Abstract
Gonadotropin-releasing hormone (GnRH) plays a major role in the hypothalamic-pituitary-gonadal (HPG) axis, and synthesis and secretion of GnRH are regulated by gonadal steroid hormones. Disruptions in androgen levels are involved in a number of reproductive defects, including hypogonadotropic hypogonadism and polycystic ovarian syndrome. Androgens down-regulate GnRH mRNA synthesis in vivo and in vitro via an androgen receptor (AR)-dependent mechanism. Methyltrienolone (R1881), a synthetic AR agonist, represses GnRH expression through multiple sites in the proximal promoter. In this study, we show AR also represses GnRH transcription via the major enhancer (GnRH-E1). A multimer of the −1800/−1766 region was repressed by R1881 treatment. Mutation of two bases, −1792 and −1791, resulted in decreased basal activity and a loss of AR-mediated repression. AR bound to the −1796/−1791 sequence in electrophoretic mobility shift assays, indicating a direct interaction with DNA or other transcription factors in this region. We conclude that AR repression of GnRH-E1 acts via multiple AR-responsive regions, including the site at −1792/−1791.
Keywords: GnRH, androgen receptor, repression, hypothalamus, enhancer
1. Introduction
Serum gonadotropin levels are regulated by neuroendocrine feedback of gonadal steroid hormones at the level of the hypothalamus and pituitary. One potential cause of improper gonadotropin-releasing hormone (GnRH) action is the disruption of androgen feedback to the hypothalamus. A number of reproductive defects involve alterations in androgen levels. For example, androgen levels are lower in males with hypogonadotropic hypogonadism, and polycystic ovarian syndrome is characterized by an increase in androgen levels in women.
Androgen exerts its effects via the androgen receptor (AR), a member of the nuclear receptor superfamily. AR mediates a number of biological effects of androgens, including spermatogenesis, sexual differentiation and maturation, and gonadotropin regulation. GnRH expression is decreased by androgens in vivo (Roselli et al., 1990; Toranzo et al., 1989) and in vitro (Belsham et al., 1998; Shakil et al., 2002); the precise mechanisms of this repression remain unclear. The low abundance of nuclear receptors make co-localization with the small, dispersed, heterogeous GnRH neuron population difficult, although GnRH neurons have been shown to express estrogen receptor (ER)-β in vivo (Herbison and Pape, 2001; Hrabovszky et al., 2000; Hrabovszky et al., 2001).
The GnRH-expressing neuronal cell line, GT1, is an excellent model system for the study of GnRH synthesis in response to steroid hormone treatments. The GT1 cell line was created using a GnRH-SV40 T-antigen transgene in mice (Mellon et al., 1990). GT1 cells express GnRH mRNA and display pulsatile secretion of GnRH peptide with the 30 min interpulse interval found in vivo in the mouse (Chappell et al., 2003; Martinez de la Escalera et al., 1992; Wetsel et al., 1992). They also extend neurites ending in growth cones or contacts with other cells (Mellon et al., 1990) and express neuronal markers, including pre-synaptic vesicle proteins (Mellon et al., 1992). GT1 cells have been shown to expresses androgen receptor (AR), ER-α, ER-β, and progesterone receptor A (PRA) (Navarro et al., 2003; Poletti et al., 2001; Roy et al., 1999). 5α-dihydrotestosterone (DHT) was shown to repress GnRH mRNA levels in GT1-7 cells (a clone of the GT1 cell line) (Belsham et al., 1998). Previously, we showed that liganded AR represses GnRH transcription through multiple sites in the GnRH proximal promoter (GnRH-P), including a sequence containing a cluster of Octamer-binding transcription factor-1 (Oct1), Pre-B cell leukemia transcription factor (Pbx)/Prep, and NK2 homeobox 1 (Nkx2.1) binding sites (Brayman et al., 2012).
The aim of this study was to determine whether other conserved regions of the GnRH regulatory region are involved in GnRH transcriptional repression by androgen. In addition to GnRH-P, the 5000 bp regulatory region contains three highly-conserved enhancers, GnRH-E1, GnRH-E2 and GnRH-E3 (Iyer et al., 2010). We found that R1881, an AR agonist, repressed GnRH expression through GnRH-P and GnRH-E1, but not through GnRH-E2. Moreover, repression was stronger when both GnRH-P and GnRH-E1 were present. Chromatin immunoprecipitation (ChIP) assays showed increased interaction between AR and GnRH-E1 after treatment with R1881. A reporter containing four copies of the −1800/−1766 GnRH-E1 sequence was repressed by R1881, and mutation of a site previously shown to be involved in O-tetradecanoylphorbol-13-acetate (TPA)-mediated repression of full-length GnRH-E1 also resulted in a loss of androgen repression. Electrophoretic mobility shift assay (EMSA) experiments showed AR to be part of protein complexes binding to the −1796/−1791 sequence, which overlaps both the TPA-responsive site and a putative hormone response element (HRE). Collectively, our findings show that interaction of AR with GnRH-E1 causes repression of GnRH gene expression, and further elucidates the mechanism of AR-mediated repression of GnRH1 gene transcription.
2. Materials and Methods
2.1. Plasmids and cloning
The expression plasmids used were: rat AR, pSG5-rAR (Ikonen et al., 1998); rat AR with a point mutation in the DNA binding domain (DBD), AR-C562G (Ikonen et al., 1994); human AR and human AR with a point mutation regarding in truncation of the ligand binding domain, AR1-640 (Ceraline et al., 2004). The reporter plasmids used have been described previously: those containing GnRH-P, GnRH-E1, GnRH-E2, Rous sarcoma virus enhancer (eRSVe) and/or promoter (RSVp) (Givens et al., 2004; Nelson et al., 2000); 4×1800/1766 (Rave-Harel et al., 2005); 5’ and 3’ truncations of GnRH-E1 and mut1792/1791 (Tang et al., 2005); mutOct1a, mutOct1b, and mutOct1ab (Belsham and Mellon, 2000; Tang et al., 2005). The mutOct1a (taatgtaaaa changed to tactgtacaa) and mut1790/1788 (cagcaggagg changed to cagactgagg) mutations in 4×1800/1766 were created by cloning synthesized oligonucleotides into RSVp/pGL3-luc. The 4×1616/1589 reporter was created by inserting a synthesized oligonucleotide containing four copies of the sequence from −1616 to −1589 into TK/pGL3-luc. The −1796/−1794 mutation in GnRH-E1 was created using the QuikChange® Site Directed Mutagenesis kit (Stratagene, La Jolla, CA) using the following primer set: sense, 5' - TGA GCA GCA GTT AGC CCC ACA TCA CTC CTG CTG AGA TTT TAC ATT - 3'; antisense, 5' - AAT GTA AAA TCT CAG CAG GAG TGA TGT GGG GCT AAC TGC TGC TCA - 3' (mutated bases underlined). The 4×1800/1766mut1796/1794 plasmid was created by inserting an oligonucleotide containing the −1796/−1794 mutation in four repeats of the −1800/−1766 sequence into TK/pGL3-luc. The −1790/−1788 mutation in GnRH-E1 was created using the following primers: sense, 5' – GCA GTT AGC CCC ACA GTC CTC AGT CTG AGA TTT TAC ATT AGG GCA - 3'; antisense, 5' - TGC CCT AAT GTA AAA TCT CAG ACT GAG GAC TGT GGG GCT AAC TGC - 3' (mutated bases underlined). Primers and oligonucleotides were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA).
2.2. Cell culture and transfections
GT1-7 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Cellgro, Mediatech, Inc., Herndon, VA), supplemented with 10% (v/v) fetal bovine serum (FBS; Gemini Bio-Products, Sacramento, CA), penicillin (100 units/ml) and streptomycin (0.1 mg/ml) (Invitrogen, Carlsbad, CA) at 37 C with 5% CO2. One day before transfection, cells were plated in 24-well plates. Cells were transfected with FuGene 6 reagent (Roche Applied Science, Indianapolis, IN) using 400 ng of AR expression plasmid (except in TPA experiments), 400 ng of the indicated reporter plasmid, and 100 ng of the internal control (Herpes simplex virus thymidine kinase 109 bp promoter upstream of the β-galactosidase gene, TK-βgal). For R1881 experiments, media was changed to phenol red free DMEM, supplemented with 2% (v/v) charcoal/dextran-treated (cs-) FBS (Gemini) and 2 mM Glutamax (Invitrogen) approximately 5 h after transfection. Twenty-four h after transfection, cells were treated with 100 nM R1881 (Sigma, St. Louis, MO) or ethanol vehicle (0.1% [v/v]) in phenol red-free DMEM, supplemented with 2% (v/v) cs-FBS and 2 mM Glutamax, or with 100nM O-tetradecanoylphorbol-13-acetate (TPA) or ethanol vehicle (0.1% [v/v]) in phenol red-free DMEM, as indicated. Cells were lysed 24 h after treatment and assayed for luciferase and β-galactosidase expression as previously described (Lawson et al., 1996). In all transfection experiments, luciferase values were normalized to the values from a co-transfected TK-βgal reporter to control for transfection efficiency, and the data represent the mean, ± SEM, of at least three experiments done in quadruplicate.
2.3. Electrophoretic mobility shift assay
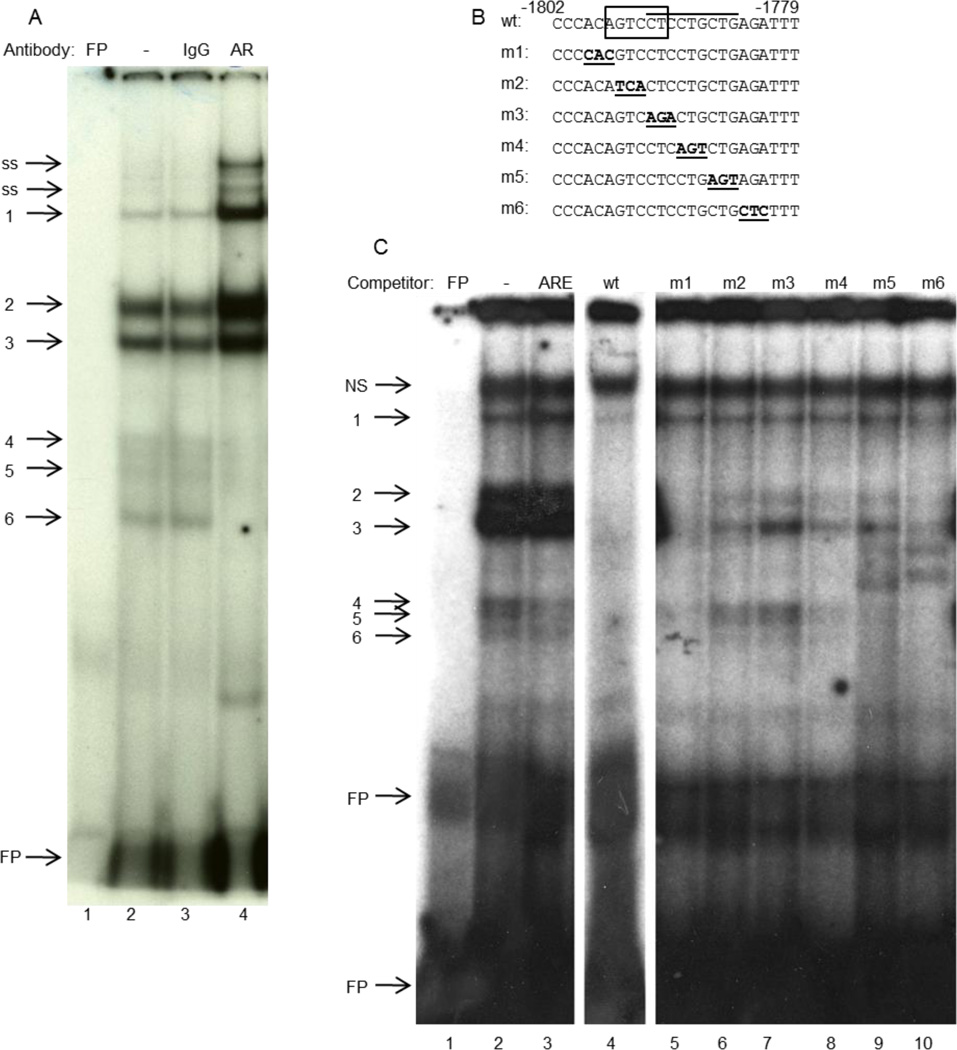
GT1-7 cells were transfected with FuGENE 6 reagent in 10 cm dishes with 8 µg expression vector in phenol red-free DMEM containing 10% (v/v) cs-FBS and 2 mM Glutamax. Forty-eight h after transfection, cells were treated for 2 h with 100 nM R1881 in phenol red-free DMEM containing 2% (v/v) cs-FBS and 2 mM Glutamax. Nuclear extracts were prepared as described previously (Schreiber et al., 1989), and protein concentration was determined by Bradford assay (Bradford, 1976). Baculovirus AR was made as described (Spady et al., 2004). Oligonucleotide probe was labeled and purified as previously described (Tang et al., 2005). Reactions were performed as described (Brayman et al., 2012). The sense strand sequences of the double-stranded oligonucleotide probes and competitors were: −1802/−1779, 5’-CCCACAGTCCTCCTGCTGAGATTT-3’; ARE, 5’-GTCTGGTACAGGGTGTTCTTTTTG-3’. Sequences of mutated competitors are indicated in Fig. 5B.
Fig. 5.
AR-containing complexes bind to the −1796/−1791 sequence of GnRH-E1. (A), Nuclear extracts from GT1-7 cells that had been transfected with AR and treated with R1881 were incubated with a radiolabeled probe containing the −1802/−1779 region of GnRH-E1, with or without antibody. Lane 1: free probe (FP); Lane 2: probe with nuclear extract; Lane 3, rabbit IgG; Lane 4, anti-AR antibody. Bands 4–6 represent AR-containing complexes; ss: super-shifted AR. (B), Sequences of the wild type (wt) and mutant oligonucleotide competitors. Box: putative HRE; overline: TPA-responsive site; bold/underline: mutations. (C), Nuclear extracts from GT1-7 cells that had been transfected with AR were incubated with a radiolabeled probe containing the −1802/−1779 region of GnRH-E1, with or without indicated unlabeled competitor. Lane 1: free probe (FP); Lane 2: probe with nuclear extract; Lane 3: unlabeled androgen response element (ARE) competitor; Lane 4: wild type (wt) unlabeled competitor; Lanes 5–10: indicated mutant unlabeled competitor. Bands 4–6 represent AR-containing complexes; NS: non-specific band.
2.4. Chromatin immunoprecipitation
GT1-7 cells were transfected and treated as described for EMSA (above). Two hours after addition of R1881, the nuclear fraction was obtained as described (Iyer et al., 2010). Chromatin was sonicated to an average length of 200 bp and immunoprecipitation was performed using the ChIP Assay Kit (Millipore, Temecula, CA) and an anti-AR antibody (PG-21; Upstate, Lake Placid, NY), as per the manufacturer’s instructions. Polymerase chain reaction (PCR) was performed using primers as described (Iyer et al., 2010) and as indicated in figure legends. The primers used were: mouse GnRH-E1 (amplifies the region between −1814 and −1653) sense, 5’-GCCAAACACCACAGTCTTCTCTTGAGTGAC-3’ and antisense, 5’- CTGGCACAAAGAGCAAAAGAACCTCCTCTC-3’; GnRH Intron 3 (amplifies the region between +3623 and +3820) sense, 5’- CGGTGACTTCAATTTCCACACCCAATGGAC-3’ and antisense, 5’- CATTAGCCGCGTAAAGGATGACGCTGTGAG-3’. Data are representative of three independent experiments. Real time PCR was performed on the chromatin using the GnRH-E1 primers, as described (Brayman et al., 2012).
2.5. Statistical analysis
Statistical analysis was performed using JMP version 9 (SAS Institute, Cary, NC). All relative luciferase units were optimally transformed by the method of Box and Cox. Data were analyzed by Student’s t test. A P value of less than 0.05 was the requirement for declaring significance.
3. Results
3.1. AR represses the GnRH regulatory region through GnRH-E1 and GnRH-P
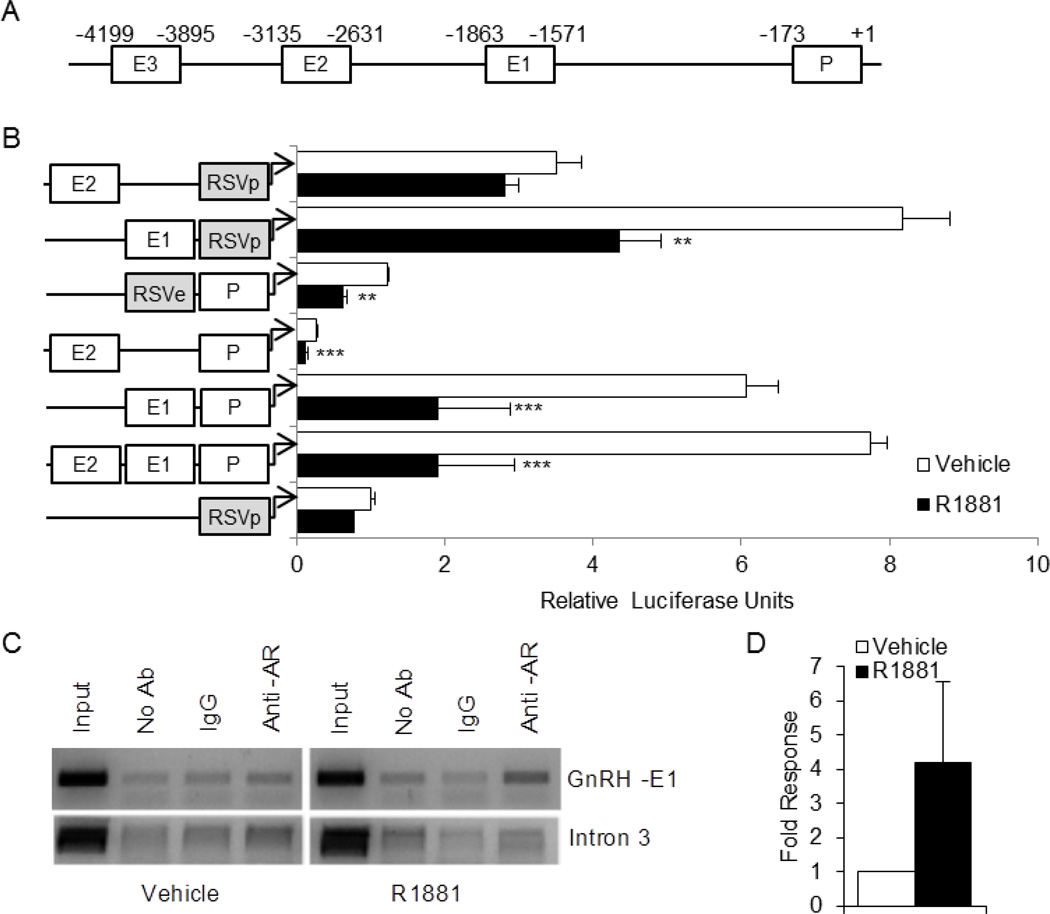
We previously identified four evolutionarily conserved regions of the 5000 bp GnRH regulatory region (Fig. 1A): enhancer 3 (GnRH-E3, −4199/−3895 bp upstream of the transcriptional start site), enhancer 2 (GnRH-E2, −3135/−2631), enhancer 1 (GnRH-E1, −1863/−1571), and the proximal promoter (GnRH-P, −173/+1) (Iyer et al., 2010). AR repression of GnRH transcription was retained in serial truncations of the 5000 kb regulatory region through −173 bp, indicating that the GnRH-P is involved (Brayman et al., 2012). However, we did not explore whether the upstream enhancers are also involved in AR-mediated repression.
Fig. 1.
AR associates with the enhancer 1 region of the GnRH regulatory region and represses transcription. (A), Schematic of the −5 kb GnRH regulatory region, including the locations of the known enhancers and proximal promoter. (B), GT1-7 cells were transiently transfected with luciferase reporter constructs containing the GnRH-P (P), GnRH-E1 (E1), and/or GnRH-E2 (E2) regions, and/or the RSV promoter (RSVp) or enhancer (RSVe), as indicated, along with the AR expression vector. Cells were treated for 24 h with 100 nM R1881 (closed bars) or ethanol vehicle (open bars) and subjected to luciferase assay. Data are shown as relative luciferase units, relative to vehicle-treated RSVp, and represent the mean, ± SEM, of at least three experiments done in quadruplicate. **P<0.01, and ***P<0.001 versus vehicle. (B), GT1-7 cells were transiently transfected with AR and treated for 2 h with 100 nM R1881 or ethanol vehicle. Nuclei were extracted and subjected to chromatin immunoprecipitation with no antibody (no Ab), an antibody specific for AR (anti-AR), or IgG control. The resulting chromatin was analyzed by PCR with primers to GnRH-E1 or Intron 3. The gels shown were representative of three independent experiments. (C), Quantitative real time PCR was performed on the chromatin samples using primers to GnRH-E1.
GT1-7 cells were transiently transfected with an AR expression plasmid, along with luciferase reporter genes containing different combinations of GnRH-P, GnRH-E1, and GnRH-E2. Although GT cells have been shown to express AR (Poletti et al., 2001), GT1-7 cells lose AR expression after only a few passages; transfection with exogenous AR was required for response in our hands (Brayman et al., 2012). We utilized the non-responsive, heterologous Rous Sarcoma Virus enhancer or promoter, RSVe or RSVp, to substitute for the GnRH enhancers or promoter to isolate the activity of each element. GnRH-E3 was not evaluated because truncations showed that the far upstream regions, including GnRH-E3, were not involved (Brayman et al., 2012). Transfected cells were treated with or without 100 nM R1881 (a synthetic AR agonist) for 24 h. As expected, transcription from GnRH-P was repressed by R1881 treatment, regardless of the enhancer used (RSVe, GnRH-E1 or GnRH-E2) (Fig. 1B). Interestingly, GnRH-E1, but not GnRH-E2, was repressed when evaluated on the neutral RSV promoter. For the rest of this study, we chose to evaluate the mechanism of repression by AR using GnRH-E1 upstream of RSVp to avoid compensation by the androgen-responsive GnRH promoter.
To determine whether AR interacts with endogenous chromatin in the GnRH-E1 region in GT1-7 cells, ChIP assays were performed. GT1-7 cells were transfected with AR expression plasmid and treated with 100 nM R1881 (or ethanol vehicle) for 2 hours prior to nuclei isolation. An antibody specific for AR was used to immunoprecipitate chromatin associated with AR. The resulting isolated DNA was used in a PCR with primers specific for GnRH-E1 (or intron 3 as a control) (Iyer et al., 2010), and PCR products were visualized via gel electrophoresis. AR interaction with GnRH-E1, but not GnRH intron 3, increased upon treatment with R1881 (Fig. 1C). This was confirmed by real time PCR analysis of the isolated DNA (Fig. 1D). Thus, liganded AR interacts, directly or indirectly, with the androgen-regulated GnRH-E1 regulatory region in this GnRH neuronal cell line.
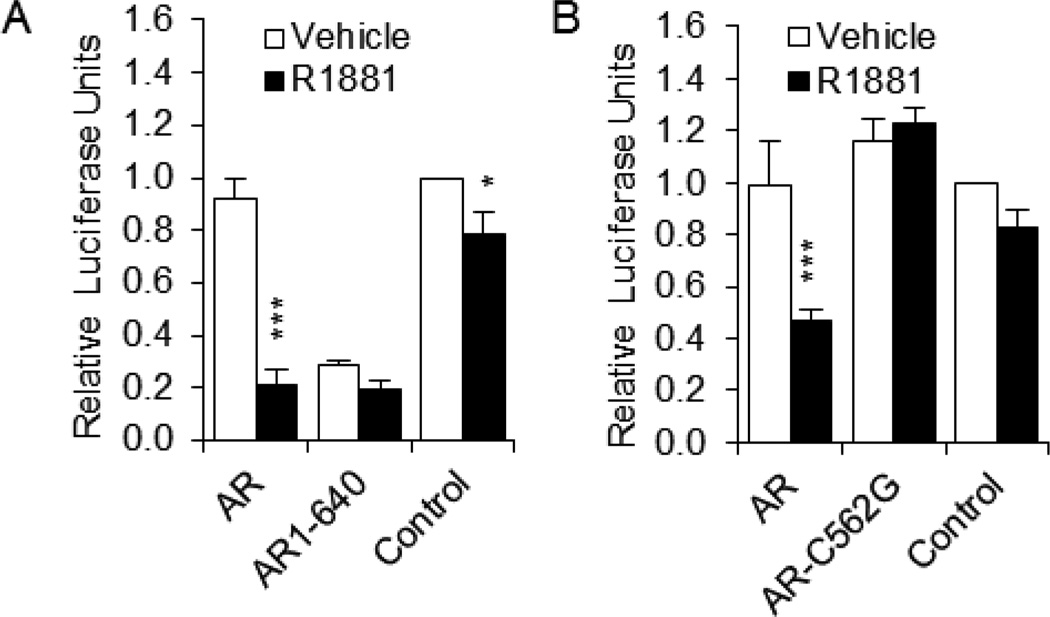
3.2. The AR DNA-binding domain is required for repression of GnRH-E1
AR contains three distinct regions that are involved in its activity: the amino-terminal activation domain, the DNA-binding domain (DBD), and the carboxy-terminal ligand-binding domain (LBD). To determine which domains are involved in repression of GnRH-E1, GT1-7 cells were transiently transfected with the GnRH-E1 reporter plasmid (GnRH-E1 on RSVp) and wild-type AR, AR lacking the LBD (AR1-640), or AR with a point mutation in the DBD (AR-C562G). AR1-640 has been shown to be active even in the absence of ligand (Brayman et al., 2012; Ceraline et al., 2004). AR1-640 was constitutively active and repressed GnRH-E1 transcriptional activity, regardless of treatment (Fig. 2A, compare AR1-640 to Control). Thus, the LBD is not required for repression of GnRH-E1. In contrast, AR-C562G was unable to repress GnRH-E1 transcriptional activity (Fig. 2B), indicating that DBD interaction with chromatin or with another transcription factor is required for repression of GnRH-E1 by AR. This is similar to previous findings that show that the DBD, but not LBD, of AR is required for repression of GnRH-P (Brayman et al., 2012).
Fig. 2.
The AR DNA-binding domain, but not the ligand-binding domain, is required for repression of GnRH-E1. GT1-7 cells were transiently transfected with (A), GnRH-E1 on RSVp and wild-type human AR or AR lacking the LBD (AR1-640), or (B), GnRH-E1 on RSVp and wild-type rat AR or AR containing a single bp mutation in the DBD (AR-C562G). Control: empty vector (no AR). Cells were treated for 24 h with 100 nM R1881 (closed bars) or ethanol vehicle (open bars) and subjected to luciferase assay. Data are expressed relative to vehicle-treated control. *P<0.05, ***P<0.001 versus vehicle.
3.3. Multiple regions are involved in AR repression of GnRH-E1
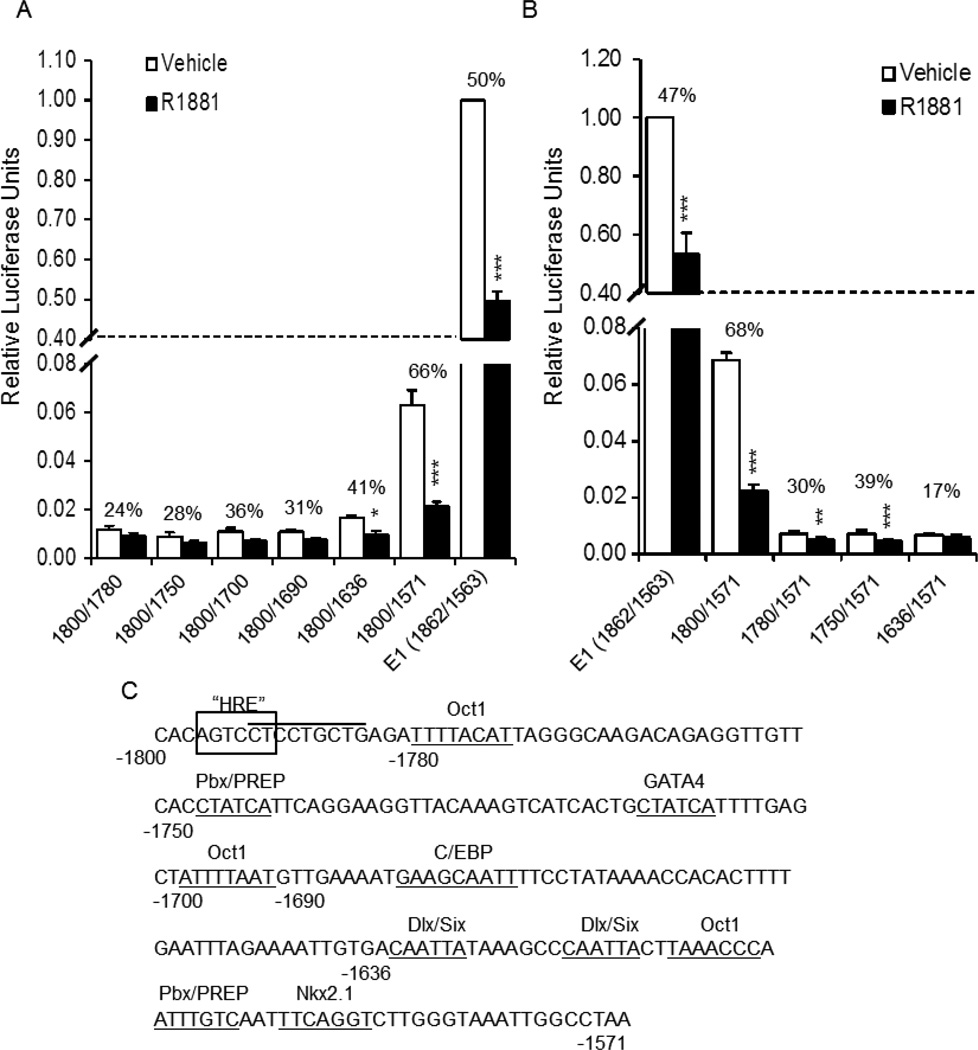
Truncations of GnRH-E1 were utilized to determine what region(s) of GnRH-E1 is involved in AR repression. E1 (−1862/−1563) was first reduced to −1800 to −1571, the E1 core region defined in Tang et al. (Tang et al., 2005). Though basal expression was reduced somewhat (Fig. 3A and B), repression remained. Further deletion from either the 3’ or 5’ end resulted in a dramatic reduction in transcriptional activity, but repression was still present (Fig. 3). Truncation of the 3’ end, from −1800/−1571 to −1800/−1636, removed several known transcription factor binding sites, including a binding site cluster of Oct1, Pbx/Prep, and Nkx2.1 (Clark and Mellon, 1995; Rave-Harel et al., 2004), and resulted in partial abrogation of AR repression (66% versus 41% repressed; Fig. 3A). Interestingly, a similar binding site cluster was found to be sufficient for AR repression of GnRH-P (Brayman et al., 2012). However, a multimer of the −1616 to −1589 sequence was not repressed by liganded AR (Supplemental Fig. 1A), indicating that, unlike the similar GnRH promoter cluster, this region of GnRH-E1 is not sufficient for repression.
Fig. 3.
Sequences between −1636 and −1571, and between −1800 and −1780, are involved in AR-mediated repression of GnRH-E1. GT1-7 cells were transiently transfected with the AR expression plasmid and either the (A), 3’ truncations or (B), 5’ truncations of the GnRH-E1 reporter plasmid on RSVp. Cells were treated with 100 nM R1881 (closed bars) or ethanol vehicle (open bars) for 24 h and subjected to luciferase assay. Data are shown as relative luciferase units, relative to vehicle-treated GnRH-E1, and represent the mean, ± SEM, of at least three experiments done in quadruplicate. Numbers above each set of bars represent percent repression by R1881. *P<0.05, **P<0.01 and *** P<0.001 versus vehicle-treated. (C), Sequence and relative locations of known transcription factor binding sites in GnRH-E1. Box: putative HRE; overline: TPA-responsive site; underline: known transcription factor binding sites.
Truncation of the core GnRH-E1 from the 5’ end, from −1800/−1571 to −1780/−1571, also decreased both basal activity and AR repression (68% versus 30% repressed; Fig. 3B). This truncation deleted part of an Oct1 binding site. To determine if Oct1 binding sites were involved in repression by R1881, mutation of Oct1 sites (Oct1a at −1781/−1774 and Oct1b at −1701/−1694) were made, both singly and in combination, within the full GnRH-E1 sequence. Mutations of these Oct1 sites did not affect repression (Supplemental Fig. 1B). We conclude that repression of GnRH-E1 by androgen involves sites in both the −1636/−1571 and −1800/−1780 regions, but the Oct1a and Oct1b sites do not appear to play a role.
3.4. AR represses a multimer of the −1800/−1766 region, and repression requires the TPA-responsive region
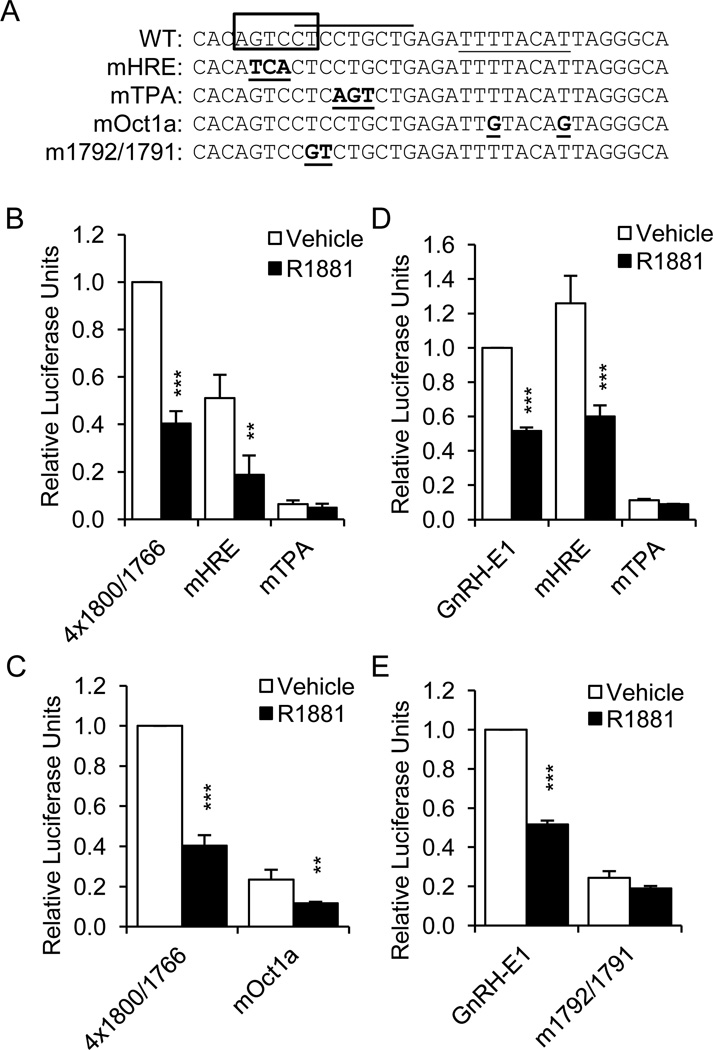
Since deletion of the sequence from −1800 to −1780 resulted in a decrease in AR-mediated repression, we chose to examine this region in more detail. Interestingly, −1797/−1792 was identified by Transcription Element Search Software (TESS) (Schug and Overton, 1997) as a putative nuclear receptor hormone response element (HRE) half-site; no other half-site was identified nearby. This putative site (AGTCCT) is completely conserved between mouse and rat. To determine if this region is sufficient for AR-mediated repression, a multimer containing four copies of the −1800/−1766 sequence, upstream of RSVp, (4×1800/1766) was transiently transfected into GT1-7 cells along with an AR expression plasmid. The multimer was significantly repressed by R1881 treatment (Fig. 4B). Mutation of the Oct1 binding site in this multimer (mutOct1) dramatically reduced basal activity, but did not abolish AR-mediated repression (Fig. 4C).
Fig. 4.
The −1800/−1766 region of GnRH-E1 is sufficient for androgen repression, and mutation of the TPA-responsive site abrogates repression of GnRH-E1. (A), Sequences of the −1800/−1766 region and mutations. Box: putative HRE; overline: TPA-responsive site; underline: Oct1-binding site; bold/underline: mutations. (B), GT1-7 cells were transiently transfected with the AR expression plasmid and a multimer of the −1800/−1766 sequence (4×1800/1766), or this multimer with 3-bp mutations in the putative HRE (mHRE, −1796/−1794) or the TPA-responsive site (mTPA, −1790/−1788). (C), GT1-7 cells were transiently transfected with the AR expression plasmid and 4×1800/1766, or this multimer with mutation in the Oct1-binding site (mOct1a). (D), GT1-7 cells were transiently transfected with the AR expression plasmid and GnRH-E1 on RSVp, or GnRH-E1 with 3-bp mutations in the putative HRE (mHRE) or the TPA-responsive site (mTPA). (E), GT1-7 cells were transiently transfected with the AR expression plasmid and GnRH-E1 on RSVp, or GnRH-E1 with a different mutation in the TPA-responsive site (m1792/1791). Cells were treated with 100 nM R1881 (closed bars) or ethanol vehicle (open bars) for 24 h and subjected to luciferase assay. Data are shown as relative luciferase units, relative to vehicle-treated 4×1800/1766 or GnRH-E1, and represent the mean, ± SEM, of at least three experiments done in quadruplicate. **P<0.01 and *** P<0.001 versus vehicle-treated.
The −1800/−1780 region was also shown to be involved in TPA repression of GnRH-E1 (Tang et al., 2005). The TPA response was mapped to a site spanning −1793 to −1785. Mutation of three bases of the putative HRE that do not overlap the TPA-responsive site (mHRE, −1796/−1794) decreased basal expression from 4×1800/1766, but not repression by R1881 (Fig. 4B). On the other hand, mutation of three bases within the TPA-responsive site (mTPA, −1790/−1788) dramatically reduced basal activity, but the multimer was no longer repressed by R1881. We conclude that the −1800/−1766 region is sufficient for AR repression and the TPA-responsive site may play a role.
Previous findings show that a two base-pair mutation at −1792/−1791 reduced basal activity of GnRH-E1 and abrogated TPA repression (Tang et al., 2005). To determine whether the TPA-responsive sequence is important in androgen repression of the full GnRH-E1, mutations were made within GnRH-E1 and tested on the RSV promoter. Mutation of the putative HRE had no effect on either basal activity or repression by AR (Fig. 4D, mHRE). Conversely, mutation of the TPA-responsive site eliminated repression by R1881 (mTPA, Fig. 4D). Similar results were obtained when two other bases within the more 5’ region of the TPA-responsive site were mutated (Fig. 4E), indicating that this binding site is also involved in AR-mediated repression of GnRH-E1.
3.5. AR and TPA repress GnRH-E1 via different mechanisms
Since repression of GnRH-E1 by both TPA and androgen requires the TPA-responsive site, we hypothesized that they might act via similar mechanisms to achieve this repression. However, unlike androgen, TPA activated the 4×1800/1766 multimer (Supplemental Fig. 2B), indicating that the two treatments utilize distinct mechanisms. Mutation of the TPA-responsive (Supplemental Fig. 2B) or Oct1 site (Supplemental Fig. 2C) did not affect TPA induction of the multimer, but mutation of the putative HRE (mHRE) resulted in repression of the multimer by TPA (Supplemental Fig. 2B). Mutation of the putative HRE in the context of the full enhancer did not affect TPA-mediated repression (Supplemental Fig. 2D). As expected, mutation of the TPA-responsive site eliminated repression of GnRH-E1 by TPA (Supplemental Fig. 2D and E).
3.6. AR binds to the −1802/−1779 region of GnRH-E1
To determine whether AR interacts with the −1800/−1766 sequence, electrophoretic mobility shift assay (EMSA) was performed. Nuclear extracts from GT1-7 cells that had been transiently transfected with AR expression plasmid and treated with R1881 were incubated with a radiolabeled oligonucleotide probe containing the −1802/−1779 sequence. Protein-DNA complexes were separated by polyacrylamide gel electrophoresis. Three bands were observed (Fig. 5A, lane 2, bands 4–6) that were super-shifted by an antibody specific for AR (lane 4, bands ss), but not normal IgG (lane 3). The AR antibody did not bind the probe in the absence of nuclear extract (data not shown). Thus, AR binds the −1802/−1779 sequence of GnRH-E1. Unlabeled oligonucleotide containing the consensus androgen response element (ARE) competed with the trio of bands 4–6 that was supershifted by anti-AR antibody (Fig. 5C, lane 3), further confirming that these protein complexes include AR. Other proteins bind this sequence, as well, as shown by bands 1, 2 and 3. Baculovirus-expressed AR from whole insect cell extracts bound to a consensus ARE but was unable to bind the −1802/−1779 probe (data not shown), indicating that other proteins that are expressed by GT1-7 cells are required for AR binding.
To determine where within the −1802/−1779 sequence AR binds, unlabeled oligonucleotides containing three-base pair scanning mutations were utilized (Fig. 5B). Unlabeled mutant oligonucleotides m2 and m3 were unable to compete for binding of bands 4–6 (lanes 6 and 7), indicating that AR interacts with the GnRH-E1 DNA sequence at −1796/−1791. The m2 mutation corresponds to the mHRE mutation in Fig. 4, and m3 corresponds to the −1792/−1791 mutation in Supplemental Fig. 2 though it includes a third mutated base pair at −1793. Band 3 likely corresponds to the protein found by Tang et al. (Tang et al., 2005) to be important for TPA repression of the GnRH-E1 through this region since its competition pattern is similar and indicates that it binds to region of −1793/−1785. Thus, AR interacts with the −1796/−1791 sequence of GnRH-E1 to repress transcriptional activity in the presence of androgen.
4. Discussion
A significant amount of work has been done to evaluate the mechanisms of gonadal steroid hormone regulation of the GnRH promoter (Chandran et al., 1999; Nelson et al., 1998; Pak et al., 2006). We previously showed that androgens repress GnRH transcription via multiple sites in GnRH-P (Brayman et al., 2012). Here, as the first report of hormonal regulation of the proximal enhancer region, we show that GnRH-E1 is repressed by AR in response to agonist by a mechanism that is dependent on the AR DNA but not ligand binding domain (DBD and LBD, respectively).
The highly conserved enhancers, GnRH-E1 and GnRH-E2, and the −173 bp promoter, GnRH-P, were evaluated separately and in combination for regulation by AR. Any reporter plasmid containing GnRH-E1 and/or GnRH-P was significantly repressed by R1881 treatment. Like GnRH-P (Brayman et al., 2012), AR association with GnRH-E1 chromatin in GT1-7 cells was increased upon treatment with R1881, and AR repression of GnRH-E1 was dependent on the AR DBD. Thus, these data indicate that the AR DBD interacts either directly with chromatin or indirectly through association with another transcription factor bound to GnRH-E1.
Truncation analysis of GnRH-E1 suggested that multiple sites in GnRH-E1 are involved in androgen repression. However, any truncation of the enhancer resulted in a dramatic reduction in basal activity, confounding analysis of further repression by androgens. It is possible that truncation results in such a low level of basal expression that transcription cannot be further repressed. Nonetheless, removal of the sequence between −1636 and −1571, and that between −1800 and −1780, reduced repression by AR. Both of these sequences contain Oct1 binding sites, and the −1636/−1571 region contains a cluster of Oct1, Pbx/Prep, and Nkx2.1 binding sites that is similar to the one shown to be involved in androgen repression of GnRH-P (Brayman et al., 2012). However, unlike GnRH-P, the binding site cluster alone was not sufficient for AR-mediated repression of GnRH-E1. The two sequences (−1616/−1589 and −106/−91) share only 37% homology (Myers and Miller, 1988), perhaps explaining this difference.
A multimer of the −1800/−1766 sequence was repressed by liganded AR. Mutation of the Oct1 site in the multimer did not affect repression, consistent with our observation that mutation of that Oct1 site in the full GnRH-E1 did not alleviate repression. AR bound in vitro to an oligonucleotide probe containing the −1802/−1779 sequence. The high mobility of the complexes observed in EMSA suggests that AR binds to this region as a monomer, rather than as a dimer. AR bound specifically to the sequence at −1796/−1791 (GTCCTC), which overlaps the putative HRE half-site at −1797/−1792 (AGTCCT) as well as the TPA-responsive binding site at −1793/−1785 (CTCCTGCTG). Mutation of the GTC at −1796/−1794 only partially affected binding of the TPA-responsive complex (Tang et al., 2005), but was unable to compete for AR binding (see m2 in Fig. 5C). However, mutation of this GTC did not affect AR-mediated repression of either the −1800/−1766 multimer or the full GnRH-E1. On the other hand, a mutation of three base pairs, −1790 to −1788, which also overlapped the TPA-responsive site, did relieve repression in the −1800/−1766 multimer. Mutation of this site did not disrupt AR binding (see m4 in Fig. 5C), suggesting that either the TPA-responsive region is important in AR-mediated repression, or that the dramatic reduction in basal expression did not allow further repression by R1881. AR does not repress GnRH-E1 via the same mechanism as TPA targets (e.g., activator protein-1, AP-1), since TPA treatment activated the 4×1800/1766 multimer.
Since the −1792/−1791 sequence was also bound by AR in EMSA (see m3 in Fig. 5C) and overlaps the TPA-responsive site (Tang et al., 2005), we determined that mutation of these two base pairs in GnRH-E1 was capable of relieving AR-mediated repression. While this mutation also reduced basal transcription levels to approximately 20% of GnRH-E1, it is unlikely that this reduction was responsible for the loss of repression, since the −1800/−1571 truncation resulted in approximately 94% lower basal activity but was still significantly repressed by R1881 (see Fig. 3A). We concluded that AR binding to the region around −1792/−1791, or interaction with another transcription factor bound to that site, is important for androgen-mediated repression of GnRH-E1. AR likely requires interaction with another protein to bind to GnRH-E1, possibly as a monomer, since baculovirus-expressed AR alone was unable to bind.
Androgen represses GnRH gene expression through both GnRH-P and GnRH-E1, but AR regulation of the two regions appears to be via distinct mechanisms. Repression of GnRH-P by androgen involves a cluster of Oct1, Pbx/PREP, and Nkx2.1 binding sites, although other sites also contribute to repression (Brayman et al., 2012). Interaction of all of these transcription factors may be important for regulation of GnRH-P. Although a similar binding site cluster is present in GnRH-E1, it is not sufficient for AR-mediated repression. Instead, AR interacts with chromatin in GT1-7 cells in culture and by direct interactions with a site within the 5’ end of the enhancer that is indispensable for enhancer activity (Tang et al., 2005). We further determine that repression of GnRH gene expression by androgen is maximal in the presence of both GnRH-P and GnRH-E1, suggesting the coordinate actions of these regulatory regions provides a particularly effective repression mechanism for androgens via the AR.
Dysregulation of the GnRH gene leads to improper levels of the GnRH peptide hormone and thus abnormal communication between the hypothalamus, pituitary, and gonads. This report indicates that AR can repress GnRH via its proximal enhancer in addition to its promoter region. Identifying the proteins and regions involved in AR-mediated GnRH repression could lead to novel protein targets for the treatment of reproductive disorders.
Supplementary Material
Highlights.
-
-
AR-mediated repression of GnRH gene expression involves enhancer 1.
-
-
AR represses GnRH enhancer 1 via interaction with the −1796/−1791 region.
-
-
Our study provides a new mechanism involved in the repression of GnRH by androgens.
Acknowledgements
The authors would like to thank Jorma Palvimo (University of Kuopio, Kuopio, Finland) for the pSG5-rAR and pSG5-rAR-C562G plasmids, Xiang-Dong Fu (University of California, San Diego) for the human AR and AR1-640 plasmids, and Xiuping Yu (Vanderbilt University, Nashville, TN) for technical advice regarding ChIP assays. The authors would also like to thank Varykina Thackray and Christine Glidewell-Kenney for manuscript editing and many helpful discussions.
This work was supported by NIH grants R01 DK044838, R01 HD072754, and R01 HD020377 (to P.L.M.) and by NICHD/NIH through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (P.L.M.). P.L.M. was partially supported by P30 DK063491, P30 CA023100, and P42 ES101337. M.J.B. was partially supported by F32 HD058460 and T32 HD007203. P.A.P. was partially supported by the Doris Howell Foundation.
Abbreviations
- cs
charcoal/dextran-treated
- DBD
DNA-binding domain
- DHT
5α-dihydrotestosterone
- GnRH-E
GnRH enhancer
- GnRH-P
GnRH promoter
- HPG
hypothalamic-pituitary-gonadal
- LBD
ligand-binding domain
- MMTV
mouse mammary tumor virus
- Nkx2.1
NK2 homeobox 1
- Oct-1
octamer-binding transcription factor-1
- Pbx
Pre-B cell leukemia transcription factor
- PMSF
phenylmethylsulfonyl fluoride
- RSVe
Rous sarcoma virus enhancer
- RSVp
RSV promoter
- TK
thymidine kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belsham DD, Evangelou A, Roy D, Duc VL, Brown TJ. Regulation of gonadotropin-releasing hormone (GnRH) gene expression by 5alpha-dihydrotestosterone in GnRH-secreting GT1-7 hypothalamic neurons. Endocrinology. 1998;139:1108–1114. doi: 10.1210/endo.139.3.5846. [DOI] [PubMed] [Google Scholar]
- Belsham DD, Mellon PL. Transcription factors Oct-1 and C/EBP β (CCAAT/enhancer binding protein-β) are involved in the glutamate/nitric oxide/cyclic guanosine 5'-monophosphate-mediated repression of gonadotropin-releasing hormone gene expression. Mol. Endocrinol. 2000;14:212–228. doi: 10.1210/mend.14.2.0418. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brayman MJ, Pepa PA, Berdy SE, Mellon PL. Androgen receptor repression of GnRH gene transcription. Mol. Endocrinol. 2012;26:2–13. doi: 10.1210/me.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceraline J, Cruchant MD, Erdmann E, Erbs P, Kurtz JE, Duclos B, Jacqmin D, Chopin D, Bergerat JP. Constitutive activation of the androgen receptor by a point mutation in the hinge region: a new mechanism for androgen-independent growth in prostate cancer. Int. J. Cancer. 2004;108:152–157. doi: 10.1002/ijc.11404. [DOI] [PubMed] [Google Scholar]
- Chandran UR, Warren BS, Baumann CT, Hager GL, DeFranco DB. The glucocorticoid receptor is tethered to DNA-bound Oct-1 at the mouse gonadotropin-releasing hormone distal negative glucocorticoid response element. J. Biol. Chem. 1999;274:2372–2378. doi: 10.1074/jbc.274.4.2372. [DOI] [PubMed] [Google Scholar]
- Chappell PE, White RS, Mellon PL. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J. Neurosci. 2003;23:11202–11213. doi: 10.1523/JNEUROSCI.23-35-11202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ME, Mellon PL. The POU homeodomain transcription factor Oct-1 is essential for activity of the gonadotropin-releasing hormone neuron-specific enhancer. Mol. Cell. Biol. 1995;15:6169–6177. doi: 10.1128/mcb.15.11.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens ML, Kurotani R, N R-H, Miller NLG, Mellon PL. Phylogenetic footprinting reveals functional upstream regions of the gonadotropin-releasing hormone gene that enhance cell-specific expression. Mol. Endocrinol. 2004;18:2950–2966. doi: 10.1210/me.2003-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front. Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszán T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141:3506–3509. doi: 10.1210/endo.141.9.7788. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- Ikonen T, Palvimo JJ, Janne OA. Heterodimerization is mainly responsible for the dominant negative activity of amino-terminally truncated rat androgen receptor forms. FEBS Lett. 1998;430:393–396. doi: 10.1016/s0014-5793(98)00701-7. [DOI] [PubMed] [Google Scholar]
- Ikonen T, Palvimo JJ, Kallio PJ, Reinikainen P, Janne OA. Stimulation of androgen-regulated transactivation by modulators of protein phosphorylation. Endocrinology. 1994;135:1359–1366. doi: 10.1210/endo.135.4.7925097. [DOI] [PubMed] [Google Scholar]
- Iyer AK, Miller NL, Yip K, Tran BH, Mellon PL. Enhancers of GnRH transcription embedded in an upstream gene use homeodomain proteins to specify hypothalamic expression. Mol. Endocrinol. 2010;24:1949–1964. doi: 10.1210/me.2010-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson MA, Whyte DB, Mellon PL. GATA factors are essential for activity of the neuron-specific enhancer of the gonadotropin-releasing hormone gene. Mol. Cell. Biol. 1996;16:3596–3605. doi: 10.1128/mcb.16.7.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez de la Escalera GM, Choi ALH, Weiner RI. b1-adrenergic regulation of the GT1 gonadotropin-releasing hormone (GnRH) neuronal cell lines: Stimulation of GnRH release via receptors positively coupled to adenylate cyclase. Endocrinology. 1992;131:1397–1402. doi: 10.1210/endo.131.3.1354602. [DOI] [PubMed] [Google Scholar]
- Mellon PL, Wetsel WC, Windle JJ, Valença MM, Goldsmith PC, Whyte DB, Eraly SA, Negro-Vilar A, Weiner RI. Immortalized hypothalamic gonadotropin-releasing hormone neurons. In: Chadwick DJ, Marsh J, editors. Functional Anatomy of the Endocrine Hypothalamus. Chichester: Wiley & Sons Ltd; 1992. pp. 104–126. [DOI] [PubMed] [Google Scholar]
- Mellon PL, Windle JJ, Goldsmith P, Padula C, Roberts J, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990;5:1–10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- Myers EW, Miller W. Optimal alignments in linear space. Comput Appl Biosci. 1988;4:11–17. doi: 10.1093/bioinformatics/4.1.11. [DOI] [PubMed] [Google Scholar]
- Navarro CE, Abdul Saeed S, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ. Regulation of cyclic adenosine 3',5'-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotropin-releasing hormone neurons. Mol. Endocrinol. 2003;17:1792–1804. doi: 10.1210/me.2003-0040. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Eraly SA, Mellon PL. The GnRH promoter: Target of transcription factors, hormones, and signaling pathways. Mol. Cell. Endocrinol. 1998;140:151–155. doi: 10.1016/s0303-7207(98)00043-4. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Lawson MA, Kelley CG, Mellon PL. Neuron-specific expression of the rat gonadotropin-releasing hormone gene is conferred by interactions of a defined promoter element with the enhancer in GT1-7 cells. Mol. Endocrinol. 2000;14:1509–1522. doi: 10.1210/mend.14.9.0521. [DOI] [PubMed] [Google Scholar]
- Pak TR, Chung WC, Roberts JL, Handa RJ. Ligand-independent effects of estrogen receptor beta on mouse gonadotropin releasing hormone (GnRH) promoter activity. Endocrinology. 2006;147:1924–1931. doi: 10.1210/en.2005-1297. [DOI] [PubMed] [Google Scholar]
- Poletti A, Rampoldi A, Piccioni F, Volpi S, Simeoni S, Zanisi M, Martini L. 5Alpha-reductase type 2 and androgen receptor expression in gonadotropin releasing hormone GT1-1 cells. J. Neuroendocrinol. 2001;13:353–357. doi: 10.1046/j.1365-2826.2001.00635.x. [DOI] [PubMed] [Google Scholar]
- Rave-Harel N, Givens ML, Nelson SB, Duong HA, Coss D, Clark ME, Hall SB, Kamps MP, Mellon PL. TALE homeodomain proteins regulate gonadotropin-releasing hormone gene expression independently and via interactions with Oct-1. J. Biol. Chem. 2004;279:30287–30297. doi: 10.1074/jbc.M402960200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rave-Harel N, Miller NLG, Givens ML, Mellon PL. The Groucho-related gene family regulates the gonadotropin-releasing hormone gene through interaction with the homeodomain proteins MSX1 and OCT1. J. Biol. Chem. 2005;280:30975–30983. doi: 10.1074/jbc.M502315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Kelly MJ, Ronnekleiv OK. Testosterone regulates progonadotropin-releasing hormone levels in the preoptic area and basal hypothalamus of the male rat. Endocrinology. 1990;126:1080–1086. doi: 10.1210/endo-126-2-1080. [DOI] [PubMed] [Google Scholar]
- Roy D, Angelini NL, Belsham DD. Estrogen directly represses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-alpha (ERalpha)- and ERbeta-expressing GT1-7 GnRH neurons. Endocrinology. 1999;140:5045–5053. doi: 10.1210/endo.140.11.7117. [DOI] [PubMed] [Google Scholar]
- Schreiber E, Matthias P, Müller M, Schaffner W. Rapid detection of octamer binding proteins with mini-extracts prepared from a small number of cells. Nucl. Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug J, Overton GC. Technical Report CBIL-TR-1997-1001-v0.0. School of Medicine, University of Pennsylvania, Computational Biology and Informatics Laboratory; 1997. TESS: Transcription Element Search Software on the WWW. [Google Scholar]
- Shakil T, Hoque AN, Husain M, Belsham DD. Differential regulation of gonadotropin-releasing hormone secretion and gene expression by androgen: membrane versus nuclear receptor activation. Mol. Endocrinol. 2002;16:2592–2602. doi: 10.1210/me.2002-0011. [DOI] [PubMed] [Google Scholar]
- Spady TJ, Shayya R, Thackray VG, Ehrensberger L, Bailey JS, Mellon PL. Androgen regulates FSHβ gene expression in an activin-dependent manner in immortalized gonadotropes. Mol. Endocrinol. 2004;18:925–940. doi: 10.1210/me.2003-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Mazur M, Mellon PL. The protein kinase C pathway acts through multiple transcription factors to repress gonadotropin-releasing hormone gene expression in hypothalamic GT1-7 neuronal cells. Mol. Endocrinol. 2005;19:2769–2779. doi: 10.1210/me.2004-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toranzo D, Dupont E, Simard J, Labrie C, Couet J, Labrie F, Pelletier G. Regulation of pro-gonadotropin-releasing hormone gene expression by sex steroids in the brain of male and female rats. Mol. Endocrinol. 1989;3:1748–1756. doi: 10.1210/mend-3-11-1748. [DOI] [PubMed] [Google Scholar]
- Wetsel WC, Valença MM, Merchenthaler I, Liposits Z, López FJ, Weiner RI, Mellon PL, Negro-Vilar A. Intrinsic pulsatile secretory activity of immortalized LHRH secreting neurons. Proc. Natl. Acad. Sci. USA. 1992;89:4149–4153. doi: 10.1073/pnas.89.9.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.