Abstract
The context preexposure facilitation effect (CPFE) is a variant of contextual fear conditioning in which context learning and context-shock associations occur on separate occasions. The CPFE with an immediate shock emerges between Postnatal Day (PND) 17 and 24 in the rat and depends on hippocampal NMDA-receptor function in PND 24 rats (Schiffino et al., 2011). This study investigated this ontogenetic effect further and reports three findings: First, the CPFE is absent on PND19 but emerges modestly in rats given exposure on PND 21. Second, the absence of the CPFE on PND17 does not reflect inability to consolidate the context-shock association established on the training day. Lastly, the CPFE on PND 24 requires exposure to the combined features of the context. These results are the first to show that the early development of contextual fear conditioning depends on conjunctive representations and that processes underlying the CPFE begin to emerge around PND 21 in the rat.
Keywords: Ontogeny, Hippocampus, Context, Memory, Spatial cognition
Introduction
In standard contextual fear conditioning, animals are placed in a conditioning chamber and, after some time (e.g., 120 sec), receive a brief foot-shock. When reintroduced to the chamber a day later, animals demonstrate species-typical freezing behavior, indicative of conditioned fear. The discovery that auditory-cue and standard contextual fear conditioning dissociate during development (Rudy, 1993) has stimulated the use of contextual fear conditioning in studies of the ontogeny of hippocampus-dependent memory (Burman, Murawski, Schiffino, Rosen & Stanton, 2009; Dumas & Rudy, 2010; Raineki, Holman, Debiec, Bugg, Beasley and Sullivan 2010; Rudy, 1993; Rudy & Morledge, 1994; Schiffino, Murawski, Rosen & Stanton, 2011; Stanton, 2000). The emergence of auditory fear conditioning by Postnatal Day (PND) 16–18, followed by contextual fear conditioning around PND 21–23 (Burman et al., 2009; Dumas & Rudy, 2010; Rudy, 1993; Rudy & Morledge, 1994; Schiffino et al., 2011; Stanton, 2000), was historically attributed to the ontogenetic emergence of hippocampal function (Rudy, 1993), which was then seen as necessary for fear conditioning to contextual but not discrete cues (Kim & Fanselow, 1992; Phillips & LeDoux, 1992). However, evidence soon emerged against this view (e.g., Maren, Aharonov & Fanselow, 1997) and it is now clear that the hippocampus is not critically involved in standard contextual fear conditioning (see Rudy, 2009, for recent review). Context conditioning is now thought to be mediated by two distinct competing associative systems (Fanselow, 2000; Maren, 2001; Rudy, 2009; Rudy, Huff, & Matus-Amat, 2004; Rudy & O’Reilly, 2001). One is a “features-based”, in which rats learn about each feature of the context independently of the other features and the additive perception of the features serves as the conditioned stimulus (CS) for fear conditioning (Rudy, 2009). This system is thought to rely on parahippocampal cortical regions without depending on the hippocampal formation (Rudy & O’Reilly, 2001; Rudy, 2009). The other system is the conjunctive system, which additionally relies on the hippocampal formation to combine the features of context into an integrated “whole,” or “conjunctive representation” that serves as the CS during context conditioning (Fanselow, 1986; 1990; Rudy & O’Reilly, 1999; Rudy, 2009; Teyler & DiScenna, 1986; Teyler & Rudy, 2007).
The role of these two associative systems has been analyzed by using a variant of standard contextual fear conditioning, a paradigm known as the context preexposure facilitation effect (CPFE, Rudy, 2009; Rudy et al., 2004). In the CPFE, the acquisition of the context representation and the association of that representation with shock occur on separate occasions (Fanselow, 1990). Context preexposure occurs on the first day; fear conditioning via an immediate foot shock delivered in the same context occurs on the second day, and the test of contextual fear occurs on the third day. During this test, considerable freezing is displayed in rats preexposed to the training context when compared to control rats that were not preexposed (which show the immediate shock deficit, Fanselow, 1986, 1990). One advantage of the CPFE paradigm is that it is easier to manipulate aspects of context learning apart from fear learning. For example, on the preexposure day, rats can encounter the individual features of context either together, to support conjunctive learning, or separately, to preclude this learning, in order to empirically determine the role of conjunctive learning in contextual fear conditioning. Conjunctive learning is required for the CPFE in rats 30 days and older (Rudy & O’Reilly, 1999) but it is currently not known whether this is true at younger ages (Pugh & Rudy, 1996; see Discussion). Another advantage of the CPFE is that consolidation of the context memory itself can be examined apart from consolidation of the contextual fear memory, as the spatial learning and affective learning episodes occur on separate days. In standard contextual fear conditioning, freezing exhibited immediately following shock delivery has been used to examine short-term retention of fear that is not subject to consolidation over a retention interval. This learning does not depend on the hippocampus (Kim et al., 1992) and is present as early as PND 18 in the rat (Rudy & Morledge, 1994). In contrast, retention of this learning at least a day later depends on the hippocampus (Kim et al., 1992) and emerges between PND 18 and 23 (Rudy, 1993). It has been argued that developmental differences in consolidation of the conjunctive context representation account for developmental differences in context conditioning (Rudy & Morledge, 1994), but the role that consolidation of context-shock associations may play has not been examined early in ontogeny. Another advantage of the CPFE is that it is more sensitive to hippocampal injury than standard contextual fear conditioning (Fanselow, 2010; Rudy, 2009). Both tasks are completely disrupted by retrograde hippocampal lesions. However, anterograde lesions eliminate the CPFE but only impair standard context conditioning under certain circumstances (Fanselow, 2010; Maren et al., 1997; Rudy, 2009; Wiltgen, Sanders, Anagnostaras, Sage & Fanselow, 2006). Additionally, hippocampal inactivation at any stage of the CPFE---preexposure, training, or testing---disrupts the effect (Matus-Amat, Higgins, Barrientos, & Rudy, 2004), indicating that the hippocampus is critically involved in multiple aspects of this variant of contextual fear conditioning. For these reasons, the CPFE is clearly the paradigm of choice in studies that use context conditioning to understand the ontogeny of hippocampus-dependent memory.
Recently, this lab has shown that the CPFE emerges between PND 17 and 24 and is disrupted by intrahippocampal infusion of an NMDA-receptor antagonist prior to the preexposure phase (Schiffino et al., 2011). These findings indicate that a form of context learning that depends on hippocampal plasticity emerges by PND 24. The present study extends this work by examining additional questions surrounding the ontogeny of the CPFE. First, we asked at what age between PND 17 and 24 does the CPFE first emerge (Experiment 1). Second, we asked whether the emergence of the CPFE reflects consolidation of the context representation or consolidation of the context-shock association (Experiment 2). Finally, we asked whether the CPFE is based on elemental or conjunctive associations at the age when it first emerges (Experiment 3).
General Method
Subjects
Subjects were Long-Evans weanling rats born to dams bred at the University of Delaware. Litters were culled to 8 pups (typically 4 male, 4 female) on PND 3. No more than 1 same-sex littermate was assigned to a given experimental condition. Litters were weaned on PND 21, except where noted. Dams were housed with their litters in clear polypropylene cages measuring 8″ high × 18″ long × 9″ wide in an animal colony, which operated according to NIH guidelines. The colony room was maintained on a 12:12 h light/dark cycle. The age of birth (PND 0) was determined by daily monitoring of the dams. Following weaning, pups were housed with same-sex littermates and provided ad libitum food and water. Except where noted, animals were singly housed in individual cages 2 days prior to experimentation. All Pre-exposure, Training and Testing sessions occurred in the afternoon between 2:00 and 6:00 p.m. (see ‘Behavioral Procedure’).
Apparatus
The apparatus has been described previously (Burman et al., 2009; Schiffino et al., 2011). Conditioning occurred in 1 of 4 identical Plexiglas conditioning chambers situated under a fume hood, which provided the only source of overhead lighting and low-level background noise. The sides and ceiling of the chambers were transparent, except for 1 wall of each chamber, which prevented viewing of adjacent pups. Each chamber consisted of a grid floor (0.5-cm diameter bars situated 1.25-cm apart) connected to a shock-generator that delivered the US (two 2 s 1.5 mA foot shocks, separated by 1 s; Schiffino et al., 2011). Pre-exposure sessions took place in these conditioning chambers or in a set of alternate chambers. These chambers were wire mesh cages housed within BRS-LVE sound-attenuating shells used for eyeblink conditioning (Brown & Stanton, 2008). They were situated in a different room and differed from the conditioning chambers in size and texture (Schiffino et al., 2011).
Conditioned fear was assessed by measuring freezing during the contextual fear tests. Freezing was defined as the cessation of all visible movement except for respiration. The data were analyzed using FreezeFrame software (Actimetrics, Wilmette, IL) as previously described (Burman et al., 2009).
Experiment 1
The purpose of Experiment 1 was to determine at what point in ontogeny pups begin to acquire a conjunctive representation of the conditioning context. Because the CPFE develops between PND 17 and 24 (Schiffino et al. 2011), Experiment 1 sought to examine intermediate ages (PND 19 vs. PND 21) to further determine when performance in this task appears during ontogeny. Subjects were preexposed to the training context (Pre) or an alternate context (No-Pre) on either PND 19 or 21.
Method
Subjects were 41 pups (22 male, 19 female), which were the offspring of 10 dams. The pups were assigned to the following conditions: 19-Pre (6 males, 4 females), 19-No Pre (6 males, 5 females), 21-Pre (5 males, 6 females) and 21-No Pre (5 males, 4 females).
All handling of animals and behavioral procedures were identical to those previously used in this lab by Schiffino et al. (2011, Experiment 2). The CPFE procedure took place in three phases: preexposure, training and testing, occurring about 24-hr apart. On the preexposure day (PND 19 or 21), pups were preexposed for 5 minutes to either the conditioning context (Pre) or an alternate context (No Pre) which was situated in a completely different room and consisted of the same mesh chambers that were used as a control context in our previous studies (Schiffino et al., 2011). For the Pre Group, prior to each preexposure, training and testing session, pups were weighed, placed into individual opaque transport cages and were carted to a holding area across from the training room while the conditioning chamber was cleaned with a 5% ammonium hydroxide solution before the start of each phase (Schiffino et al., 2011). Animals in the No Pre group were carted and placed in the alternate chambers for a total time period approximately equal to that of the Pre Group. For all experiments, Treatment group was counterbalanced across Age.
Twenty-four hours following preexposure, all pups were trained in the conditioning context. Pups in the Pre group were trained in the same chamber as the preexposure phase. In order to ensure immediate delivery of the shocks, pups were placed in the conditioning chamber one at a time. The placement-to-shock interval was less than 5 s. This procedure has been shown to facilitate context conditioning in preexposed animals during the weanling period, while 30 s of context exposure is sufficient to support conditioning in standard contextual fear (Burman et al., 2009). Following the immediate shocks, pups were removed as quickly as possible and returned to their home cage.
Twenty-four hours following training, all pups were returned to the conditioning context and all procedures were identical to the preexposure phase for the Pre group. All pups were tested in the same chamber location that they encountered on the training day.
In order to determine the emergence of the CPFE within a narrow range of ages, pups preexposed on PND 19, trained on PND 20 and tested on PND 21 were compared to pups preexposed on PND 21, trained on PND 22 and tested on PND 23. Thus, a 2 (Preexposure Group: Pre vs. No Pre) × 2 (Preexposure Age: PND 19 vs. PND 21) × 2 (Sex) factorial design was used.
In order to maximize litter sampling and ensure undisturbed litters between age groups, pups were cross-fostered on PND 19 or PND 21, prior to the pre-exposure session. Accordingly, 8 pups per dam were run on PND 19 or PND 21 without duplicating sex or litter for any experimental factor. Additionally, in order to control for the influence of the weaning procedure during the CPFE protocol, pups from both age groups remained with the dam until completion of the experiment.
Results and Discussion
On seven instances, the data from same sex littermates were averaged. Data from three animals were removed as outliers (scores exceeding +/− 2 standard deviations from other animals in the group). One outlier was removed from each of the 4 conditions, with the exception of the 19-No Pre condition.
In addition to both PND 19 and 21 Pre and No-Pre groups, the PND 17 and 24 Pre and No Pre groups from Schiffino et al. (2011; Experiment 2) were included in the analysis for purposes of comparison. ANOVA results indicated no main effect or interaction of Sex (all ps >.2), thus the data are collapsed across this variable, and analyzed via 4 (Preexposure Age) × 2 (Preexposure Group) ANOVA. As shown in Figure 1, the CPFE was absent on PND 17 and 19, as indicated by the absence of freezing in both the Pre and No Pre groups, while a modest CPFE was demonstrated on PND 21, with greater freezing in the Pre group relative to the No Pre group. Additionally, a substantial CPFE was observed for the PND 24 age group. Statistically, there was a main effect of Preexposure Group (F(1, 63 = 20.94, p<.001) and Preexposure Age (F(3, 63 = 10.33, p<.001), reflecting greater freezing in the Pre groups and in the PND21 and 24 rats. Additionally, there was a significant Preexposure Group × Preexposure Age interaction (F(3,63 = 7.24, p < .001). Post hoc Newman-Keuls test confirmed that Pre group differences were not evident for the PND 17 and 19 age groups (all p-values >.7). However, compared to their No Pre counterparts, both PND 21 and 24 Pre groups showed significant increases in freezing (PND 21: p < .05; PND 24: p < .001), and the PND 24 Pre group froze more than the 21 Pre group (p < .003). Thus, a modest CPFE effect appears to emerge by PND21 which continues to develop through PND24.
Figure 1.
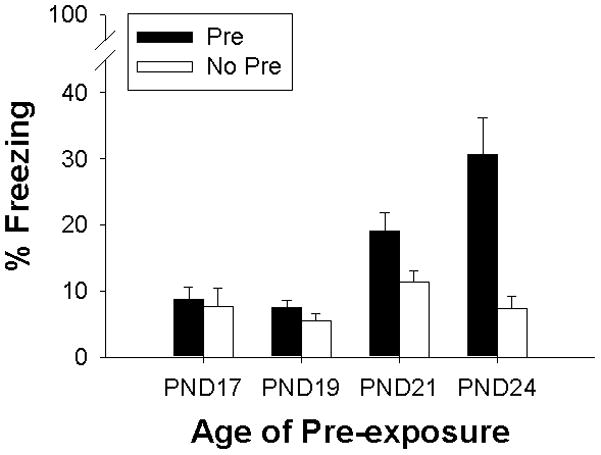
Mean percent freezing in Experiment 1 for Pre (Black) and No-Pre (White) groups across Pre-exposure Age (PND17, 19, 21 and 24). Bars represent standard errors of the mean. PND17 and 24 data are reproduced from Schiffino et al., (2011; Experiment 2). Significant facilitation of conditioning by context pre-exposure was observed on PND24 to a greater degree than PND21.
Experiment 2
The purpose of Experiment 2 was to determine whether the ontogeny of the CPFE reflects an age difference in consolidation efficacy of a conjunctive representation of context encoded on the pre-exposure day or of the context-shock association encoded on the training day. When shock occurs after 2 minutes of context exposure, post-shock freezing occurs at high and equivalent levels in both PND 18 and 23 rats (Rudy & Morledge, 1994) suggesting contexts are encoded and temporarily associated with shock at both ages via a non-hippocampal mechanism (e.g. Kim et al., 1992). A day later, only the PND 23 rats showed freezing to context, suggesting failure of PND 18 rats to consolidate the context representation or context-shock association during the 24-hr retention interval (Rudy & Morledge, 1994). It is currently unknown whether the failure of preweanling rats to show the CPFE occurs for a similar reason. To address this question, we examined post-shock freezing on the training day in groups that had been pre-exposed to the training or the alternate context on PND 17 or PND 24. If consolidating a conjunctive representation underlies the ontogeny of the CPFE, then post-shock freezing would be observed in the older but not the younger age group. In contrast, if post-shock freezing is observed in both age groups, it would suggest that the ontogeny of the CPFE reflects a failure of the younger group to consolidate the context-shock association between the training and testing day.
Method
Subjects were (17 male, 17 female), offspring of 17 dams bred and reared as described in Experiment 1. Pups were assigned to the following groups: 24-Pre (5 males, 6 females), 24-No Pre (4 males, 4 females), 17-Pre (4 males, 4 females), and 17-No Pre (4 males, 3 females). Again, no more than a single same-sex littermate was assigned to a particular group.
The methods for Experiment 2 were as described in Experiment 1 for the apparatus and all handling of animals (except that rats preexposed on PND 24 were weaned on PND 21; Schiffino et al., 2011). The preexposure and training procedure was also identical except that, following the completion of shock presentation during training, pups remained in the conditioning chamber for 5 minutes, during which freezing data were recorded. Thus, a 2 (Group: Pre vs. No Pre) × 2 (Pre-exposure Age: PND17 vs. PND24) × 2 (Sex: male vs female) factorial design was used.
Results & Discussion
Since there were no main or interaction effects of sex (all p-values>.5), the data are shown collapsed across this variable (Figure 2). Post-shock freezing appeared only in the rats pre-exposed to the training context on PND 24. There was a main effect of Group (F(1,30 = 11.47, p<.002), and Age (F(1,30 = 4.31, p=.046), as well as a Group × Age interaction (F(1, 30= 8.75, p<.007). Post-hoc Newman-Keuls tests confirmed that pups pre-exposed to the conditioning context on PND24 showed significantly greater levels of freezing than all other groups (all ps<.002). Thus, contextual information acquired during preexposure can be retrieved and associated with an immediate shock and thereby elicit post-shock freezing on the training day in PND24 but not PND17 rats.
Figure 2.
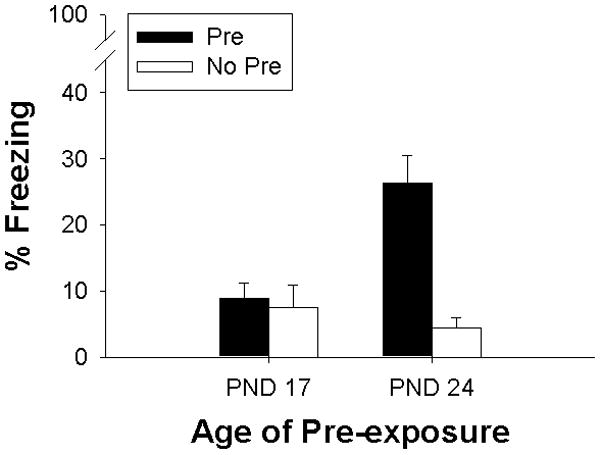
Mean percent freezing in Experiment 2 for Pre (Black) and No-Pre (White) groups across Pre-exposure Age (PND17, 24). Bars represent standard errors of the mean. Facilitation of conditioning by context pre-exposure was only observed on PND24.
These results suggest that conjunctive representations of the context are consolidated and retrieved from memory 24-hour later during training in weanling but not pre-weanling rats. Postulating age differences in consolidation of context-shock associations following training is not necessary to account for the ontogeny of the CPFE.
Experiment 3
In order to further determine if weanling animals form a conjunctive versus elemental representation of the context during pre-exposure sessions, three different pre-exposure groups were tested in our CPFE paradigm---Context, Features, and Control (cf., Rudy & O’Reilly, 1999). Pups in the Context (or Pre) group were exposed to the conditioning chamber during pre-exposure, while pups in the Features group were pre-exposed to single elements of the context on separate occasions, and pups in the Control (or No-Pre) group were pre-exposed to a context which shared no common elements with the conditioning chamber. The question of interest was whether pups would show evidence of contextual fear conditioning when preexposed to the conditioning context versus separable elements of the conditioning context. If context conditioning is based on conjunctive representations, then only the former group would show elevated freezing relative to the control group, whereas an elemental representation of the context would lead to elevated freezing in both groups, relative to controls.
Method
Subject were 15 male and 14 female offspring of 7 dams bred and reared as in the previous experiments. Pups were assigned to the following groups: Features (5 males, 4 females), Context (5 males, 5 females) and Control (5 males, 5 females). No more than one same-sex littermate was assigned to a given group. All subjects were 24 days old at the start of the CPFE protocol (exposure day).
The methods for Experiment 3 were as described in Experiment 1 for the apparatus and all handling of animals except that three pre-exposure contexts were created that consisted of one “element” of the conditioning context. Context A was the conditioning context, consisting of metal rods and clear Plexiglas walls situated in a spatial location. Context B only contained the rod element from context A, in which white paper was placed over the walls in order to preclude reliance upon distal cues. Additionally, Context B was situated in another room in order to separate the spatial location feature of the conditioning context. Context C only contained the transparent wall element of context A, in which mesh inserts were placed over the rod floor. Context C was also situated in another room. Context D was a clear mouse tub (7″ high, 12″ long, 8″ wide), which contained a mesh lid covering and was placed in the same position in the same room as Context A. Thus, Context D only shared the same spatial location feature of Context A. Lastly, Context E (the alternate context from Experiment 1 and 2) shared no features in common with Context A.
On the pre-exposure day, pups were pre-exposed successively to either Context A, E and E (Context group), Context B, C, and D, (order counterbalanced, Features group) or Context E, E and E (Control group). Each pre-exposure experience was 5-min in duration and separated by 30-min. Pups were run 4 at a time such that all 3 pre-exposure groups were included (Context, Features, and Control). Prior to each pre-exposure, training and testing session, pups were weighed, placed into individual opaque transport cages and were carted to a holding area across from the training room while the corresponding chamber (Context A, B, C, or D) was cleaned with 5% ammonium hydroxide solution as in Experiments 1 and 2.
During training, pups in the Context group were trained in the same chamber as the pre-exposure phase and pups in the Features group were trained in the same spatial location as the spatial location feature they encountered on the pre-exposure day. In order to ensure immediate delivery of the shock, pups were placed in the conditioning chamber one at a time. Following the immediate shock, pups were removed as quickly as possible and returned to their home cage.
Twenty-four hours following training, all pups were returned to the conditioning context for testing, as in Experiments 1 and 2.
Results & Discussion
One pup was excluded from analysis for procedural error, and 4 pups were excluded for meeting the criteria for a statistical outlier (± 2 standard deviations from the group mean). One outlier was from the Features group, 2 outliers were from the Context group and 1 was from the Control group.
Since there was no main effect or interaction of Sex (all p-values>.09), the data are shown collapsed across this variable (Figure 3). Only the Context (or Pre) group showed elevated freezing, indicating that pre-exposure to the separate features of the conditioning context did not enhance freezing during the testing phase. There was a main effect of group F(2, 35 = 11.28, p<.001) and post-hoc Newman-Keuls tests confirmed that pups pre-exposed to the conditioning context froze significantly more than pups in the other two groups (all p-values <.001), which did not differ significantly from each other.
Figure 3.
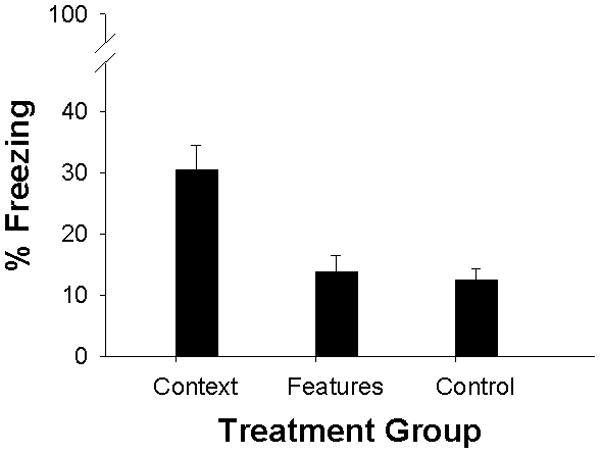
Mean percent freezing in Experiment 3 for Context (Pre), Features, and Control (No-Pre) groups. Bars represent standard error of the mean. Facilitation of conditioning was only observed in the Context (Pre) group, which encountered all features combined on PND24.
These results indicate that conditioning to the context is only evident when all features of the context are experienced together on the pre-exposure day in the CPFE paradigm. This accords with Rudy & O’Reilly’s (1999) finding that adolescent rats must form a conjunctive representation of the context in order to associate it with shock. The present results suggest that pups as young as PND 24 also form a conjunctive representation of the context.
Discussion
The ontogeny of the context pre-exposure facilitation effect (CPFE) was investigated in order to further examine the behavioral and developmental mechanisms of contextual fear learning. Experiment 1 found an absence of the CPFE in rats given context pre-exposure on PND 19 compared to pre-exposure on PND 21, suggesting that this form of context processing has not developed by PND 17 or 19 but is beginning to emerge around PND 21 in the rat. Experiment 2 indicated that the absence of the CPFE on PND 17 does not reflect inability to consolidate context learning established on the training day since a test of post-shock freezing on the training day failed to reveal contextual fear in this age group. Rather, it appears that PND 17 rats either cannot form the conjunctive representation on the preexposure day; or they cannot consolidate or retrieve contextual representations 24 hours later on PND 18; or they cannot associate these representations with shock during training. Experiment 3 determined that the CPFE required presentation of the combined features of the context on the pre-exposure day, indicating that context conditioning on PND 24 depends on conjunctive rather than elemental associations (Rudy, 2009).
Experiment 3 showed that combined exposure to the component features of context is necessary for CPFE performance when it first emerges during the weanling period. Because individual elements of the context experienced at varying times did not support the CPFE, it appears that the early ontogeny of contextual fear conditioning depends on conjunctive associations (Rudy, 2009). This is the first report to demonstrate that contextual cues are represented in a conjunctive manner in weanling rats. An earlier attempt to examine this issue with a context vs. features design found that context conditioning in both PND 18 and 23 rats was apparently based on elemental rather than conjunctive associations (Pugh & Rudy, 1996). The procedures in this study differed in important ways from those used here. First, the context was divided into two elements (wall color and floor texture) rather than the three elements used in this study. Second, some of these elements (black walls) are known to support feature-based fear conditioning in preweanling rats (Jagielo, Miller, Spear Smith, & Spear, 2003). Third, Pugh & Rudy (1996) exposed rats to the context for 120 sec on the training day prior to shock whereas training involved immediate shock in the present study. All of these procedural differences make fear conditioning based on feature-based associations more likely relative to the procedures used in the present study (see Schiffino et al., 2011, p. 196, for related discussion). When an immediate shock is used during training, the CPFE is thought to depend on the process of pattern completion which retrieves the conjunctive representation of context on the training day so that it can become associated with immediate shock (Rudy, 2009). Because pattern completion is a property of hippocampus-dependent conjunctive associations (Lee, Jerman, & Kesner, 2005; Fellini, Florian, Courtey & Roullet; Hoang & Kesner, 2008; Gold & Kesner, 2008; Iordanova, Burnett, Good & Honey, 2011; Squire, 1992), a feature-based CPFE should not be possible when training involves immediate shock (Rudy, 2009). The present findings support that assertion. The present findings resemble those obtained in PND 31 rats which showed a CPFE following preexposure to combined but not separate presentations of contextual features (Rudy & O’Reilly, 1999). In that study, the context was divided into 3 elements (as in the present study), but training consisted of shock delivered after 120-s of context exposure rather than immediate shock. Interestingly, the features group in that study showed a (nonsignificant) trend toward greater freezing relative to the non-preexposed control group (about 15 versus 9%, respectively). The absence of that trend in the present study supports the view that immediate shock reduces the role of feature-based associations in the CPFE. We previously reported that the CPFE established in PND 24 rats with the identical procedures employed here is disrupted by blockade of hippocampal NMDA-receptors on the preexposure day (Schiffino et al., 2011). The present findings confirm that context learning on the preexposure day depends on conjunctive associations. Together, these findings indicate that a form of context conditioning that is mediated by hippocampus-dependent conjunctive learning emerges during the weanling period in the rat.
In Experiment 2, a test of post-shock freezing revealed context conditioning in PND 24 but not PND 17 rats. Thus, PND 17 pups do not fail to show the CPFE because they can form a context-shock association on the training day but are unable to consolidate this association after training or retrieve it during testing. Rather, it appears that PND 17 pups are unable to acquire and/or consolidate contextual representations formed during the pre-exposure session or to retrieve and associate this representation with shock during the training session. Previous investigations implicate the amygdala in learning and consolidation of post-shock freezing (Wallace & Rosen, 2001). The fact that PND 18 rats show discrete-cue fear conditioning (e.g., Rudy, 1993) as well as post-shock freezing following a standard contextual fear conditioning trial (Rudy & Morledge, 1994) makes it unlikely that undeveloped associative functions of the amygdala account for the absence of post-shock freezing in the younger age group in the present study (cf. Moriceau, Roth & Sullivan, 2010, for further data on amygdala function in preweanling rats). To our knowledge, the present study is the first to use post-shock freezing as a measure of context fear following immediate shock in the CPFE paradigm. In contrast to post-shock freezing seen during a standard context conditioning trial, the freezing in the present study depends on the process of pattern completion. Given that immediate shock fails to produce contextual fear conditioning in the No-Pre control groups, the sensory/visual features that are experienced just prior to immediate shock onset do not generate representations that can be associated with shock (the immediate shock deficit, Fanselow, 1986). However, such representations are available to preexposed rats via the apparently rapid pattern completion process (discussed above). Thus, age differences in pattern completion may account for the findings of Experiment 2. It is also possible that age differences in forming or consolidating conjunctive representations on the pre-exposure day are important. Indeed, hippocampus-dependent consolidation in the CPFE is evident from studies showing an increase in conditioned freezing with an increase in retention interval from pre-exposure to training (Rudy & Wright-Hardesty, 2005). The present findings are also consistent with the view that there are differences between pre- and postweanling rats in consolidation of hippocampus-dependent, conjunctive representations of context (Rudy & Morledge, 1994).
The present study found that the CPFE is absent on PND 17 and 19, emerges modestly on PND 21, and is robust by PND 24 (Experiment 1). We previously reported that the CPFE does not get larger between PND 24 and 31 (Schiffino et al., 2011). The CPFE and standard contextual fear conditioning develop in parallel with each other (Raineki et al., 2010; Schiffino, et al., 2011) and with other spatial memory tasks (e.g., Rudy & Paylor, 1988; Rudy, Stadler-Morris, & Albert, 1987; Green & Stanton, 1989), suggesting that they engage common spatial cognitive functions of the hippocampus that emerge around weaning in the rat. However, these findings should not be taken as evidence that the hippocampus is not functional during the preweanling period. A number of tasks are impaired by hippocampal damage in PND 16–18 rats (see Stanton, 2000, for review). Indeed, hippocampal LTP, which is thought to underlie cognitive functions of the hippocampus, has been demonstrated two weeks after birth in CA1 and 3 weeks after birth in the dentate gyrus (Harris & Teyler, 1984; Bekenstein & Lothman, 1991). Moreover, hippocampal place fields have been demonstrated as early as PND16 (Langston, Ainge, Couey, Canto, Bjerknes, Witter, Moser, & Moser, 2010; Willis, Cacucci, Burgess & O’Keefe, 2010). The point in ontogeny when hippocampal function is expressed behaviorally depends on task parameters and on the development of other neurobehavioral systems that interact with the hippocampus (Stanton, 2000). Thus, in addition to hippocampal function, it is likely that interactions of the hippocampus with parahippocampal regions and/or the amygdala determine when contextual fear conditioning emerges during ontogeny (Schiffino et al., 2011).
Acknowledgments
The authors would like to thank Dr. Jeffrey B. Rosen for graciously providing access to the fear conditioning equipment used in these experiments. We would also like to thank William B. Schreiber and Lisa B. Dokovna for technical assistance. This work was supported by the University of Delaware and by NIH grant RO1-AA014288-01.
References
- Bekenstein JW, Lothman EW. An in vivo study of the ontogeny of long-term potentiation (LTP) in the CA1 region and in the dentate gyrus of the rat hippocampal formation. Brain Res Dev Brain Res. 1991;63(1–2):245–251. doi: 10.1016/0165-3806(91)90084-v. [DOI] [PubMed] [Google Scholar]
- Burman MA, Murawski NJ, Schiffino FL, Rosen JB, Stanton ME. Factors governing single-trial contextual fear conditioning in the weanling rat. Behav Neurosci. 2009;123(5):1148–1152. doi: 10.1037/a0016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas TC, Rudy JW. Development of the hippocampal memory system: Creating networks and modifiable synapses. In: Blumberg MS, Freeman JH Jr, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York, NY: Oxford University Press; 2010. pp. 587–606. [Google Scholar]
- Fanselow Associative vs topographical accounts of the immediate shock-freezing deficit in rats: Implications for the response selection rules governing species-specific defensive reactions. Learning & Motivation. 1986;17:16–39. [Google Scholar]
- Fanselow Factors governing one-trial contextual conditioning. Anim Learn Behav. 1990;18(3):264–270. [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110(1–2):73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. From contextual fear to a dynamic view of memory systems. Trends in Cognitive Sciences. 2010;14:7–17. doi: 10.1016/j.tics.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RJ, Stanton ME. Differential ontogeny of working memory and reference memory in the rat. Behav Neurosci. 1989;103(1):98–105. doi: 10.1037//0735-7044.103.1.98. [DOI] [PubMed] [Google Scholar]
- Gold AE, Kesner RP. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus. 2005;15(6):808–814. doi: 10.1002/hipo.20103. [DOI] [PubMed] [Google Scholar]
- Harris KM, Teyler TJ. Developmental onset of long-term potentiation in area CA1 of the rat hippocampus. J Physiol. 1984;346:27–48. doi: 10.1113/jphysiol.1984.sp015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang LT, Kesner RP. Dorsal hippocampus, CA3, and CA1 lesions disrupt temporal sequence completion. Behav Neurosci. 2008;122(1):9–15. doi: 10.1037/0735-7044.122.1.9. [DOI] [PubMed] [Google Scholar]
- Iordanova MD, Burnett DJ, Good M, Honey RC. Pattern memory involves both elemental and configural processes: Evidence from the effects of hippocampal lesions. Behav Neurosci. doi: 10.1037/a0023762. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS, DeCola JP, Landeira-Fernandez J. Selective impairment of long-term but not short-term conditional fear by the N-methyl-D-aspartate antagonist APV. Behav Neurosci. 1992;106:591–596. doi: 10.1037//0735-7044.106.4.591. [DOI] [PubMed] [Google Scholar]
- Langston RF, Ainge JA, Couey JJ, Canto CB, Bjerknes TL, Witter MP, Moser EI, Moser MB. Development of the spatial representation system in the rat. Science. 2010;328(5985):1576–1580. doi: 10.1126/science.1188210. [DOI] [PubMed] [Google Scholar]
- Lee I, Jerman TS, Kesner RP. Disruption of delayed memory for a sequence of spatial locations following CA1- or CA3-lesions of the dorsal hippocampus. Neurobiol Learn Mem. 2005;84(2):138–147. doi: 10.1016/j.nlm.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88(2):261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24(10):2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Sullivan RM. Rodent model of infant attachment learning and stress. Developmental Psychobiology. 2010;52:651–660. doi: 10.1002/dev.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. New York: Oxford University Press; 1978. [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Rudy JW. A developmental analysis of contextual fear conditioning. Dev Psychobiol. 1996;29(2):87–100. doi: 10.1002/(SICI)1098-2302(199603)29:2<87::AID-DEV1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Raineki C, Holman PJ, Debiec J, Bugg M, Beasley A, Sullivan RM. Functional emergence of the hippocampus in context fear learning in infant rats. Hippocampus. 2010;20(9):1037–1046. doi: 10.1002/hipo.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behav Neurosci. 1993;107(5):887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal- hippocampal system. Learning & Memory. 2009;16(10):573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav Neurosci. 1994;108(2):227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav Neurosci. 1999;113(5):867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn Affect Behav Neurosci. 2001;1(1):66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O’Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116(4):530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28(7):675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Paylor R. Development of interocular equivalence of place learning in the rat requires convergence sites established prior to training. Behav Neurosci. 1987;101(5):732–734. doi: 10.1037//0735-7044.101.5.732. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Wright-Hardesty K. The temporal dynamics of retention of a context memory: something is missing. Learn Mem. 2005;12(2):172–177. doi: 10.1101/lm.84005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiol Learn Mem. 2011;95(2):190–198. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME. Multiple memory systems, development and conditioning. Behav Brain Res. 2000;110(1–2):25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, DiScenna P. The hippocampal memory indexing theory. Behav Neurosci. 1986;100:147–154. doi: 10.1037//0735-7044.100.2.147. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, Rudy JW. The hippocampal indexing theory and episodic memory: Updating the index. Hippocampus. 2007;17:1158–1169. doi: 10.1002/hipo.20350. [DOI] [PubMed] [Google Scholar]
- Wallace KJ, Rosen JB. Neurotoxic lesions of the lateral nucleus of the amygdala decrease conditioned fear but not unconditioned fear of a predator odor: comparison with electrolytic lesions. J Neurosci. 2001;21(10):3619–3627. doi: 10.1523/JNEUROSCI.21-10-03619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26(20):5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


