Abstract
Protein tyrosine kinase-7 (PTK7) was recently identified as a surface protein expressed on hematopoietic cells. To determine if PTK7 is a useful biomarker in clinical practice for acute leukemia immunophenotyping and detection, we examined the PTK7 expression in human bone marrow and thymic specimens. Our results show that PTK7 expression in normal thymic T cells is tightly regulated during the maturational process, but in T cell acute lymphoblastic leukemia (T-ALL) the expected temporal relationship of expression between PTK7 and other maturational T cell markers is lost or disrupted. In addition, nearly all T-ALL cases expressed higher PTK7 levels than mature T cells in the human bone marrow specimens. Therefore, in conjunction with other T cell markers, PTK7 has utility as a biomarker for detecting minimal residual disease of T-ALL in the bone marrow.
Keywords: acute myelogenous leukemia, T cell acute lymphoblastic leukemia, aptamer, PTK7, and flow cytometry
Introduction
Cancers behave differently due to differences at the molecular levels. Development of new molecular biomarkers to detect tumor cells can enhance diagnosis and tailor personalized individual therapy. To facilitate the discovery of potential new biomarkers, a cell-based method of Systematic Evolution of Ligands by Exponential enrichment (SELEX) was developed to use live tumor cells as targets to select specific DNA aptamer probes (1-3). In previous studies, we used cultured T-ALL cells as targets to develop specific DNA aptamer probes. One of the selected DNA aptamer probes, Sgc8, was further labeled as a probe to enrich and to identify its target membrane protein, PTK7 (4).
PTK7, also known as colon carcinoma kinase-4 (CCK-4), is a receptor protein tyrosine kinase-like molecule that contains a catalytically inactive tyrosine kinase domain (5). PTK7 was first identified in melanocytes (6, 7), and was shown to have high expression levels in several tumors, including colon, gastric and lung cancers (8-11). PTK7 was found to play a role in regulating neural development and planar cell polarity in vertebrates (12, 13) and in morphogenetic cell movements during embryonic development (14-16). PTK7-deficient cells exhibited weakened β-catenin/T-cell factor transcriptional activity on Wnt3 stimulation, indicating that PTK7 acts as an important conserved modulator of multiple Wnt pathways in normal and possibly pathological conditions, including cancer (14, 17-19).
Recently, PTK7 expression has been identified in normal hematopoietic and acute myelogenous leukemia (AML) cells, and as an independent prognostic survival factor in AML patients treated with induction chemotherapy (20). In this study, we examined PTK7 expression levels in bone marrow hematopoietic cells, immature thymic T cells, acute lymphoblastic leukemia (ALL) cells, and AML cells to explore whether PTK7 has biomarker potential for immunophenotyping maturing thymic T cells and T-ALL cells, and for T-ALL minimal residual disease detection after chemotherapy.
Materials and methods
Sample collection and initial preparation
All clinical samples were submitted for pathological evaluation to the Shands Hospital Hematopathology Laboratory, University of Florida (UF), Gainesville, Florida, United States (USA). The studies were approved by the UF Institutional Review Board. The presented data includes nine cases of precursor B cell acute lymphoblastic leukemia (B-ALL), twenty-three cases of precursor T-ALL, and nineteen cases of AML. Fourteen cases of non-malignant human bone marrow and ten cases of normal thymi were also used for the studies. For comparison of PTK7 levels in non-treated and treated T-ALL cases, all post-therapy data were collected after bone marrow recovery, which ranged from 4 weeks to one year after therapy.
Bone marrow and peripheral blood samples were received in EDTA. Thymic or lymphoid tissues were received fresh, and cell suspensions were prepared according to established protocols by mincing the tissue with scalpels in RPMI media. Erythrocytes in all specimens were lysed by incubating with lysing solution (15 mM NH4Cl, 1.0 mM KHCO3, 10 mM EDTA, and pH 7.4) for 10 min at room temperature. The cells were then resuspended in phosphate-buffered saline with 0.1% NaN3 (PBS), and washed twice in PBS.
Cell surface staining, data acquisition and data analysis
The fluorochromes used include allophycocyanin (APC), fluorescein isothiocyanate (FITC), phycoerythrin (PE), and peridinin chlorophyll protein (PerCP). The Anti-PTK7-PE antibody (clone 188B, Miltenyi Biotec, Germany) was used for the determination of PTK7 expression levels in combination with other fluorochrome-conjugated monoclonal antibodies in the following combinations (FITC-PE-PERCP-APC): CD10-PTK7-CD45-CD19, CD1A-PTK7-CD3-CD7, CD1A-PTK7-CD4-CD8, CD1A-PTK7 CD2-CD7, CD1A-PTK7-CD5-CD3, CD11b-PTK7-CD45-CD34, and CD34-PTK7-CD45-CD117. PTK7-APC was added in later for detecting T-ALL-MRD in order to make panels according to phenotypes of individual T-ALL cases. The commonly used panels for T-ALL detection are CD4-CD8-CD3-CD7, CD5-CD7-CD45-CD3, CD2/CD5-CD7-CD123-PTK7 and CD1a-CD7-CD45-CD117/CD34. The anti-PTK7 antibodies have been tittered to optimize signal-to-noise ratio. In addition, we used many other antibodies (CD34, CD117, CD13, CD11b, CD33, HLA-DR, CD11c, CD38, CD19, CD20, CD10, CD23, CD1a, CD2, CD3, CD5, CD4, CD8, CD7, CD56, CD57, CD15, CD14 or CD64) in our clinical panels to aid identify different cell populations.
Cells (3 × 105) were incubated with fluorophore-conjugated antibodies in 200 μl of Hanks balanced salt solution (Mediatech Cellgro, Herndon, Virginia, USA) with 5% human AB serum (Bio-Whittaker, Walkersville, Maryland, USA) for 15 minutes on ice without light exposure. Subsequently, the cells were washed twice with PBS by centrifugation at 500g for 5 minutes and resuspended in a final volume of 250 μL of PBS. Four-color Flow cytometry was performed using a FACScalibur flow cytometer (Becton Dickinson [BD], San Jose, California, USA) equipped with a 488-nm argon laser, a 635-nm diode laser, and CellQuest software (BD). Thirty-thousand events were collected for thymic or lymphoid tissue specimens, and 300 000 events were collected for bone marrow or peripheral blood specimens. Daily calibration of the instrument was performed using standardized CaliBRITE Beads (BD) with FACSComp Software (BD).
The data analysis was performed using FCS Express software (De Novo Software, Los Angeles, California, USA, http://www.denovosoftware.com/site/FCSExpress.shtml). Initial cell subpopulations were established using the levels of CD45 expression and side-scatter (SSC) properties (21, 22). For normal bone marrow, different subpopulations were identified based on their surface immunophenotype and light scatter properties as follows: immature B cells were defined as the CD19+/CD10+, dim CD20+ or CD20−, and dim CD45+ population, the myeloblasts as the CD34+ and CD117+ population; the monocytes (mature and immature) as the CD64+, CD14+, bright CD45+ with intermediate SSC population, granulocytes (mature and immature) as CD15+, CD34−/CD117− with high SSC population, and the mature lymphocytes as the CD20+ or CD3+, bright CD45+ with low SSC population. The leukemic cells in clinical specimens were defined according their immunophenotypes generated for clinical diagnosis, through panels containing four-color combinations of 27-35 antibodies covering multiple T, B or myeloid antigens. The geometric mean fluorescence intensity (MFI) and standard deviation (SD) of PTK7 or isotype controls were determined for individually gated subpopulations.
For mixing studies, we spiked variable numbers of T-ALL cells into normal human bone marrow samples to assess whether PTK7 can aid the detection of T-ALL cells in a background of normal bone marrow cells. Antibodies against PTK7, T cell antigens, CD45, CD123 and CD34 are used for flow cytometry studies and 500 000 events are collected.
Statistic analysis
GraphPad Software (San Diego, California, the United State) was used for statistical analyses. The t test and One-way Analysis of Variance (ANOVA) test was used to compare PTK7 levels of the different cell populations and corresponding isotype controls. The paired t test was used for the comparison of PTK7 levels before and after therapy. Unless stated otherwise, results are given as mean ± SD.
Results
Expression of PTK7 by maturing hematopoietic cells and acute leukemia cells
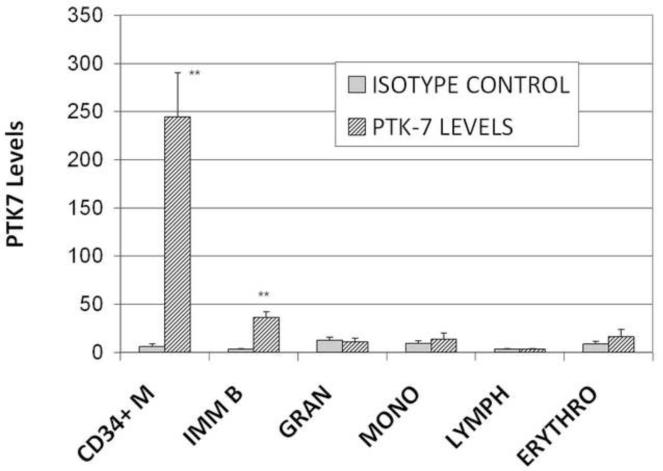
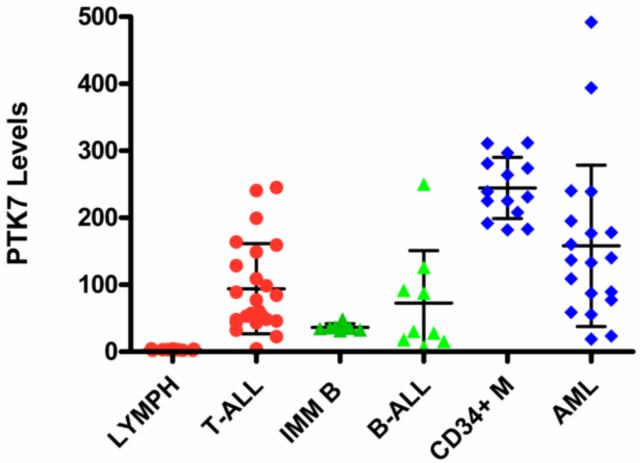
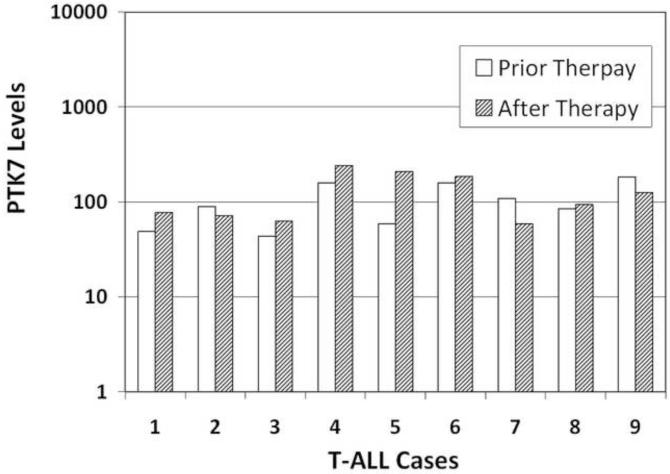
To determine if PTK7 can be used as a biomarker for immunophenotyping immature T cells or acute leukemia, we first determined if PTK7 is selectively expressed in different human bone marrow cell populations. Using flow cytometry and fluorescence-conjugated antibodies, we were able to separate the bone marrow cells into multiple subpopulations (CD34+ myeloblasts, maturing erythroid precursors, maturing granulocytes, maturing monocytes, immature B cells and mature lymphocytes), and were able to determine the PTK7 expression levels of each subpopulation. As shown in Figure 1, PTK7 expression was primarily seen on CD34+/CD117+ early myeloid progenitor cells and to lesser extent on early immature B cells. The maturing erythroid, granulocytic, monocytic, mature B- and T-cells did not show significant expression of PTK7, indicating that the expression of PTK7 is markedly decreased during maturation. Subsequently, we compared the levels of PTK7 expression in the three major types of acute leukemia cases and their normal counterparts in human bone marrow. Variable levels of PTK7 expression were noted in all three major types of acute leukemia: B-ALL, T-ALL and AML cases (Figure 2) and it is difficult to use PTK7 expression levels to differentiate B-ALL or AML cells from their normal counterparts, normal immature B precursors or CD34+ myeloid precursors, respectively. However, nearly all of the studied T-ALL cases had significantly higher PTK7 expression levels than those of normal mature T cells in bone marrow (P<0.01) (Figure 2). Notably, immature T cell precursors with detectable PTK7+ were not identified in the normal human bone marrow specimens. In addition, we compared PTK7 expression in non-treated and treated T-ALL cases, and the PTK7 levels of T-ALL cells following therapy were not changed significantly (Figure 3).
Figure 1.
PTK7 expression in non-malignant human bone marrow cells. The MFI and SD of PTK7 are shown for the individual cell populations. CD34+M: CD34+ myeloblasts; IMM B: immature B cells; GRAN: granulocytes; MONO: monocytes; LYMPH: mature lymphocytes; and ERYTHRO: nucleated erythroid precursors. Error bars indicate SD; ** P<0.01
Figure 2.
Comparison of PTK7 expression in mature lymphocytes, non-malignant precursor cells and acute leukemia cells. The PTK7 fluorescence levels for subpopulations of individual cases are illustrated. LYMPH: mature lymphocytes; CD34+M: CD34+ myeloblasts; and IMM B: immature B cells. Error bars indicate SD; * P<0.05; **P<0.01.
Figure 3.
Comparison of PTK7 levels in T-ALL cases before and after therapy. The PTK7 MFI of individual cases is illustrated. The paired t-test was used for the comparison of PTK7 levels before and after therapy. The two-tailed P value is 0.3588, considered not significant.
Expression of PTK7 by normal human thymic immature T precursors and T-ALL cells
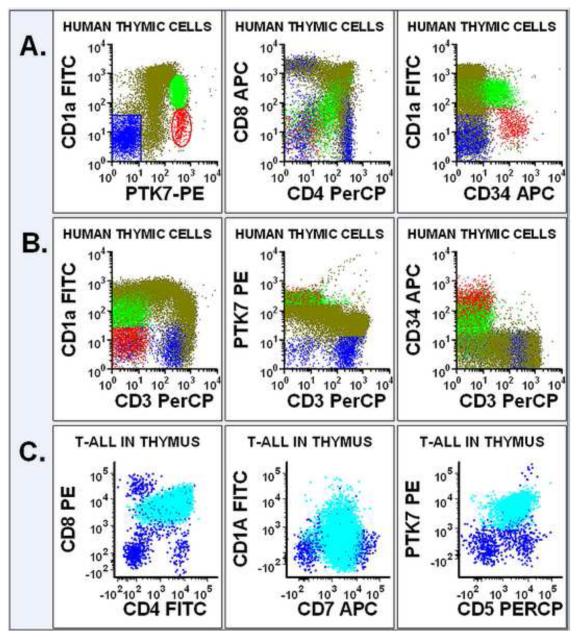
Due to the fact that virtually all of the T-ALL cases studied invariably expressed PTK7, we examined the PTK7 expression of immature T cells in normal human thymus. Figure 4A illustrates immature human T cells at different maturational stages in a typical fresh thymic specimen. The normal mature and immature T cells are well separated in the histogram plot of PTK7 vs. CD1a (Figure 4A). The immature T cells in the early stage of maturation express relatively high levels of PTK7, CD34, and dim CD1a without expression of CD4 or CD8 (red dots in Figure 4A). The immature T cells in the mid-stage of maturation show relatively consistent levels of PTK7 with decreased levels of CD34 and increased levels of CD1a, CD4 and CD8 (green dots in Figure 4A). The immature T cells in the late stage of maturation express PTK7, CD4 and CD8 with decreasing levels of CD1a (Olive dot in Figure 4A). During the process of T cell maturation, the immature T cells gradually gained CD3 and lost CD1a and PTK7 (Figure 4B)
Figure 4.
PTK7 Expression in non-malignant immature thymic T cells and T-ALL cells. (A): The non-malignant thymic T cells are separated according to CD1a and PTK7 levels into early stage (red), mid stage (green), late stage (olive) and mature T cells (blue). The expression of CD4, CD8 and CD34 of T cells each stage is also shown. (B): The temporal relationship between the expression levels of CD3, CD1a, PTK7 and CD34. (C): The immunophenotype of a representative case of T-ALL cells (cyanide) arising in thymus is shown.
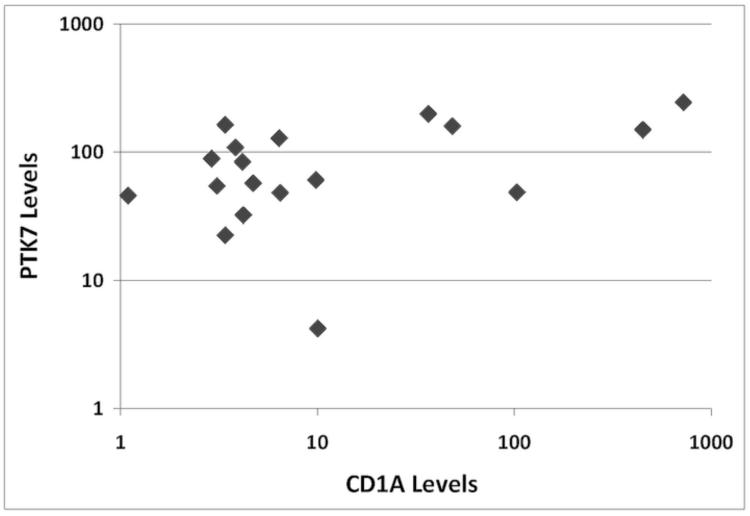
As shown in Figure 4C, T-ALL arising in the thymus had different expression patterns of PTK7, CD1a and T cell antigens, The levels and/or temporal relationship of PTK7, CD1a, or T cell antigen expression as seen in normal maturing thymic T cells is lost in T-ALL cells. We compared the expression of PTK7 and CD1a in eighteen T-ALL cases. Almost all T-ALL cases expressed PTK7; however, only a subset of the T-ALL cases expressed significant levels of CD1a (Figure 5). Thus, the differential expression patterns formed by PTK7 and CD1a or other T cell antigens will not only be valuable for the evaluation of the T cell maturation process, but also can be used to differentiate non-malignant immature T cells from T-ALL cells.
Figure 5.
Comparison of PTK7 levels and CD1a expression in T-ALL cases. The leukemia cells were selectively gated according to their immunophenotype generated by clinical flow cytometric analysis. The MFIs of PTK7 or CD1a expression of individual T-ALL cases are illustrated.
Using PTK7 as a biomarker to aid detection of T-ALL in clinical specimens
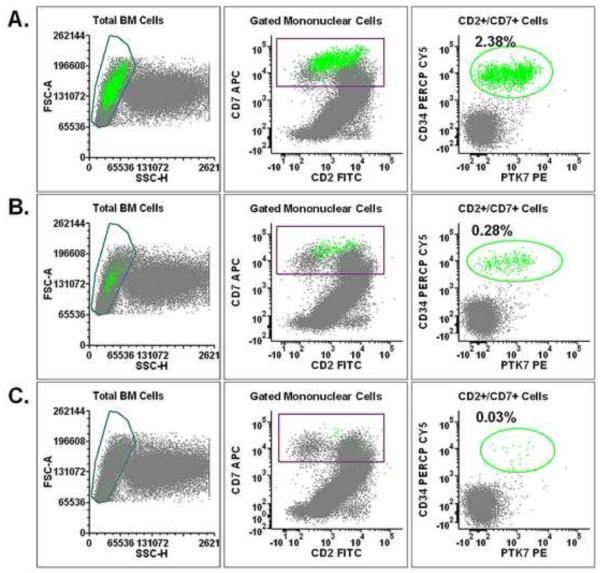
PTK7 may potentially be used as a biomarker for detecting T-ALL cells in bone marrow specimens and thymic tissues for two reasons: 1) A significant number of immature T cells is not detected in human bone marrow specimens by routine clinical flow cytometry analysis, and 2) PTK7 is more consistently expressed in immature T cells of different maturational stages than CD34 or CD1a. To assess whether PTK7 can be used in flow cytometry panels to detect a low frequency of T-ALL cells, we spiked variable numbers of T-ALL cells into normal human bone marrow specimens, and detected T-ALL cells against the background of normal bone marrow cells. As illustrated in Figure 6, a panel of monoclonal antibodies including PTK7 can detect as low as 0.03% T-ALL cells. As increasing numbers of T-ALL cells were added, tight clusters of T-ALL cells can be easily identified via the sequential gating of mononuclear cells, T cells, and CD34+/PTK+ cells (Figure 6).
Figure 6.
Detection of T-ALL cells spiked into normal bone marrow cells. T-ALL cells are mixed into normal human bone marrow cells to final concentrations of 3%, 0.3% and 0.03%. T-ALL cells (green) are identified from the background normal cells by sequential gating on mononuclear cells, T cells and CD34+/PTK7+ cells.
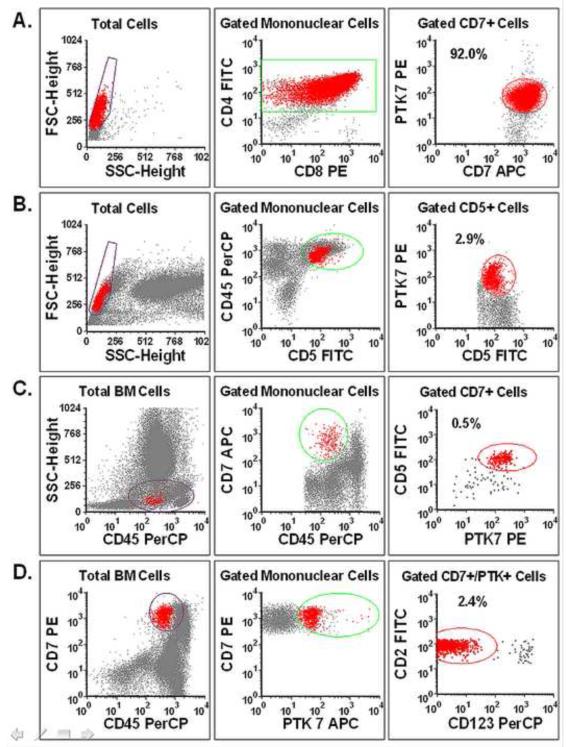
Following chemotherapy, we used PTK7 and other T cells markers in flow cytometry panels to detect residual T-ALL cells in patients’ clinical specimens. The histograms in Figure 7 illustrate three examples utilizing PTK7 and other available surface markers for residual T-ALL detection. In combination with other T cell markers, PTK7 helps separate immature T cells from normal mature T cells and other cell populations before (Figure 7A) and after therapy (Figure 7B). It is necessary to use PTK7+ in combination with antibodies for T cell antigens in order to demonstrate the aberrant expression of PTK7 or T cell antigens. For instance, the CD45 expression of the PTK7+/CD5+ cells (red) is much lower than that of mature T cells (blue) (Figure 7C). Also, CD123 may be used to exclude PTK7+ dendritic cells because the plasmacytoid dendritic cells express high levels of CD123 (23) and a small number of CD123+ plasmacytoid dendritic cells in bone marrow may express PTK7 with dim CD7 (Figure 7D).
Figure 7.
Using PTK7 as a biomarker for immunophenotyping and detecting T-ALL in human bone marrow specimens. T-ALL cells in all of the histograms are identified and shown as red dots. (A) and (B) show a representative case of T-ALL, (A): Before therapy and (B): After therapy. (C) and (D) show residual T-ALL cell detection in the bone marrow specimens of two other representative cases. Because the expression of T cell antigens differs in individual T-ALL cases, different immunophenotypic panels and gating strategies were applied to detect the residual T-ALL cells.
Discussion
PTK7 was initially identified in non-hematopoietic cells, and it was shown to regulate cell migration and planar cell polarity (12, 13). Increasing evidence suggests the role of the planar cell polarity receptor PTK7 in hematologic diseases (20, 24, 25). We were incidentally led to PTK7 when it was identified as a target protein of the Sgc8 DNA aptamer that was selected, to recognize a T-ALL cell line (CCRF-CEM) (4). A recently published study showed PTK7 was expressed in more than two-thirds of AMLs, and its expression was correlated with myeloid lineage differentiation. In addition, PTK7 expression was correlated with increased resistance to apoptosis in leukemic cell lines and primary AML blasts (20). In the current study, we compared PTK7 expression levels in normal bone marrow precursors and acute leukemia cells of all three lineages (AML, B-ALL and T-ALL). In addition to confirming earlier results by other investigators (20), we demonstrated that PTK7 was expressed in early immature B precursors in human bone marrow and in immature T cells in the thymus. We have also shown that the expression of PTK7 in B-ALL and AML cases varied significantly.
Even though PTK7 is a protein marker that is found in multiple subpopulations of hematopoietic cells, it can still be a useful biomarker in clinical practice. There are few biomarkers that are exclusively expressed in a single type of hematopoietic cells and that can be used to distinguish leukemic cells from their normal counterparts. In clinical practice, we often rely on recognizable patterns of surface protein expression and the temporal relationships between multiple biomarkers to build windows to distinguish leukemic cells from normal cells. For instance, most flow cytometry laboratories use the combination of CD38, CD56 and CD45 to detect neoplastic plasma cells in myeloma patients, despite that CD56 is a typical marker used for NK cells. Neither CD38 nor CD56 are specific for bone marrow cells, but the majority of neoplastic plasma cells display coexpression of CD38 and CD56. Therefore, CD56, a commonly used NK cell marker, can be used for the detection of myeloma cells in clinical practice in conjunction with other markers.
Since PTK7 is expressed in normal bone marrow cell subpopulations (early B cell and myeloid precursors), it is difficult to use PTK7 as a biomarker to separate leukemic cells of B-ALL or AML from normal bone marrow precursors unless the leukemic cells show markedly increased or decreased levels of PTK7. However, the situation is quite different in the setting of T-ALL. Although the early T cell precursors are thought to be present in bone marrow before they migrate into thymus, PTK7+ immature T cells are virtually non-detectable in human bone marrow specimens at the level of clinical flow cytometry studies. In addition, regardless of their origin (e.g. blood, bone marrow, or thymus), leukemic cells from nearly all T-ALL cases expressed higher levels of PTK7 than those of mature T cells in the human bone marrow specimens, and the PTK7 levels of T-ALL cells were not changed significantly after chemotherapy. Thus, the differential expression of PTK7 on T-ALL and bone marrow mature T cells creates a window for us to use combination panels, including PTK7 and T cell markers, to separate the T-ALL cells from normal bone marrow cells. This was supported by the mixing studies we performed by spiking different numbers of T-ALL cells into normal human bone marrow samples (Figure 4). In these studies, the analysis of PTK7 expression aided us to detect low levels of T-ALL cells in a background of normal bone marrow cells. Under the same principle, one may use CD1a, CD34, and other T cells antigens to detect T-ALL cells in bone marrow specimens. Unfortunately, we often see in our clinical practice that many T-ALL cases lack expression of immature T cell markers, such as CD1a, CD34 or CD117. In addition, we also demonstrated the PTK7 expression of immature thymic T cells and its temporal relationship with CD34, CD1a, and T cell antigens. PTK7 is expressed by very early precursor T cells (CD34+, CD4− and CD8−) and cortical immature T cells (CD34−, CD4+ and CD8+). In our clinical practice, knowing the temporal relationship between PTK7 and CD1a or other T cells antigens during T cell maturation helps to differentiate T-ALL cells from non-malignant immature thymic T cells in thymic specimens. Thus, PTK7 is a useful marker that enhances our detection of T-ALL minimal residual disease.
It should be noted that the detection of minimal residual disease of acute leukemia is still a challenging process in clinical practice because aberrant antigens may be expressed differently in each leukemia case. Most importantly, the development of new biomarkers creates an opportunity for us to distinguish leukemic cells from normal cells, aiding leukemia diagnosis, prognosis, follow-up, and the eventual evolution into targeted therapy.
Acknowledgments
This work was supported by a grant of the National Institutes of Health (CA129311) to Y.L..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Authorship
Contribution: G.J., designed, performed research experiments and analyzed the data; M.Z., B.Y and M.Y., performed research experiments; S.A. and C.C., performed data analysis and interpretation, B.L., performed data analysis drafting the paper; Y.L., designed research experiments, analyzed and interpreted the data, and wrote the paper.
Reference List
- (1).Shangguan D, Li Y, Tang Z, Cao ZC, Chen HW, Mallikaratchy P, et al. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc Natl Acad Sci U S A. 2006 Aug 8;103(32):11838–43. doi: 10.1073/pnas.0602615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Cerchia L, Giangrande PH, McNamara JO, de F V. Cell-specific aptamers for targeted therapies. Methods Mol Biol. 2009;535:59–78. doi: 10.1007/978-1-59745-557-2_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Shangguan D, Cao ZC, Li Y, Tan W. Aptamers evolved from cultured cancer cells reveal molecular differences of cancer cells in patient samples. Clin Chem. 2007 Jun;53(6):1153–5. doi: 10.1373/clinchem.2006.083246. [DOI] [PubMed] [Google Scholar]
- (4).Shangguan D, Cao Z, Meng L, Mallikaratchy P, Sefah K, Wang H, et al. Cell-specific aptamer probes for membrane protein elucidation in cancer cells. J Proteome Res. 2008 May;7(5):2133–9. doi: 10.1021/pr700894d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Boudeau J, Miranda-Saavedra D, Barton GJ, Alessi DR. Emerging roles of pseudokinases. Trends Cell Biol. 2006 Sep;16(9):443–52. doi: 10.1016/j.tcb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- (6).Park SK, Lee HS, Lee ST. Characterization of the human full-length PTK7 cDNA encoding a receptor protein tyrosine kinase-like molecule closely related to chick KLG. J Biochem. 1996 Feb;119(2):235–9. doi: 10.1093/oxfordjournals.jbchem.a021228. [DOI] [PubMed] [Google Scholar]
- (7).Lee ST, Strunk KM, Spritz RA. A survey of protein tyrosine kinase mRNAs expressed in normal human melanocytes. Oncogene. 1993 Dec;8(12):3403–10. [PubMed] [Google Scholar]
- (8).Mossie K, Jallal B, Alves F, Sures I, Plowman GD, Ullrich A. Colon carcinoma kinase-4 defines a new subclass of the receptor tyrosine kinase family. Oncogene. 1995 Nov 16;11(10):2179–84. [PubMed] [Google Scholar]
- (9).Saha S, Bardelli A, Buckhaults P, Velculescu VE, Rago C, St CB, et al. A phosphatase associated with metastasis of colorectal cancer. Science. 2001 Nov 9;294(5545):1343–6. doi: 10.1126/science.1065817. [DOI] [PubMed] [Google Scholar]
- (10).Gorringe KL, Boussioutas A, Bowtell DD. Novel regions of chromosomal amplification at p21, 5p13, and 12q14 in gastric cancer identified by array comparative genomic hybridization. Genes Chromosomes Cancer. 2005 Mar;42(3):247–59. doi: 10.1002/gcc.20136. [DOI] [PubMed] [Google Scholar]
- (11).Endoh H, Tomida S, Yatabe Y, Konishi H, Osada H, Tajima K, et al. Prognostic model of pulmonary adenocarcinoma by expression profiling of eight genes as determined by quantitative real-time reverse transcriptase polymerase chain reaction. J Clin Oncol. 2004 Mar 1;22(5):811–9. doi: 10.1200/JCO.2004.04.109. [DOI] [PubMed] [Google Scholar]
- (12).Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004 Jul 1;430(6995):93–8. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- (13).Shnitsar I, Borchers A. PTK7 recruits dsh to regulate neural crest migration. Development. 2008 Dec;135(24):4015–24. doi: 10.1242/dev.023556. [DOI] [PubMed] [Google Scholar]
- (14).Peradziryi H, Tolwinski NS, Borchers A. The many roles of PTK7: A versatile regulator of cell-cell communication. Arch Biochem Biophys. 2012 Jan 3; doi: 10.1016/j.abb.2011.12.019. [DOI] [PubMed] [Google Scholar]
- (15).Peradziryi H, Kaplan NA, Podleschny M, Liu X, Wehner P, Borchers A, et al. PTK7/Otk interacts with Wnts and inhibits canonical Wnt signalling. EMBO J. 2011 Sep 14;30(18):3729–40. doi: 10.1038/emboj.2011.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wagner G, Peradziryi H, Wehner P, Borchers A. PlexinA1 interacts with PTK7 and is required for neural crest migration. Biochem Biophys Res Commun. 2010 Nov 12;402(2):402–7. doi: 10.1016/j.bbrc.2010.10.044. [DOI] [PubMed] [Google Scholar]
- (17).Puppo F, Thome V, Lhoumeau AC, Cibois M, Gangar A, Lembo F, et al. Protein tyrosine kinase 7 has a conserved role in Wnt/beta-catenin canonical signalling. EMBO Rep. 2011 Jan;12(1):43–9. doi: 10.1038/embor.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Golubkov VS, Chekanov AV, Cieplak P, Aleshin AE, Chernov AV, Zhu W, et al. The Wnt/planar cell polarity protein-tyrosine kinase-7 (PTK7) is a highly efficient proteolytic target of membrane type-1 matrix metalloproteinase: implications in cancer and embryogenesis. J Biol Chem. 2010 Nov 12;285(46):35740–9. doi: 10.1074/jbc.M110.165159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Golubkov VS, Aleshin AE, Strongin AY. Potential relation of aberrant proteolysis of human protein tyrosine kinase 7 (PTK7) chuzhoi by membrane type 1 matrix metalloproteinase (MT1-MMP) to congenital defects. J Biol Chem. 2011 Jun 10;286(23):20970–6. doi: 10.1074/jbc.M111.237669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Prebet T, Lhoumeau AC, Arnoulet C, Aulas A, Marchetto S, Audebert S, et al. The cell polarity PTK7 receptor acts as a modulator of the chemotherapeutic response in acute myeloid leukemia and impairs clinical outcome. Blood. 2010 Sep 30;116(13):2315–23. doi: 10.1182/blood-2010-01-262352. [DOI] [PubMed] [Google Scholar]
- (21).Borowitz MJ, Guenther KL, Shults KE, Stelzer GT. Immunophenotyping of acute leukemia by flow cytometric analysis. Use of CD45 and right-angle light scatter to gate on leukemic blasts in three-color analysis. Am J Clin Pathol. 1993 Nov;100(5):534–40. doi: 10.1093/ajcp/100.5.534. [DOI] [PubMed] [Google Scholar]
- (22).Nicholson JK, Hubbard M, Jones BM. Use of CD45 fluorescence and side-scatter characteristics for gating lymphocytes when using the whole blood lysis procedure and flow cytometry. Cytometry. 1996 Mar 15;26(1):16–21. doi: 10.1002/(SICI)1097-0320(19960315)26:1<16::AID-CYTO3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- (23).Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000 Dec 1;165(11):6037–46. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- (24).Lhoumeau AC, Puppo F, Prebet T, Kodjabachian L, Borg JP. PTK7: a cell polarity receptor with multiple facets. Cell Cycle. 2011 Apr 15;10(8):1233–6. doi: 10.4161/cc.10.8.15368. [DOI] [PubMed] [Google Scholar]
- (25).Haines CJ, Giffon TD, Lu LS, Lu X, Tessier-Lavigne M, Ross DT, et al. Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J Exp Med. 2009 Feb 16;206(2):275–85. doi: 10.1084/jem.20080996. [DOI] [PMC free article] [PubMed] [Google Scholar]