Abstract
Background
Recent studies suggest a role for vitamin D in innate immunity, including the prevention of respiratory tract infections (RTIs). We hypothesize that serum 25-hydroxyvitamin D (25[OH]D) levels are inversely associated with self-reported recent upper RTI (URTI).
Methods
We performed a secondary analysis of the Third National Health and Nutrition Examination Survey, a probability survey of the US population conducted between 1988 and 1994. We examined the association between 25(OH)D level and recent URTI in 18 883 participants 12 years and older. The analysis adjusted for demographics and clinical factors (season, body mass index, smoking history, asthma, and chronic obstructive pulmonary disease).
Results
The median serum 25(OH)D level was 29 ng/mL (to convert to nanomoles per liter, multiply by 2.496) (interquartile range, 21–37 ng/mL), and 19% (95% confidence interval [CI], 18%–20%) of participants reported a recent URTI. Recent URTI was reported by 24% of participants with 25(OH)D levels less than 10 ng/mL, by 20% with levels of 10 to less than 30 ng/mL, and by 17% with levels of 30 ng/mL or more (P<.001). Even after adjusting for demographic and clinical characteristics, lower 25(OH)D levels were independently associated with recent URTI (compared with 25[OH]D levels of ≥30 ng/mL: odds ratio [OR], 1.36; 95% CI, 1.01–1.84 for <10 ng/mL and 1.24; 1.07–1.43 for 10 to <30 ng/mL). The association between 25(OH)D level and URTI seemed to be stronger in individuals with asthma and chronic obstructive pulmonary disease (OR, 5.67 and 2.26, respectively).
Conclusions
Serum 25(OH)D levels are inversely associated with recent URTI. This association may be stronger in those with respiratory tract diseases. Randomized controlled trials are warranted to explore the effects of vitamin D supplementation on RTI.
Upper respiratory tract infection (URTI), or the “common cold,” is the most widespread infectious disease and the most common reason for US emergency department visits and unscheduled out-patient visits.1 More than 200 viruses contribute to the clinical syndrome of cough, nasal congestion, nasal discharge, sore throat, and sneezing.2,3 Most adults in the United States experience 2 to 4 URTIs per year, and most children experience 6 to 10 per year, which carries enormous population morbidity due to the high incidence of disease and disruption caused by symptoms.4,5
Because options for curing or preventing URTIs are limited, treatment relies on symptom relief.6,7 For example, over-the-counter preparations may have modest benefit on symptom duration and severity.8 For many decades, vitamin C (ascorbic acid) has been marketed and used for the prevention and treatment of URTIs despite the lack of convincing evidence of benefit in community populations.9 However, prevention of colds/influenza and “immune boosting” remain among the top reasons that Americans take vitamin and herbal supplements.10
Although ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3) are less commonly used vitamin supplements,10 the importance of vitamin D for general health has expanded far beyond rickets.11 Indeed, vitamin D is involved in the regulation of 1000 human genes12 and, most relevant to this study, seems to have promise in the prevention of infection, including URTI. Because few foods contain ergocalciferol or cholecalciferol, sunlight exposure is the primary determinant of vitamin D status in humans.11 However, in northern latitudes, between November and March, there are insufficient UV-B rays to produce vitamin D. Cannell et al13 asserted that wintertime vitamin D insufficiency may explain seasonal variation in influenza; this argument may also apply to other RTIs.
The potential mechanism for this observation is suggested by recent evidence that vitamin D plays an important role in innate immunity, particularly through the antimicrobial peptide cathelicidin.14 Indeed, early epidemiologic studies15–22 found a strong association between rickets and RTI. Although serum 25-hydroxyvitamin D (25[OH]D) levels of at least 10 ng/mL (to convert to nanomoles per liter, multiply by 2.496) prevent rickets, levels of at least 30 ng/mL are advantageous for general health, with approximately 40 ng/mL considered optimal.23,24 Recent prospective cohort25 and case-control26,27 studies have demonstrated a consistent association between low (including nonrachitic) serum 25 (OH)D levels and RTIs. These preliminary studies, although promising, were based on small, nondiverse cohorts of patients; the association between 25(OH)D level and RTI has not been explored on a population level.
In the present study, we examine the association between serum 25(OH)D level and recent URTI in a large cross-sectional sample that represents the entire US population. The primary hypothesis is that serum 25(OH)D level is inversely associated with recent URTI.
METHODS
STUDY DESIGN AND PARTICIPANTS
Between October 18, 1988, and October 15, 1994, the National Center for Health Statistics conducted the Third National Health and Nutrition Examination Survey (NHANES III). We performed a secondary analysis of this nationally representative, cross-sectional sample of the noninstitutionalized US civilian population. We received a waiver from the Partners Human Research Committee and the Colorado Multiple Institutional Review Board as an exempt study.
Full details of the survey methods, including sampling, interview, examination, laboratory measurements, ethics approval, and informed consent, are described elsewhere.28 Briefly, the survey used a complex, stratified, multistage probability sample design. The NHANES III oversampled certain subgroups of people, including low-income persons, adolescents aged 12 to 17 years, adults 60 years and older, blacks, and Mexican Americans.
Individuals selected for participation were notified by letter approximately 10 days before the home visit for the interview. The NHANES III collected household interview data, including demographic characteristics and data on health and nutrition, for 22 266 of 27 145 invited participants (82.0%) 12 years and older. Most of these participants (19 784 [88.9%]) subsequently underwent physical and laboratory examinations in a mobile examination center or at a home visit. We limited this analysis to the 18 883 participants with reported serum 25(OH)D values, representing 195 million Americans. Because physical and laboratory examinations occurred in mobile examination centers, inclement weather was an issue during data collection. To avoid these issues and improve response, the NHANES III preferentially scheduled data collection in the lower latitudes (further south) during the winter and in the higher latitudes (further north) in the summer.29
DATA COLLECTION
From the household interview data, we analyzed information on self-reported age, sex, race/ethnicity, and socioeconomic status, as measured by the poverty to income ratio (the ratio of a family's income to the poverty threshold of a family of the same size). We recorded smoking history (current smoking and pack-years), current asthma, and chronic obstructive pulmonary disease (COPD) diagnosis from self-reported history. We defined COPD based on responses to questions on emphysema or chronic bronchitis. From the examination data, we calculated body mass index as weight in kilograms divided by height in meters squared.
Blood samples collected during the examination were centrifuged, aliquoted, and frozen to −70°C on-site. They were then shipped on dry ice to central laboratories, where they were stored at −70°C until analysis. Serum 25(OH)D levels were measured using a radioimmunoassay kit after extraction with acetonitrile (DiaSorin, Stillwater, Minnesota) by the National Center for Environmental Health (Atlanta, Georgia). We used the date of the physical examination and laboratory data collection to most accurately adjust for the effect of season on serum 25 (OH)D levels.
The primary outcome (recent URTI) was based on the response to the following question: “In the past few days, have you had a cough, cold, or other acute illness?” This question, asked during the physical and laboratory examination visit before spirometric testing, was designed to identify participants with recent URTIs, which could affect spirometry results. Selection of this outcome variable allowed for temporal proximity between 25(OH)D level and recent URTI because serum samples were also obtained during the physical and laboratory examination visit.
STATISTICAL ANALYSIS
We performed statistical analyses using Stata 9.0 (StataCorp LP, College Station, Texas). Using survey commands, we applied the recommended subsample weights for the interview plus examination data to account for unequal probabilities of selection and to accurately represent estimates for the US population. All of the results are presented as weighted values. We calculated variance based on NHANES-provided masked variance units using the Taylor series linearization method. All of the P values are 2-tailed, with P<.05 considered statistically significant.
We calculated proportions with 95% confidence intervals (CIs) for demographic factors and those thought to be related to recent URTI (ie, serum 25[OH]D level, season, body mass index, region, current cigarette smoking, smoking history in pack-years, and asthma and COPD diagnoses), overall and in the subset with recent URTI. We categorized serum 25(OH)D levels as less than 10 ng/mL (<25 nmol), 10 to less than 30 ng/mL (25–74.9 nmol/L), and 30 ng/mL or more (≥75 nmol/L).11,23 To improve interpretability of the analysis, we converted age, poverty to income ratio, body mass index, and smoking pack-year history from continuous to categorical variables. In addition, we dichotomized race to white and nonwhite based on the known effect of melanin on vitamin D synthesis in darker-skinned individuals.11 We determined univariate associations between risk factors and the outcome of recent URTI using the Pearson χ2 test for categorical variables and simple ordinal logistic regression for ordinal variables.
To evaluate the independent association between serum 25 (OH)D level and recent URTI, we created multivariable models by progressively adding covariates that might confound or alter the 25(OH)D-URTI association. Factors known to be associated with serum 25(OH)D levels30 but not directly related to URTI, such as milk intake, vitamin D supplementation, and sunlight exposure, were not included in the models owing to collinearity. Because season may affect serum 25(OH)D levels and recent URTI, we evaluated this important variable separately and with other covariates. We reported odds ratios (ORs) with 95% CIs for variables in the model. In addition, we evaluated interaction terms for asthma and COPD, conditions that we hypothesized might have clinically important effect modification on the 25(OH)D-URTI association.
RESULTS
Characteristics of the weighted NHANES III sample, stratified by recent URTI status, are given in Table 1. The participants had a median age of 38 years; 52% were female and 75% were white. Overall, the median serum 25 (OH)D level was 29 ng/mL (interquartile range, 21–37 ng/mL). Serum 25(OH)D levels of less than 10 ng/mL were present in 2% (95% CI, 2%–2%) of the population and of 10 to less than 30 ng/mL in 53% (95% CI, 51%–55%). The proportion of participants who reported recent URTI was 19% (95% CI, 18%–20%).
Table 1.
Characteristics of the Study Population, Overall and in the Subset With Recent URTI
| Variable | Observations, No.a | US Population Estimate (95% CI), in Millionsa,b | Recent URTI (95% CI),% | P Valuec |
|---|---|---|---|---|
| Total | 18 883 | 195 | 19 (18–20) | |
| Serum 25(OH)D, ng/mL | ||||
| <10 | 684 | 4 (3–4) | 24 (20–29) | <.001 |
| 10 to <30 | 12 302 | 104 (99–108) | 20 (19–22) | |
| ≥30 | 5897 | 87 (78–97) | 17 (15–19) | |
| Season | ||||
| Winter, Dec–Feb | 4342 | 33 (22–45) | 26 (23–29) | <.001 |
| Spring, Mar–May | 5246 | 39 (27–51) | 18 (16–21) | |
| Summer, June–Aug | 4504 | 66 (44–87) | 13 (11–15) | |
| Fall, Sept–Nov | 4646 | 56 (37–74) | 21 (19–24) | |
| Age, y | ||||
| 12–19 | 2955 | 26 (24–29) | 22 (19–24) | .11 |
| 20–39 | 6496 | 78 (72–84) | 18 (17–20) | |
| 40–59 | 4262 | 53 (49–58) | 18 (16–20) | |
| ≥60 | 5163 | 37 (33–41) | 19 (17–21) | |
| Sex | ||||
| Male | 8840 | 94 (87–96) | 18 (16–19) | .04 |
| Female | 10 043 | 101 (99–102) | 20 (18–22) | |
| Race | ||||
| White | 7428 | 146 (141–151) | 19 (17–20) | .26 |
| Nonwhite | 11 455 | 48 (44–52) | 20 (18–22) | |
| Poverty to income ratio | ||||
| ≤1 | 4387 | 25 (22–28) | 22 (18–25) | .046 |
| >1 | 12 744 | 157 (154–160) | 18 (17–20) | |
| BMI | ||||
| <20 | 1900 | 21 (19–23) | 21 (18–24) | .55 |
| 20–24.9 | 6542 | 75 (68–81) | 19 (17–21) | |
| 25–29.9 | 6028 | 59 (54–63) | 17 (15–20) | |
| ≥30 | 4336 | 40 (36–44) | 20 (18–22) | |
| Region | ||||
| Northeast | 2510 | 40 (35–44) | 17 (15–18) | <.001 |
| Midwest | 3672 | 47 (42–51) | 16 (14–18) | |
| South | 8245 | 67 (59–75) | 24 (22–26) | |
| West | 4456 | 41 (26–56) | 17 (14–20) | |
| Current cigarette smoking | ||||
| Yes | 4249 | 50 (46–54) | 27 (25–30) | <.001 |
| No | 14 634 | 145 (142–148) | 16 (15–17) | |
| Cigarette pack-years | ||||
| 0 | 10 918 | 103 (100–106) | 17 (16–18) | <.001 |
| 1–9 | 3893 | 42 (39–46) | 21 (19–24) | |
| 10–19 | 1372 | 17 (16–19) | 21 (18–25) | |
| ≥20 | 2700 | 32 (29–35) | 21 (19–23) | |
| Current diagnosis of asthma | ||||
| Yes | 939 | 11 (9–12) | 26 (22–29) | <.001 |
| No | 17 944 | 184 (183–185) | 18 (17–20) | |
| Current diagnosis of COPD | ||||
| Yes | 1159 | 13 (12–15) | 33 (29–38) | <.001 |
| No | 17 724 | 181 (180–183) | 18 (17–19) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CI, confidence interval; COPD, chronic obstructive pulmonary disease; 25(OH)D, 25-hydroxyvitamin D; URTI, upper respiratory tract infection.
SI conversion factor: To convert 25(OH)D to nanomoles per liter, multiply by 2.496.
Some variables do not sum to the total because of missing data.
Estimates are based on weighted data from the Third National Health and Nutrition Examination Survey.
The P values are based on the Pearson χ2 test for categorical variables and on simple ordinal logistic regression for ordinal variables, with 2-tailed P < .05 considered statistically significant.
The proportions of participants with recent URTI, stratified by characteristics, are also given in Table 1. The median serum 25(OH)D level was slightly lower in participants reporting URTI (28 ng/mL) compared with those without URTI (29 ng/mL) (P<.001). Other characteristics associated with recent URTI included winter and fall seasons, age 12 to 19 years compared with age 20 years and older, female sex, low socioeconomic status (poverty to income ratio, ≤1), South region, current cigarette smoking, more than 0 cigarette pack-years, asthma, and COPD (P<.05 for all).
Compared with individuals with serum 25(OH)D levels of 30 ng/mL or more, those with levels less than 10 ng/mL or 10 to less than 30 ng/mL had 55% and 27% higher odds of recent URTI, respectively (Table 2, model 1). In further stratification of the group with 25(OH)D levels of 30 ng/mL or more, those with levels of 30 to less than 40 ng/mL had similar odds of recent URTI compared with those with levels of 40 ng/mL or more (OR, 0.99; 95% CI, 0.81–1.21). After adjusting for season, lower serum 25 (OH)D levels were still associated with a significantly higher URTI (Table 2, model 2). Indeed, the 25(OH)D-URTI association was consistent across all seasons (Figure). Changing the months included for each season did not materially alter the results (data not shown). When all the covariates were added to the model, serum 25(OH)D level retained its significant inverse relationship with URTI (Table 2, model 3). Winter season, younger age, current cigarette smoking, asthma, and COPD also were associated with significantly higher odds of URTI.
Table 2.
Associations Between Serum 25(OH)D Level and Recent URTI in Unadjusted and Multivariable Models
| OR (95% CI)a |
|||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Serum 25(OH)D, ng/mL | |||
| <10 | 1.55 (1.18–2.05)b | 1.37 (1.06–1.76)b | 1.36 (1.01–1.84)b |
| 10 to <30 | 1.27 (1.11–1.47)b | 1.20 (1.05–1.38)b | 1.24 (1.07–1.43)b |
| ≥30 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Season | |||
| Winter, Dec–Feb | 1.54 (1.25–1.89)b | 1.48 (1.12–1.96)b | |
| Spring, Mar–May | 1 [Reference] | 1 [Reference] | |
| Summer, June–Aug | 0.69 (0.56–0.85)b | 0.66 (0.52–0.84)b | |
| Fall, Sep–Nov | 1.22 (1.00–1.50)b | 1.25 (0.99–1.57) | |
| Age, per ↑ 10 y | 0.95 (0.91–0.98)b | ||
| Female sex | 1.13 (0.97–1.32) | ||
| White race | 1.11 (0.94–1.32) | ||
| Poverty to income ratio | 0.98 (0.94–1.01) | ||
| BMI | 1.00 (0.99–1.01) | ||
| Region | |||
| Northeast | 1 [Reference] | ||
| Midwest | 1.26 (0.95–1.68) | ||
| South | 1.17 (0.92–1.49) | ||
| West | 1.21 (0.90–1.61) | ||
| Current cigarette smoking | 1.81 (1.55–2.12)b | ||
| Smoking history, per ↑ 10 pack-years | 1.01 (0.98–1.04) | ||
| Asthma | 1.24 (1.00–1.53)b | ||
| COPD | 1.87 (1.47–2.38)b | ||
Abbreviations: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; 25(OH)D, 25-hydroxyvitamin D; OR, odds ratio; URTI, upper respiratory tract infection.
SI conversion factor: To convert 25(OH)D to nanomoles per liter, multiply by 2.496.
Based on multivariable logistic regression models for the outcome of recent URTI. Model 1 includes serum 25(OH)D alone; model 2, serum 25(OH)D plus season; and model 3, serum 25(OH)D plus all covariates.
P< .05 for the association between the predictor variable and the outcome of recent URTI adjusted for all other variables in the model.
Figure.
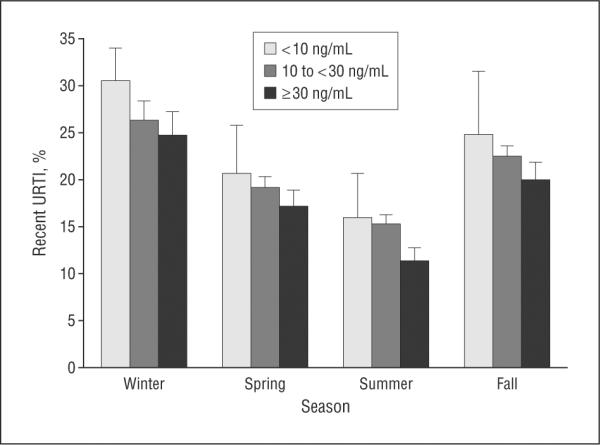
Participants with recent upper respiratory tract infection (URTI) stratified by serum 25-hydroxyvitamin D level (to convert to nanomoles per liter, multiply by 2.496) and season. Error bars represent standard errors of estimates.
Compared with participants without asthma, a similar proportion of participants with asthma had serum 25 (OH)D levels less than 10 ng/mL (both groups, 2%; P=.87). Participants with vs without COPD were more likely to have serum 25(OH)D levels less than 10 ng/mL (3% vs 2%, P=.04), but this association was not significant after adjusting for demographics, season, and smoking status (P=.12). Among asthmatic participants, those with 25 (OH)D levels less than 10 ng/mL had a higher reported rate of recent URTI than did those with levels of 30 ng/mL or more (59% vs 22%, P<.001). A similar association observed in COPD participants was also observed by 25 (OH)D strata (45% vs 31%, respectively), although the association was not statistically significant (P=.20).
While controlling for all other covariates, serum 25 (OH)D levels less than 10 ng/mL were associated with 5.67 higher odds of recent URTI in participants with asthma compared with levels of 30 ng/mL or more and 2.26 higher odds in participants with COPD. In comparison, serum 25(OH)D levels less than 10 ng/mL were associated with only 1.24 higher odds of URTI in participants without asthma and 1.27 higher odds in those without COPD. When individually adding interaction terms to the final multivariable model, the interaction term for COPD was not significant (P for interaction=.30) but was significant for asthma (P for interaction=.007).
COMMENT
To our knowledge, this is the first population-based study to evaluate and demonstrate an association between serum 25(OH)D level and URTI. The association seems to be robust, with a clinically and statistically significant association present in all seasons and when controlling for potential confounders. The dose-response association between lower levels of vitamin D and recent URTI provides further credibility to the findings.
Although early clinical studies14–22 supported an association between rickets found with serum 25(OH)D levels less than 10 ng/mL and RTI, more recent studies25–27 have found an effect of low (including nonrachitic) serum 25(OH)D levels. For example, Laaksi et al25 found that young male Finnish soldiers with serum 25(OH)D levels of less than 16 ng/mL at baseline had a 63% increased risk of absence from duty due to RTI than did soldiers with serum 25(OH)D levels 16 ng/mL or more during the following 6 months. In addition, case-control studies from India26 and Turkey27 reported an association between serum 25(OH)D levels of less than 20 ng/mL and acute lower RTI in children and neonates, respectively.
In this study, we also found that individuals with respiratory tract diseases (ie, asthma and COPD) are of particular interest. Effect modification by asthma, and possibly COPD, on the association between serum 25(OH)D level and recent URTI indicates that the role of vitamin D may be of greater importance for individuals with these common conditions. Potential explanations may be that those with respiratory tract diseases are more susceptible to RTI31,32 and have increased frequency of lower respiratory tract symptoms of higher severity and longer duration.33,34 These findings are particularly important given the high morbidity from asthma and COPD exacerbation and merit further investigation.
Preliminary studies on vitamin D supplementation and RTI also have been promising. Two interventional cohort studies with 600 to 700 IU daily of vitamin D from cod liver oil and multivitamin supplementation35 and 60 000 IU weekly from a vitamin D or calcium supplement36 found a decrease in RTIs in children receiving supplementation. One randomized controlled trial37 of bone loss in postmenopausal black women found that 7.7% of women randomized to receive 800 to 2000 IU of vitamin D daily reported respiratory tract symptoms during 3-year follow-up compared with 25.0% in the control group. Another substudy38 of a randomized controlled trial for fracture prevention found a nonsignificant 10% reduced odds of wintertime infection in participants randomized to receive 800 IU of vitamin D daily (P=.23). However, these studies were post hoc analyses of adverse events reported in studies of skeletal health, and dedicated trials are warranted.
The emerging role of vitamin D in innate immunity provides a plausible mechanism for the inverse association between serum 25(OH)D level and URTI.14 The only known human cathelicidin, hCAP-18 (LL-37), enhances microbial killing in phagocytic vacuoles, acts as a chemoattractant for neutrophils and monocytes, and has a defined vitamin D-dependent mechanism.39,40 Pathogenic antigens interact with toll-like receptors on macrophages to upregulate the expression of genes that code for the vitamin D receptor and for the 1α-hydroxylase enzyme that converts 25(OH)D to the biologically active 1,25-dihydroxyvitamin D.41,42 In turn, 1,25-dihydroxyvitamin D interacts with the promoter of the gene for cathelicidin,43 which enhances the production of hCAP-18.44,45
The presence of sufficient levels of 25(OH)D, the major circulating form of vitamin D, is necessary to activate hCAP-18 expression and to enhance macrophage function and innate immunity.45 In studies of Mycobacterium tuberculosis, Liu et al42 found a direct association of serum 25(OH)D levels with cathelicidin expression and killing of intracellular M tuberculosis. Furthermore, black individuals, known to have increased susceptibility to tuberculosis infection, had low serum 25(OH)D levels and inefficient cathelicidin messenger RNA induction, but supplementation of 25(OH)D to the reference range restored induction of cathelicidin messenger RNA.42
The results from our article provide additional evidence from a large, diverse population of the inverse association between serum 25(OH)D level and RTI. Although serum 25(OH)D levels of less than 10 ng/mL were associated with the highest rate of recent URTI, serum 25(OH)D levels of 10 to less than 30 ng/mL also were associated with higher adjusted odds of URTI compared with levels of 30 ng/mL or more. Although individuals with serum 25(OH)D levels of less than 10 ng/mL have the least efficient cathelicidin expression, serum 25 (OH)D levels of 30 ng/mL or more may be necessary for the optimal induction of cathelicidin messenger RNA.42 Although comparison of groups with 25(OH)D levels of 30 to less than 40 ng/mL and 40 ng/mL or more suggested a possible plateau of effect after approximately 30 ng/mL, this analysis was underpowered to evaluate whether higher 25(OH)D levels (ie, 40 ng/mL) provide additional benefit.
Current recommendations for vitamin D supplementation (200–600 IU/d) are unlikely to achieve optimal serum 25(OH)D levels (ie, approximately 30–40 ng/mL) in most of the US population.46 Randomized controlled trials of higher-dose vitamin D supplementation (≥1000 IU/d), particularly in the winter season, at higher latitudes, and for individuals with respiratory tract diseases, will help clarify the role of vitamin D supplementation in the reduction of RTI.
This study has some potential limitations. We could not control for all potential confounders, but we selected covariates most likely to be associated with serum 25(OH)D level and recent URTI. Unmeasured confounders may have an effect on the association between 25(OH)D level and URTI. Thus, causal inference is not possible, and we cannot exclude that the results are merely an epiphenomenon; randomized controlled trials are valuable for determining the causal nature of an observed association. Reverse causation (ie, lower serum 25[OH]D levels due to recent URTI and, thus, decreased outdoor activity) seems unlikely because the half-life of serum 25(OH)D is 2 to 3 weeks11 and URTIs last only 3 to 4 days,3 but it may affect the enhanced vitamin D-URTI association in participants with asthma and COPD owing to longer symptom duration in those individuals.
The primary outcome (recent URTI) is a relatively crude and self-reported measure that may include other non-RTIs; however, limitations on the accuracy of this outcome would be expected to reduce the magnitude of associations. Serum samples were collected at only 1 point in time and were preferentially collected in northern states in the summer and in southern states in the winter. As a result, serum 25(OH)D levels are likely higher than would be expected by random sampling across all seasons. Although the overall serum 25(OH)D level and the recent URTI rate may be affected, it is unlikely that their association would be materially different. Nevertheless, we encourage efforts to replicate these findings in independent samples.
In conclusion, serum 25(OH)D levels have an independent, inverse association with recent URTI. Although 25(OH)D levels less than 30 ng/mL and URTI were higher in the winter season, the inverse association was present throughout the year. Individuals with respiratory tract diseases, such as asthma, who have low serum 25(OH)D levels may be even more susceptible to RTI. Vitamin D supplementation may reduce the incidence of URTI and exacerbations of respiratory tract diseases. Randomized controlled trials are warranted to explore the direct effect of vitamin D supplementation and to establish optimal levels of serum 25(OH)D in the prevention of RTI.
Acknowledgments
Funding/Support: Dr Camargo was supported by the Massachusetts General Hospital Center for D-receptor Activation Research and grant R01 HL84401 from the National Institutes of Health, National Heart, Lung, and Blood Institute.
Role of the Sponsor The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Ginde had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Ginde and Camargo. Acquisition of data: Ginde. Analysis and interpretation of data: Ginde, Mansbach, and Camargo. Drafting of the manuscript: Ginde. Critical revision of the manuscript for important intellectual content: Ginde, Mansbach, and Camargo. Statistical analysis: Ginde and Camargo. Obtained funding: Camargo. Study supervision: Camargo.
Financial Disclosure: None reported.
REFERENCES
- 1.Burt CW, McCaig LF, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2005. Adv Data. 2007;388:1–15. [PubMed] [Google Scholar]
- 2.Monto AS. Epidemiology of viral respiratory infections. Am J Med. 2002;112(suppl 6A):4S–12S. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 3.Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51–59. doi: 10.1016/S0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163(4):487–494. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 5.Bramley TJ, Lerner D, Sarnes M. Productivity losses related to the common cold. J Occup Environ Med. 2002;44(9):822–829. doi: 10.1097/00043764-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Pratter MR. Cough and the common cold: ACCP evidence-based clinical practice guidelines. Chest. 2006;129((1)(suppl)):72S–74S. doi: 10.1378/chest.129.1_suppl.72S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simasek M, Blandino DA. Treatment of the common cold. Am Fam Physician. 2007;75(4):515–520. [PubMed] [Google Scholar]
- 8.Smith SM, Schroeder K, Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev. 2008;(1):CD001831. doi: 10.1002/14651858.CD001831.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Douglas RM, Hemilä H, Chalker E, Treacy B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2007;(3):CD000980. doi: 10.1002/14651858.CD000980.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287(3):337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 12.Tavera-Mendoza LE, White JH. Cell defenses and the sunshine vitamin. Sci Am. 2007;297(5):62–65. 68–70, 72. doi: 10.1038/scientificamerican1107-62. [DOI] [PubMed] [Google Scholar]
- 13.Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134(6):1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4(2):80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariam TW, Sterky G. Severe rickets in infancy and childhood in Ethiopia. J Pediatr. 1973;82(5):876–878. doi: 10.1016/s0022-3476(73)80087-3. [DOI] [PubMed] [Google Scholar]
- 16.Patwari A, Nabi G, Nadroo AM, Singh D, Manhas RS. Pulmonary changes in rickets in children. Indian Pediatr. 1979;16(5):413–415. [PubMed] [Google Scholar]
- 17.El-Radhi AS, Majeed M, Mansor N, Ibrahim M. High incidence of rickets in children with wheezy bronchitis in a developing country. J R Soc Med. 1982;75(11):884–887. doi: 10.1177/014107688207501112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beser E, Cakmakci T. Factors affecting the morbidity of vitamin D deficiency rickets and primary protection. East Afr Med J. 1994;71(6):358–362. [PubMed] [Google Scholar]
- 19.Muhe L, Lulseged S, Mason KE, Simoes EA. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349(9068):1801–1804. doi: 10.1016/S0140-6736(96)12098-5. [DOI] [PubMed] [Google Scholar]
- 20.Banajeh SM, al-Sunbali NN, al-Sanahani SH. Clinical characteristics and outcome of children aged under 5 years hospitalized with severe pneumonia in Yemen. Ann Trop Paediatr. 1997;17(4):321–326. doi: 10.1080/02724936.1997.11747905. [DOI] [PubMed] [Google Scholar]
- 21.Najada AS, Habashneh MS, Khader M. The frequency of nutritional rickets among hospitalized infants and its relation to respiratory diseases. J Trop Pediatr. 2004;50(6):364–368. doi: 10.1093/tropej/50.6.364. [DOI] [PubMed] [Google Scholar]
- 22.Siddiqui TS, Rai MI. Presentation and predisposing factors of nutritional rickets in children of Hazara Division. J Ayub Med Coll Abbottabad. 2005;17(3):29–32. [PubMed] [Google Scholar]
- 23.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes [published correction appears in Am J Clin Nutr. 2006;84(5):1253] Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 24.Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008;87(6):1738–1742. doi: 10.1093/ajcn/87.6.1738. [DOI] [PubMed] [Google Scholar]
- 25.Laaksi I, Ruohola JP, Tuohimaa P, et al. An association of serum vitamin D concentrations <40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86(3):714–717. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 26.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58(4):563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 27.Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers [published online November 21, 2007] Eur J Clin Nutr. doi: 10.1038/sj.ejcn.1602960. doi:10.1038 /sj.ejcn.1602960. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention . Third National Health and Nutrition Examination Survey, 1988–1994, Reference Manuals and Reports [CD-ROM] National Center for Health Statistics; Hyattsville, MD: 1996. [Google Scholar]
- 29.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 30.Scragg R, Camargo CA., Jr Frequency of leisure time physical activity and serum 25-hydroxyvitamin D levels in the U.S. population: results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2008;168(6):577–586. doi: 10.1093/aje/kwn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manolitsas ND, Trigg CJ, McAulay AE, et al. The expression of intercellular adhesion molecule-1 and the β 1-integrins in asthma. Eur Respir J. 1994;7(8):1439–1444. doi: 10.1183/09031936.94.07081439. [DOI] [PubMed] [Google Scholar]
- 32.Wark PA, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201(6):937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corne JM, Marshall C, Smith S, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359(9309):831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 34.van Elden LJ, Sachs AP, van Loon AM, et al. Enhanced severity of virus associated lower respiratory tract disease in asthma patients may not be associated with delayed viral clearance and increased viral load in the upper respiratory tract. J Clin Virol. 2008;41(2):116–121. doi: 10.1016/j.jcv.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linday LA, Shindledecker RD, Tapia-Mendoza J, Dolitsky JN. Effect of daily cod liver oil and a multivitamin-mineral supplement with selenium on upper respiratory tract pediatric visits by young, inner-city, Latino children: randomized pediatric sites. Ann Otol Rhinol Laryngol. 2004;113(11):891–901. doi: 10.1177/000348940411301108. [DOI] [PubMed] [Google Scholar]
- 36.Rehman PK. Sub-clinical rickets and recurrent infection [research letter] J Trop Pediatr. 1994;40(1):58. doi: 10.1093/tropej/40.1.58. [DOI] [PubMed] [Google Scholar]
- 37.Aloia JF, Li-Ng M. Re: epidemic influenza and vitamin D [letter] Epidemiol Infect. 2007;135(7):1095–1096. doi: 10.1017/S0950268807008308. author reply: 1097-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avenell A, Cook JA, Maclennan GS, Macpherson GC. Vitamin D supplementation to prevent infections: a sub-study of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438) Age Ageing. 2007;36(5):574–577. doi: 10.1093/ageing/afm091. [DOI] [PubMed] [Google Scholar]
- 39.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19(9):1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 40.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179(4):2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 41.Reichel H, Koeffler HP, Bishop JE, Norman AW. 25-Hydroxyvitamin D3 metabolism by lipopolysaccharide-stimulated normal human macrophages. J Clin Endocrinol Metab. 1987;64(1):1–9. doi: 10.1210/jcem-64-1-1. [DOI] [PubMed] [Google Scholar]
- 42.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 43.Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 44.Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D3. J Cyst Fibros. 2007;6(6):403–410. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Törmä H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124(5):1080–1082. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- 46.Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academy Press; Washington, DC: 1999. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board, Institute of Medicine. Vitamin D; pp. 250–287. [Google Scholar]


