 It has also been shown that electrical stimulation can enhance neurogenesis in the hippocampus.10 Whether direct stimulation of this entorhinal output to the hippocampus enhances learning is not known. However, stimulation of targets in the lateral hypothalamus in rodents during learning resulted in improved performance on tests of subsequent memory.11
The few studies involving direct electrical stimulation of the hippocampus in humans have generally shown a disruptive effect on memory. Studies have shown that stimulation of the hippocampus above the threshold for eliciting an afterdischarge (i.e., a neuronal discharge that occurs after termination of the stimulus) on the electroencephalogram (EEG) results in memory impairments.12,13 More recently, bilateral stimulation of the hippocampus during learning was shown to have a negative effect on subsequent recognition memory.14,15 To test the hypothesis that site-specific stimulation at a particular phase of information processing enhances memory performance in humans, we applied deep-brain stimulation to targets in the hippocampal and entorhinal regions in seven subjects with pharmacoresistant epilepsy while they learned locations within a novel virtual environment.
METHODS
STUDY SUBJECTS
The subjects were seven patients with pharmacoresistant epilepsy (Table 1, and Tables 1, 2, and 3 in the Supplementary Appendix, available with the full text of this article at NEJM.org) in whom intracranial depth electrodes were implanted for 7 to 10 days in order to determine the area of seizure onset for possible surgical resection. They met clinical criteria for the surgical procedure of depth-electrode placement.21,22 We implanted the electrodes stereotactically, using guidance from magnetic resonance imaging (MRI) and digital subtraction angiography.22,23 The electrodes (Adtech) had platinum contacts for EEG recording and for stimulation.
Table 1.
Clinical Characteristics of the Seven Subjects.*
| Subject No. |
Verbal IQ | Digit Span | Verbal Memory | Visual Memory |
Executive Function |
|
|---|---|---|---|---|---|---|
| WMS | CVLT percentile |
|||||
| 1 | 102 | 91 | 84 | 84 | 24 | 90 |
| 2 | — | 2 | 25 | 1 | 1 | 1 |
| 3 | 77 | 16 | 5 | 16 | 1 | 1 |
| 4 | 81 | 16 | 1 | 2 | 1 | 58 |
| 5 | 117 | 95 | 50 | 1 | 8 | 21 |
| 6 | 113 | 75 | 50 | 69 | 63 | 6 |
| 7 | 103 | 21 | 84 | 69 | 34 | 27 |
Verbal IQ (verbal IQ for Subject 2 was not obtained) and digit span (i.e., attention) were calculated with the use of the Wechsler Adult Intelligence Scale,16,17 verbal memory by means of the logical memory portion of the Wechsler Memory Scale (WMS) and the long-delay free-recall portion of the California Verbal Learning Test (CVLT),18 visual memory with the use of the 30-second delayed version of the Rey–Osterrieth Complex Figure Test,19 and executive function by means of the Trail Making Test, Part B.20
We placed the electrodes solely on the basis of clinical criteria. Six of the seven subjects had entorhinal electrodes, and five of the seven had at least one hippocampal electrode. Four of the seven subjects had both entorhinal and hippocampal electrodes implanted ipsilaterally, allowing EEG data from the hippocampus to be usefully recorded during entorhinal stimulation. Only one electrode fell within a determined seizure-onset zone (Table 2 in the Supplementary Appendix); we excluded data obtained with the use of this electrode from a repeated analysis. All research was carried out at the UCLA Medical Center; the study protocol was approved by the center’s institutional review board. The subjects provided written informed consent to participate in the study.
STIMULATION
Stimulation was current-regulated and charge-balanced, with biphasic rectangular pulses set below the threshold for afterdischarge, which was identified on the basis of pretesting (range, 1.0 to 2.0 mA). The subjects were unaware of the stimulation condition, and no subject reported noticing any effect of stimulation. Electrode contacts were stimulated through an interface with a Grass C-12 stimulator and a Telefactor relay box (both from Astro-Med) and a Stellate Harmonie 6.2ef recording system (Stellate Systems). Stimulation was bipolar, with the electrodes placed 1.5 mm apart (surface area, 0.059 cm2), with a cycle of 5 seconds on and 5 seconds off at a frequency of 50 Hz and a pulse width of 300 µsec. The current ranged from 0.5 to 1.5 mA, with stimulation ranging between 2.5 and 7.6 microcoulombs (µC) of charge per square centimeter per phase, which is well below the safe maximum used for long-term and short-term stimulation (30 and 57 µC, respectively).24,25 The impedance of the electrodes was between 1 and 4 kΩ. Limits for stimulation were a voltage of up to 3.0 V, a pulse width up to 450 µsec, and a frequency up to 130 Hz, which are considered safe and are well tolerated in patients with epilepsy who have depth electrodes in the temporal lobe26; similar stimulation levels have been used to control epileptic seizures.27 During all sessions involving deep-brain stimulation, a neurologist was present to monitor the subject and view the real-time EEG for afterdischarges.
BEHAVIORAL TASKS
The subjects completed a spatial learning task that consisted of navigation through a virtual environment to deliver passengers to stores (Fig. 1 in the Supplementary Appendix). This task has been used in several studies showing recruitment of the medial temporal lobe during navigation.28–30 The study was performed in accordance with the protocol (available at NEJM.org), which includes mention of a test of deep-brain stimulation during egocentric training; we have yet to carry out this part of the protocol.
For the entorhinal-stimulation condition of the study, we tested six subjects (all except Subject 5), each in a single testing session. For the hippocampal-stimulation condition of the study, we tested four subjects unilaterally (Subjects 2, 3, 4, and 7), and we tested Subject 5 with left and right hippocampal stimulation separately. Thus, there were six tests of retention of memory after stimulation of the entorhinal area and six tests of retention of memory after stimulation of the hippocampus. Each test consisted of four blocks of navigation trials. In each testing session, subjects learned to navigate to six stores in a virtual environment to drop off passengers; each store was repeated in each of the four blocks (for a total of 24 navigation trials for each brain region tested). During the first three blocks, stimulation was applied during navigation to three of the six stores. For each subject, stimulation was applied consistently, according to the store, across blocks. For example, during each of the first three blocks of the test, Subject 1 received deep-brain stimulation while navigating to stores 1, 3, and 5 but not to stores 2, 4, and 6; Subject 2, on the other hand, received deep-brain stimulation while navigating to stores 2, 4, and 6 but not to stores 1, 3, and 5. The order of the stores was randomized among the blocks, with stimulation and nonstimulation alternating between consecutive trials within each block. Three subjects navigated to the first store during entorhinal stimulation; the other three navigated to the first store in the absence of entorhinal stimulation. Each store occurred equally often in stimulation and nonstimulation conditions across subjects. Stimulation was applied throughout the entire trial in 5-second on–off trains; the trial duration varied, depending on the time needed for the subject to locate the store (mean [±E] trial time, 14.8±1.8 seconds). No stimulation was given during the fourth block of navigation trials.
The four blocks were separated by 2-minute intervals during which the subjects carried out two control tasks (see the Methods section in the Supplementary Appendix). These tasks were designed to determine whether any effect of stimulation on spatial learning was due to improvement in motor or perceptual abilities.
We quantified spatial learning by first calculating the shortest path length (i.e., the ideal path) from the location of passenger pickup to drop-off at the target store destination.31 Next, the actual path length was calculated. The key dependent variable in the study was the excess path length, which was calculated by subtracting the length of the ideal path from the length of the actual path to the store for each trial (Fig. 2A in the Supplementary Appendix). Shorter excess path length indicated better performance by the subject. We also quantified latency as an additional measure of navigation efficiency, which was calculated as the time (in seconds) from passenger pickup to drop-off at the store. The path lengths to stores within a condition (stimulation or nonstimulation) were not equal. However, the path lengths to stores across conditions (stimulation vs. nonstimulation) were equal.
ELECTRODE LOCALIZATION
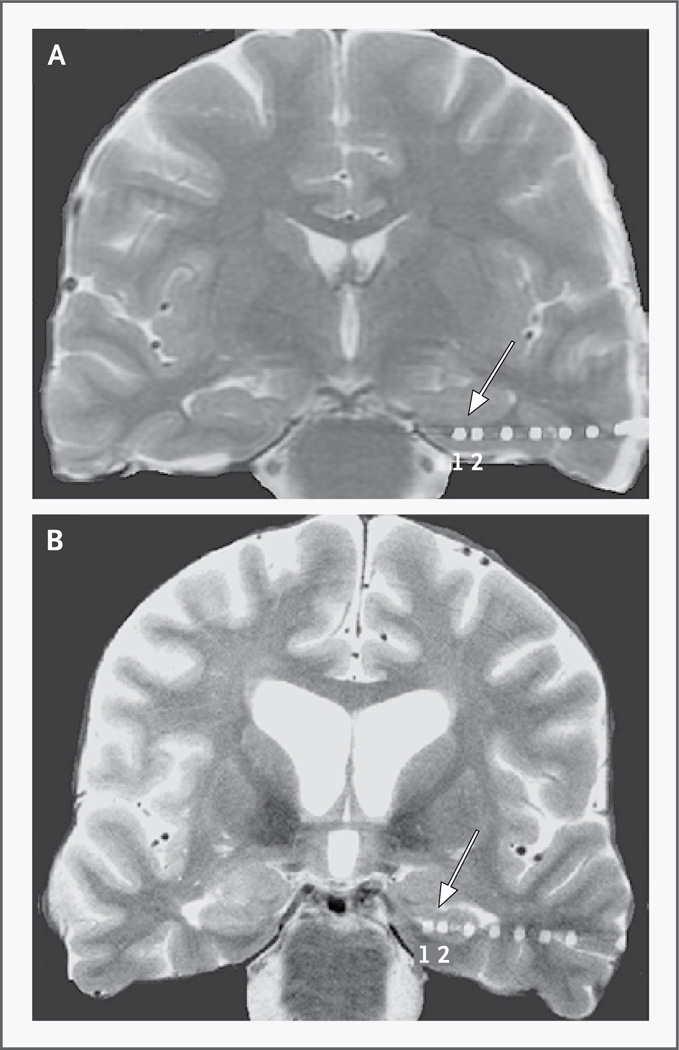
Before implantation of the depth electrodes, subjects underwent MRI with a head-only, 3-tesla scanner (Magnetom Trio, Siemens) (see the Methods section and Table 3 in the Supplementary Appendix for details of scanning parameters and electrode localization). We delivered deep-brain stimulation using the two most distal contacts of each electrode (Fig. 1). At least one contact of each electrode in the entorhinal region was within the alvear bundle, which includes the perforant pathway.
Figure 1. High-Resolution Magnetic Resonance Imaging (MRI) in Two Subjects with Implanted Electrodes.
Coronal high-resolution MRI scans show electrodes imaged on postoperative computed tomographic (CT) scans coregistered to the MRI scans. The two most distal electrodes (numbered 1 and 2, with 1 marking the most distal electrode) are shown in the left entorhinal region in one subject (Panel A) and the ipsilateral hippocampus in another subject (Panel B). For all subjects, the two most distal electrodes were used for stimulation.
ELECTROPHYSIOLOGICAL ANALYSIS
We obtained EEG data from the hippocampus in the four subjects in whom we placed electrodes in both the entorhinal region and the ipsilateral hippocampus. Each data record (sampling frequency, 200 Hz) was filtered for theta (3 to 8 Hz), alpha (9 to 14 Hz), beta (15 to 35 Hz), and gamma (36 to 100 Hz) frequency bands. To determine whether phase resetting occurred in the hippocampus after stimulation of the entorhinal region, waveforms for 5-second periods before and during each stimulation train were averaged separately for each trial. Phase resetting produces greater alignment of waves across trials and thus greater amplitude in the averaged waveform.9 We then calculated the percentage increase in theta-phase resetting for the 5-second period after the onset of stimulation, as compared with the 5-second period before the onset of stimulation. See the Methods section in the Supplementary Appendix for more details.
STATISTICAL ANALYSIS
For each stimulation site (entorhinal and hippocampal), we completed a two (stimulation vs. nonstimulation condition) by three (blocks 1, 2, and 3) repeated-measures analysis of variance for latency and excess path length. For retention block 4, we compared the median latency and median excess path length for navigation to store locations that had been learned while stimulation was applied with those learned in the absence of stimulation during blocks 1, 2, and 3, using the Wilcoxon signed-rank test, with a P value of less than 0.05 considered to indicate statistical significance. To examine effects in individual subjects, for each testing session we calculated the average percent reduction in excess path length during block 4 for each of the three locations that had been learned during periods with stimulation in blocks 1, 2, and 3, and compared the data with the average percent reduction in excess path length during block 4 for each of the three locations that had been learned without stimulation in those blocks. We performed a two-tailed binomial sign test for the entorhinal region to examine whether the number of subjects who had shorter path lengths in the stimulation condition exceeded the number expected by chance.
To compare the amplitude of the average EEG waveforms during the 5 seconds after the onset of stimulation and the 5 seconds before the onset of stimulation, we calculated the paired-sample t-statistic (i.e., t(3); 4 subjects and 3 degrees of freedom), with a P value of less than 0.05 considered to indicate statistical significance (Bonferroni-corrected for multiple frequency bands). We did the same comparison for the 5 seconds after the onset of a navigation trial and the 5 seconds before the onset of a navigation trial during trials with no stimulation. The phase-resetting analysis was repeated for each frequency range. To ensure that theta-phase resetting was not due to increases in the power of each navigation trial’s rhythm, we also compared the average of the individual trial waveform amplitudes (average theta power) in the stimulation and nonstimulation conditions (Fig. 4C in the Supplementary Appendix).
RESULTS
BEHAVIORAL TASKS
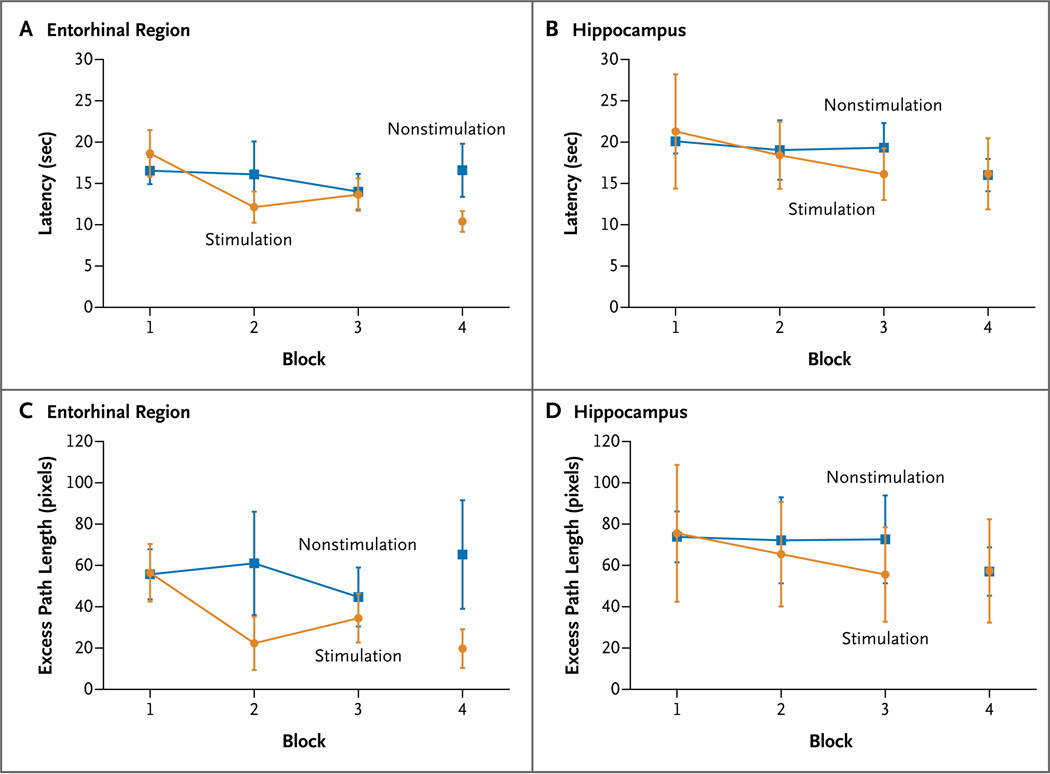
Each subject completed all blocks of trials, and each block lasted an average of 88.6±11.0 seconds. Figure 2 shows the average of the subjects’ behavioral performance during spatial learning with unilateral stimulation of the entorhinal region or hippocampus. During block 4 of the navigation trials, we tested the subjects’ memory of the store locations. In the six subjects, we observed significantly shorter latency (Fig. 2A) and shorter excess path length for locations that had been learned during stimulation of the entorhinal region in blocks 1, 2, and 3, than for locations that had been learned without stimulation (latency, z = −2.20, P = 0.03; excess path length, z = −2.20, P = 0.03; effect size, [Cohen’s] d = 1.74) (Fig. 2A and 2C, respectively). These results show that stimulation of the entorhinal region during navigation results in an enhancement of performance on subsequent navigation. In each of the six subjects, we observed enhancement of performance (i.e., reduced latency and reduced path length) of the tasks learned with entorhinal stimulation, as compared with performance of the tasks learned without such stimulation (P = 0.03). The average reduction in excess path length across the six subjects during retention for navigation learned with entorhinal stimulation was 64% (Fig. 3).
Figure 2. Behavioral Performance on Spatial Learning Tasks.
The graphs show the behavioral performance of six subjects on spatial learning tasks during stimulation and nonstimulation of the entorhinal region (Panels A and C) and the hippocampus (Panels B and D). Shown is the latency (Panels A and B) and excess path length (Panels C and D) across the six spatial learning trials for blocks 1 through 4 during stimulation and nonstimulation. I bars indicate standard errors for mean values among subjects for each condition.
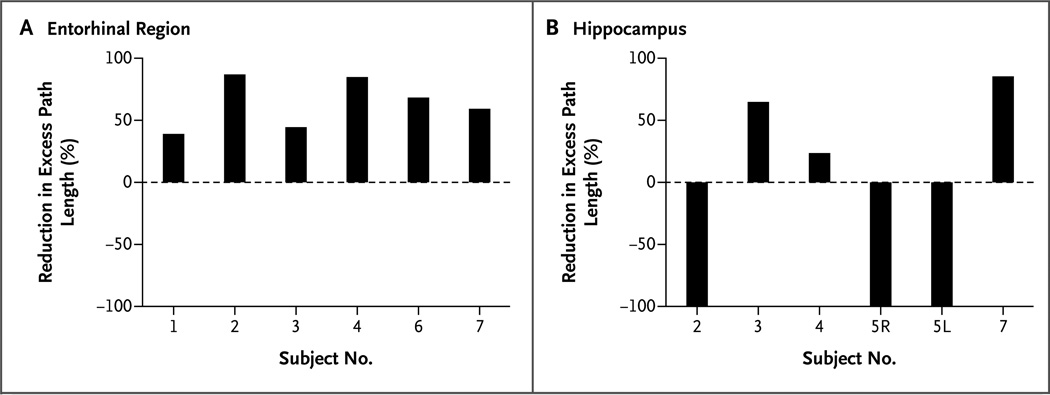
Figure 3. Reduction in Excess Path Length.
The percentage reduction in excess path length is shown for each subject during block 4 (i.e., retention) for store locations that had been learned during stimulation of the entorhinal region (Panel A) and hippocampus (Panel B) in blocks 1, 2, and 3, as compared with store locations that had been learned without stimulation. The maximum reduction that could occur was 100%, which is an excess path length of zero. The six sessions of entorhinal stimulation in all subjects (except Subject 5, who did not receive entorhinal stimulation) showed improvement in memory (reduced excess path length). The six sessions of hippocampal stimulation were from Subjects 2, 3, 4, 5, and 7; Subject 5 was tested for the right (5R) and left (5L) hippocampi separately. There was no consistent effect of stimulation of the hippocampus across subjects.
We observed improvement in memory performance across a wide range of neuropsychological test scores (Table 1, and Table 2 in the Supplementary Appendix). For example, Subject 2, whose scores on standardized tests showed impaired memory and executive function, had an 86.9% reduction in excess path length for locations learned during stimulation, as compared with those learned without stimulation. Subject 1, who performed relatively well without stimulation, had a 38.9% reduction in excess path length for locations learned during stimulation. For five of the six subjects, navigation to each of the three stores learned during stimulation was faster and shorter than navigation to each of the three stores learned without stimulation, indicating a consistent effect.
Direct hippocampal stimulation in six subjects had no effect on their performance of the spatial learning tasks, as compared with no stimulation, with respect to both latency (z = 0.52, P = 0.69) (Fig. 2B) and excess path length (z = −0.52, P = 0.69) (Fig. 2D). Excess path length was equally likely to be longer or shorter after learning with hippocampal stimulation, as compared with learning in the absence of stimulation to the hippocampus.
Neither entorhinal nor hippocampal stimulation significantly affected reaction-time performance on the guided navigation control task or the perceptual store-matching task (Fig. 3 in the Supplementary Appendix).
ELECTROPHYSIOLOGICAL DATA
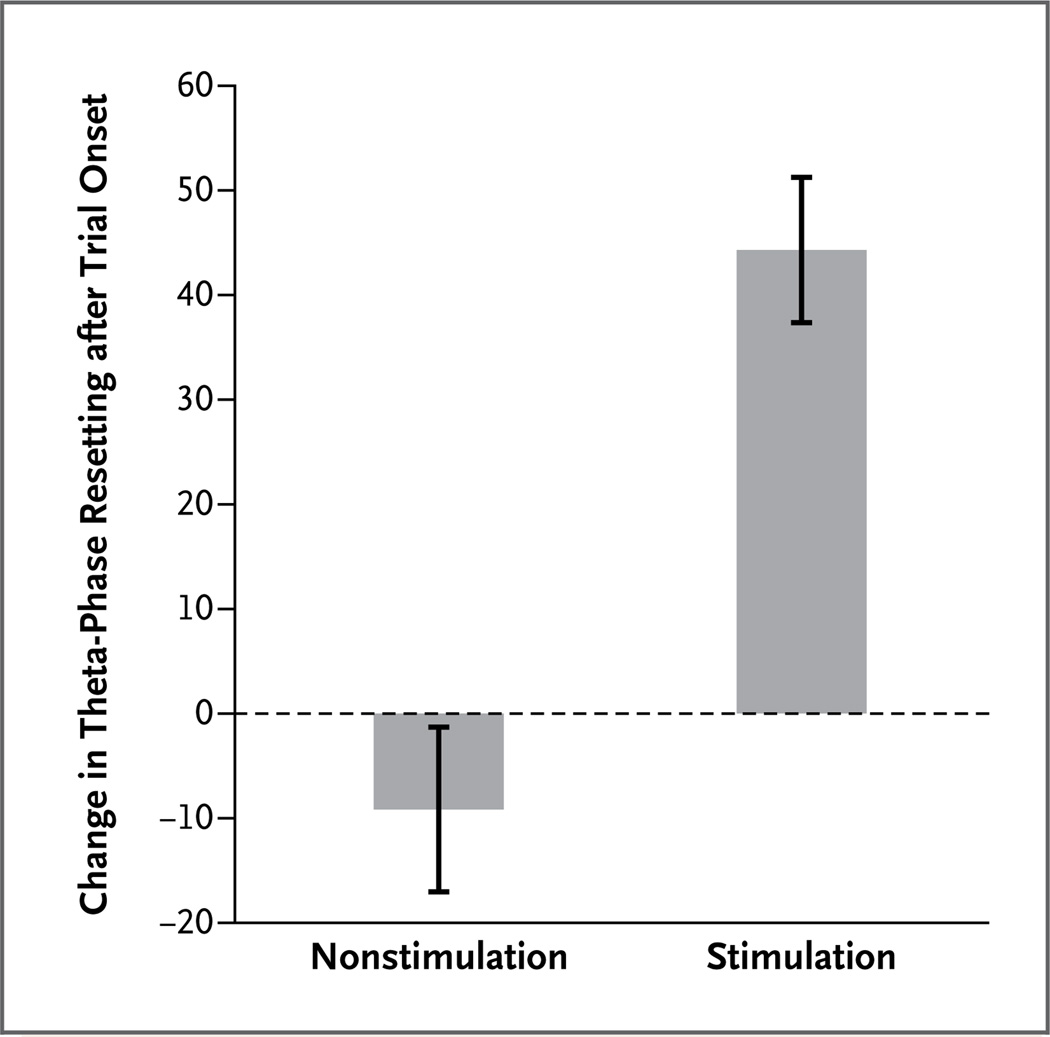
In the four subjects who had electrodes placed in the entorhinal region and ipsilateral hippocampus, the power of the averaged theta rhythm (phase resetting) after entorhinal stimulation showed an increase of 44.3±6.9% during stimulation, as compared with the period before stimulation (t(3) = 10.72, P = 0.002) (Fig. 4). There was no significant difference in the percentage change in theta-phase resetting for comparable alternating 5-second periods in nonstimulation trials (nonstimulation period vs. preceding period, t(3) = −1.35; P = 0.30). An analysis of differences in individual trials in theta power and theta-phase resetting in one subject are shown in the Methods section and Figure 4 in the Supplementary Appendix.
Figure 4. Average Theta-Phase Resetting in the Hippocampus in Four Subjects with Entorhinal and Ipsilateral Hippocampal Electrodes.
Shown is the percentage change in theta power of the average waveform (i.e., theta-phase resetting) from the 5-second period before trial onset to the 5-second period after trial onset for stimulation and nonstimulation trials. Stimulation induced a mean increase of 44.3±6.9% in theta-phase resetting, as compared with the 5-second period before stimulation-trial onset. In the nonstimulation trials, there was no significant change in theta-phase resetting after the trial onset. I bars indicate standard errors for the mean values among the subjects for each condition.
DISCUSSION
Spatial navigation depends on spatial memory. Most common tasks of daily living, such as finding one’s car in a parking lot, are critically dependent on the medial temporal lobe. Our results show that spatial learning in humans can be enhanced by electrical stimulation of the entorhinal region, a specific site within the medial temporal lobe and the chief gateway into the hippocampus. Indeed, stimulation of the entorhinal region while subjects were learning was associated with improvement in memory performance, as measured by speed and choice of route.
The subjects in this study had epilepsy, a neurologic disease that may affect memory function. It is not clear that our findings can be generalized to patients with other neurologic disorders. We did, however, observe an improvement in performance when the medial temporal lobe in persons with epilepsy was stimulated and regardless of baseline memory performance, a finding that suggests that improvement could occur in patients with other memory impairments (e.g., Alzheimer’s disease).
Whether other types of learning and memory (such as verbal or autobiographical) can be similarly enhanced awaits future study, as does the determination of the existence of laterality effects. Neuropsychological data suggest that the left medial temporal lobe is better suited to verbal learning32 and that the right medial temporal lobe is better suited to nonverbal (e.g., visuospatial) learning.33 Although two subjects in our study had stimulation in the left entorhinal area, our study is too small to support conclusions about laterality effects. Much more work is required to determine whether electrical modulation of memory circuits could be used as a therapeutic strategy to enhance function in patients with memory disturbances.
Improvement of memory performance has been observed in a single case study in which deep-brain stimulation of the hypothalamus and fornix to treat morbid obesity improved verbal recall.34 Continuous stimulation of this region over a period of 12 months has also been shown to activate the circuitry of the medial temporal lobe, as measured with EEG and positron-emission tomography in five patients with early Alzheimer’s disease,35 although memory enhancement was not shown in this group. Our findings suggest that the perforant pathway, the major source of cortical afferent input into the hippocampus, may be preferable as the site of deep-brain stimulation for memory enhancement. In fact, this is further supported by a study in rodents that was published after the completion of the present study.36
An important aspect of the memory-enhancing stimulation in this study was its application during the learning phase. This suggests that with the use of neuroprosthetic devices aimed at cognitive enhancement, stimulation may not need to be applied continuously but only when patients are attempting to learn important information. Future studies are needed to determine whether stimulation during the act of recall would also have beneficial effects.
The theta rhythm (3 to 8 Hz) is a large EEG potential recorded from the hippocampus in rodents and humans29,37 and is thought to aid formation of memories.37 It has been suggested that resetting of the phase of the theta rhythm improves memory performance by allowing the best possible encoding of novel stimuli.38 Stimulation of the perforant pathway in rodents induces resetting of the theta phase and produces favorable conditions for long-term potentiation.9,39 In four subjects in our study who had contacts implanted in the entorhinal region and ipsilateral hippocampus, we observed theta-phase resetting in the hippocampus during stimulation of the entorhinal region. In a study that used functional MRI in humans, learned information that was associated with increased spontaneous activity in the entorhinal cortex was subsequently remembered better40 than learned information with no such associated activity, suggesting that increased entorhinal input to the hippocampus can improve learning. Our preliminary results support the hypothesis that stimulation that enhances memory also induces theta-phase resetting and provide evidence supporting a possible mechanism for stimulation-induced memory enhancement in humans.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health (5T32NS07449), the National Institute of Neurological Disorders and Stroke (NS033221), and the Dana Foundation.
We thank Drs. Arne Ekstrom and Charles Wilson for their valuable advice and discussions; Kirk Shattuck and Tony Fields for technical assistance; Sakshi Aggarwal, Brooke Salaz, Irene Wainwright, and Deena Pourshaban for general assistance; and the subjects for their participation in this study.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 2.Lang AE, Lozano AM. Parkinson’s disease: second of two parts. N Engl J Med. 1998;339:1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- 3.Davis KD, Taub E, Houle S, et al. Globus pallidus stimulation activates the cortical motor system during alleviation of parkinsonian symptoms. Nat Med. 1997;3:671–674. doi: 10.1038/nm0697-671. [DOI] [PubMed] [Google Scholar]
- 4.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352:459–467. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]
- 6.Ehret A, Haaf A, Jeltsch H, Heimrich B, Feuerstein TJ, Jackisch R. Modulation of electrically evoked acetylcholine release in cultured rat septal neurones. J Neurochem. 2001;76:555–564. doi: 10.1046/j.1471-4159.2001.00030.x. [DOI] [PubMed] [Google Scholar]
- 7.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vertes RP. Hippocampal theta rhythm: a tag for short-term memory. Hippocampus. 2005;15:923–935. doi: 10.1002/hipo.20118. [DOI] [PubMed] [Google Scholar]
- 9.Williams JM, Givens B. Stimulation-induced reset of hippocampal theta in the freely performing rat. Hippocampus. 2003;13:109–116. doi: 10.1002/hipo.10082. [DOI] [PubMed] [Google Scholar]
- 10.Toda H, Hamani C, Fawcett AP, Hutchison WD, Lozano AM. The regulation of adult rodent hippocampal neurogenesis by deep brain stimulation. J Neurosurg. 2008;108:132–138. doi: 10.3171/JNS/2008/108/01/0132. [DOI] [PubMed] [Google Scholar]
- 11.Soriano-Mas C, Redolar-Ripoll D, Aldavert-Vera L, Morgado-Bernal I, Segura-Torres P. Post-training intracranial self-stimulation facilitates a hippocampus-dependent task. Behav Brain Res. 2005;160:141–147. doi: 10.1016/j.bbr.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Halgren E, Wilson CL. Recall deficits produced by afterdischarges in the human hippocampal formation and amygdala. Electroencephalogr Clin Neurophysiol. 1985;61:375–380. doi: 10.1016/0013-4694(85)91028-4. [DOI] [PubMed] [Google Scholar]
- 13.Halgren E, Wilson CL, Stapleton JM. Human medial temporal-lobe stimulation disrupts both formation and retrieval of recent memories. Brain Cogn. 1985;4:287–295. doi: 10.1016/0278-2626(85)90022-3. [DOI] [PubMed] [Google Scholar]
- 14.Coleshill SG, Binnie CD, Morris RG, et al. Material-specific recognition memory deficits elicited by unilateral hippocampal electrical stimulation. J Neurosci. 2004;24:1612–1616. doi: 10.1523/JNEUROSCI.4352-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacruz ME, Valentín A, Seoane JJ, Morris RG, Selway RP, Alarcón G. Single pulse electrical stimulation of the hippocampus is sufficient to impair human episodic memory. Neuroscience. 2010;170:623–632. doi: 10.1016/j.neuroscience.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 16.Wechsler D. Wechsler Adult Intelligence Scale, third edition (WAIS-III) San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 17.Idem. Wechsler Memory Scale, revised. New York: Psychological Corporation/Harcourt Brace Jovanovich; 2005. [Google Scholar]
- 18.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2nd ed. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 19.Meyers JE, Meyers KR. Rey Complex Figure Test and recognition trial. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 20.Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. New York: Oxford University Press; 2004. [Google Scholar]
- 21.Engel J., Jr . Appendix 2: presurgical evaluation protocols (University of California, Los Angeles) In: Engel J Jr, editor. Surgical treatment of the epilepsies. 2nd ed. New York: Raven Press; 1993. pp. 743–745. [Google Scholar]
- 22.Fried I, Wilson CW, Zhang JX, et al. Implantation of depth electrodes for EEG recording. In: De Salles AAF, Goetsch SJ, editors. Stereotactic surgery and radiosurgery. Madison, WI: Medical Physics Publishing; 1993. pp. 149–158. [Google Scholar]
- 23.Fried I, Wilson CL, Maidment NT, et al. Cerebral microdialysis combined with single-neuron and electroencephalographic recording in neurosurgical patients: technical note. J Neurosurg. 1999:91697–91705. doi: 10.3171/jns.1999.91.4.0697. [DOI] [PubMed] [Google Scholar]
- 24.Agnew WF, McCreery DB. Considerations for safety with chronically implanted nerve electrodes. Epilepsia. 1991;31(Suppl 2):S27–S32. doi: 10.1111/j.1528-1157.1990.tb05845.x. [DOI] [PubMed] [Google Scholar]
- 25.Gordon B, Lesser RP, Rance NE, et al. Parameters for direct cortical electrical stimulation in the human: histopathologic confirmation. Electroencephalogr Clin Neurophysiol. 1990;75:371–377. doi: 10.1016/0013-4694(90)90082-u. [DOI] [PubMed] [Google Scholar]
- 26.Boon P, Vonck K, De Herdt V, et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48:1551–1560. doi: 10.1111/j.1528-1167.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- 27.Jobst B. Brain stimulation for surgical epilepsy. Epilepsy Res. 2010;89:154–161. doi: 10.1016/j.eplepsyres.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Ekstrom AD, Kahana MJ, Caplan JB, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- 29.Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, Kahana MJ. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15:881–889. doi: 10.1002/hipo.20109. [Erratum, Hippocampus 2006;16:101.] [DOI] [PubMed] [Google Scholar]
- 30.Suthana NA, Ekstrom AD, Moshirvaziri S, Knowlton B, Bookheimer SY. Human hippocampal CA1 involvement during allocentric encoding of spatial information. J Neurosci. 2009;29:10512–10519. doi: 10.1523/JNEUROSCI.0621-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartley T, Maguire EA, Spiers HJ, Burgess N. The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron. 2003;37:877–888. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 32.Frisk V, Milner B. The relationship of working memory to the immediate recall of stories following unilateral temporal or frontal lobectomy. Neuropsychologia. 1990;28:121–135. doi: 10.1016/0028-3932(90)90095-6. [DOI] [PubMed] [Google Scholar]
- 33.Smith ML, Milner B. Right hippocampal impairment in the recall of spatial location: encoding deficit or rapid forgetting? Neuropsychologia. 1989;27:71–81. doi: 10.1016/0028-3932(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 34.Hamani C, McAndrews MP, Cohn M, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008;63:119–123. doi: 10.1002/ana.21295. [DOI] [PubMed] [Google Scholar]
- 35.Laxton AW, Tang-Wai DF, McAndrews MP, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol. 2010;68:521–534. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- 36.Stone SSD, Teixeira CM, DeVito L, et al. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011;31:13469–13484. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 38.Vinogradova OS, Brazhnik ES, Kitchigina VF, Stafekhina VS. Modulation of the reaction of hippocampal neurons to sensory stimuli by cholinergic substances. Neurosci Behav Physiol. 1996;26:113–124. doi: 10.1007/BF02359414. [DOI] [PubMed] [Google Scholar]
- 39.McCartney H, Johnson AD, Weil ZM, Givens B. Theta reset produces optimal conditions for long-term potentiation. Hippocampus. 2004;14:684–687. doi: 10.1002/hipo.20019. [DOI] [PubMed] [Google Scholar]
- 40.Fernández G, Brewer JB, Zhao Z, Glover GH, Gabrieli JD. Level of sustained entorhinal activity at study correlates with subsequent cued-recall performance: a functional magnetic resonance imaging study with high acquisition rate. Hippocampus. 1999;9:35–44. doi: 10.1002/(SICI)1098-1063(1999)9:1<35::AID-HIPO4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.