Abstract
An efficient method based on a rapid condensation reaction between 2–cyanobenzothiazole (CBT) and cysteine has been developed for 18F–labeling of N–terminal cysteine–bearing peptides and proteins. An 18F–labeled dimeric cRGD ([18F]CBTRGD2) has been synthesized with an excellent radiochemical yield (92% based on radio–HPLC conversion, 80% decay–corrected and isolated yield) and radiochemical purity (>99%) under mild conditions using 18F–CBT, and shown good in vivo tumor targeting efficiency for PET imaging. The labeling strategy was also applied to the site–specific 18F–labeling of a protein, Renilla lucifierase (RLuc8) with a cysteine residue at its N–terminus. The protein labeling was achieved with 12% of decay–corrected radiochemical yield and more than 99% radiochemical purity. This strategy should provide a general approach for an efficient and site–specific 18F–labeling of various peptides and proteins for in vivo molecular imaging applications.
INTRODUCTION
Positron emission tomography (PET) is a non–invasive molecular imaging technique with excellent sensitivity.1 In the past two decades, various biomolecules have been radiolabeled for PET imaging studies of receptor activity in living subjects and for disease diagnostics including tumor detection.2–5 As fluorine–18 (18F) can be easily produced in high quantities on a medical cyclotron and has an ideal half–life of 110 min for imaging subjects, it is one of commonly–used radioisotopes for producing many PET tracers. Direct 18F–fluorination however, requires harsh reaction conditions such as high temperature, pH and harmful reagent (e.g. fluorine gas), that are not suitable for most biomolecules. Therefore, an indirect 18F–labeling strategy is generally applied where the 18F is first introduced into a small organic molecule (so called 18F–prosthetic group) followed by subsequent coupling to a specific functional group (i.e., –NH2, –CO2H or –SH) in the biomolecules. Well–established 18F prosthetic groups include: 1) 18F–labeled benzaldehyde for labeling aminooxy groups via formation of an oxime bond;6–7 2) 18F–labeled activated ester and maleimide for labeling amino and thiol groups, respectively;8–11 3) [18F]fluoroazide and [18F]fluoroalkyne that can react with a biomolecule equipped with an alkyne and an azide group respectively through copper–catalyzed click chemistry.12–16 In many cases, originally designed 18F–prosthetic groups require lengthy synthetic procedures, relatively harsh reaction conditions, or difficult remote controls, which often result in poor radiochemical yield (RCY) and difficulty in purification. Recently, to overcome these problems, fast and specific ligation methods such as copper–free click reaction17–18 and the [4+2] inverse electron demand Diels–Alder reaction between tetrazine structure and trans–cyclooctene19–21 have been applied to the 18F–labeling of biomolecules.
We have previously reported a versatile bioorthogonal conjugation using 2–cyanobenzothiazole (CBT) that can rapidly react with a cysteine moiety.22–23 The observed second–order rate constant for this reaction was determined to be 9.19 M−1s−1. This condensation reaction enables rapid and site–specific fluorescent labeling of target proteins in vitro and at the surface of live cells without the need of catalysts under ambient conditions. Its rapid reaction rate along with biocompatibility makes this CBT–cysteine condensation reaction attractive for radio–labeling of biomolecules such as peptides and proteins for PET imaging applications. Herein we describe a facile and efficient 18F–labeling method using 18F–fluorinated–2–cyanobenzothiazole (18F–CBT) as a novel prosthetic group and its application to radiolabeling of a dimeric cRGD peptide for in vivo cancer targeted PET imaging. We further demonstrated the site–specific 18F–labeling of a cysteine–bearing protein using 18F–CBT, and evaluated its biodistribution in a living mouse with PET imaging.
EXPERIMENTAL SECTION
18F–labeling of tosylated CBT (3)
[18F]–Fluoride (1000 mCi) was prepared by proton bombardment of 2.5 mL [18O] enriched water target via the 18O(p,n)18F nuclear reaction. The [18F]–Fluoride was then trapped onto a Sep–Pak QMA cartridge. 18–Crown–6/K2CO3 solution (1 mL, 15:1 MeCN/H2O, 16.9 mg of 18–Crown–6, 4.4 mg of K2CO3) was used to elute the [18F]–Fluoride from QMA cartridge into a dried glass reactor. The resulting solution was azeotropically dried with sequential MeCN evaporations at 90 °C. A solution of compound 3 (2 mg in 1 mL of anhydrous MeCN) was added to the reactor and heated at 90 °C for 10 min. After cooling to 30 °C, 0.05 M HCl (2.5 mL) was added to quench the reaction mixture and prevented basic hydrolysis of the product 18F–4. The crude mixture was then purified with a semi–preparative HPLC (Phenomenex Gemini column: 10 × 250 mm, 5μ, 3 mL/min, and eluent gradient: 0–3 min 40% (0.1% TFA containing MeCN in 0.1% TFA containing H2O); 3–35 min 40–100% (0.1% TFA containing MeCN in 0.1% TFA containing H2O), Rt = 21.0 min. The collected 18F–4 was diluted with H2O (20 mL) and passed through a C18 cartridge. The trapped 18F–4 was eluted out with Et2O (2.5 mL). The Et2O was removed by helium stream and used for next reaction. The isolated radiochemical yield of 18F–4 was ca. 20% (140–150 mCi, decay–corrected to end of bombardment).
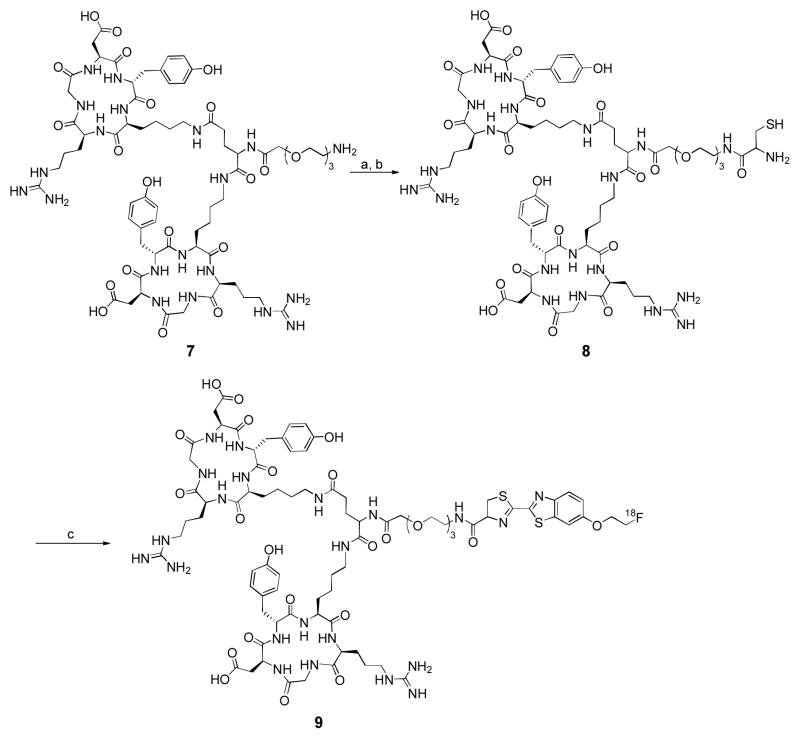
Radiosynthesis of [18F]CBTRGD2 (9)
cRGD dimer 8 (1.2 mg) was dissolved in DMF (200 μL) containing 2 equiv. of TCEP·HCl and 15 equiv. of DIPEA. The resulting solution was added to 18F–4 (40 mCi) in DMF (200 μL) at room temperature. At different time points (1, 5, 10 and 20 min), the sample was taken from the crude mixture and the reaction was quenched with 10% AcOH aqueous solution. After 20 min, the conversion yield was 92% determined by analytical HPLC. The reaction was quenched by adding 10% AcOH aqueous solution and then the crude product was purified by a semi–preparative HPLC to give [18F]CBTRGD2 9 with 80% RCY (decay–corrected to the end of synthesis). [Phenomenex Gemini column: 10 × 250 mm, 5μ, 5 mL/min, and eluent gradient: 0–50 min 10–50% (0.1% TFA containing MeCN in 0.1% TFA containing H2O), Rt = 34.4 min]. The specific radioactivity was 1.3 ± 0.15 Ci/μmol.
Radio–labeling of Cys–RLuc8 (11)
18F–4 (10.7 mCi, 7.5 μL) in DMSO solution was added to a solution of Cys–RLuc 11 (5 nmol,) in PBS buffer (150 μL, pH = 7.5 with 2 mM TECP), and stirred at 37 °C for 30 min. After the reaction, the crude mixture was diluted with PBS buffer until total volume was up to 1 mL. The crude mixture was directly loaded a NAP–10 column which was pre–conditioned with elution buffer (PBS, pH = 7.4). The crude solution (1 mL) was allowed to enter into the column completely and then 1.5 mL of elution buffer (PBS, pH = 7.4) was added into the column to collect the product [18F]CBT–RLuc 12 (12 ± 0.7%, n = 3, decay–corrected to end of synthesis, isolated yield). Overall reaction and purification steps were completed within 40 min. The specific radioactivity was 262 mCi/μmol.
microPET imaging of U87MG tumor xenografts in mice
Image analyses were carried out using a microPET R4 rodent model scanner (Siemens Medical Solutions). By tail–vein injection, each mouse was administered approximately 100 μCi of [18F]CBTRGD2 under isoflurane anesthesia. At 0.5 h, 1 h and 2 h post–injection (n = 3 group), five-minute static PET scans were acquired. For the blocking experiments, the tumor bearing mice were co–injected with 20 mg/kg mouse body weight of cRGD2 7 and [18F]CBTRGD2. PET images were acquired at 0.5 h, 1 h and 2 h post–injection (n = 3 each group). For each scan, region of interests (ROIs) were drawn over the tumor, liver and kidney by using vendor software (ASI Pro 5.2.4.0; Simens Medical Solutions) on decay–corrected whole–body coronal images. The maximum radiochemistry concentration (accumulation) within a tumor or an organ was obtained from mean pixel values within the multiple ROI volume, which were converted to percent injected dose per gram (%ID/g).
RESULTS AND DISCUSSION
Synthesis of CBT prosthetic group and its 18F–labeling procedure
To carry out 18F–labeling of the CBT structure, tosylated CBT 3 was prepared from commercially available 6–methoxy–CBT 1 (Scheme 1). The methyl group in compound 1 was removed using pyridine hydrochloride at 200 °C. The resulting hydroxyl–CBT derivative was converted to tosylate 3 by reacting with excess amount of ethylene glycol ditosylate. A 19F–analog, 19F–4, was synthesized using 2–fluoroethyl 4–methylbenzenesulfonate as a reference for HPLC characterization of 18F–4.
Scheme 1.
Synthesis of tosylated CBT 3 and a condensation reaction between 18F–4 and free cysteinea
aReagents and conditions: (a) pyridine–HCl, 200 °C, 2 h, 75%, (b) ethylene glycol di–tosylate, K2CO3, DMF, room temperature, 8 h, 53%, (c) 2–fluoroethyl 4–methylbenzenesulfonate, K2CO3, DMF, room temperature, overnight, 70 %, (d) K[18F]F, 18–Crown–6, K2CO3, MeCN, 90 °C, 10 min, 20% (decay corrected to end of bombardment, isolated yield), (e) L–cysteine, TCEP·HCl, NaHCO3, MeOH/H2O, room temperature, 1 min, 95%.
18F–labeling of tosylate 3 was first performed with traditional phase transfer catalyst (PTC) Kryptofix 222 (K222) (Fig. 1), however, an 18F–byproduct was mainly observed on radio–HPLC chromatograph instead of the desired 18F–4 (Fig. 1b), which might be due to the hydrolysis of the cyano group on CBT under this condition. Indeed, stability tests with tosylated CBT 3 and fluorinated CBT (19F–CBT) showed that the CBT compounds were hydrolyzed under K222/K2CO3 condition within 1 min and also unstable in the presence of Cs2CO3 or tetrabutylammonium bicarbonate (Fig. S1). K222 contains nucleophilic nitrogen atoms that could attack the cyano group of 3 and cause facile hydrolysis under this common radiofluorination condition. To discover alternate 18F–labeling conditions, we tested another PTC and to our delight, the CBT precursor was stable for 10 min in the presence of 18–Crown–6/K+ complex at 90 °C. Tosylated CBT 3 (2 mg) in MeCN was then added to the anhydrous 18–Crown–6/K+/[18F]F– complex and the labeling reaction was carried out at 90 °C for 10 min. Radio–HPLC chromatograph indicated by–product 18F–5 was suppressed under these conditions (Fig. 1c). Automated syntheses have been accomplished with up to 1000 mCi of radioactivity and the expected 18F–4 was obtained at a 20% RCY (decay–corrected to end of bombardment, isolated yield). Analytical HPLC revealed that the radiochemical/chemical purity of 18F–4 was more than 99%.
Figure 1.
(a) 18F–fluorination reaction of tosylated CBT 3. (b) Radiochromatography of the crude product using K222. (c) Radiochromatography of the crude product using 18–crown–6.
A condensation reaction between 18F–4 and free L–cysteine
The efficiency of condensation reaction between the prepared 18F–4 and free cysteine was subsequently investigated (Scheme 1). The reaction was carried out in MeOH/H2O (1:1, 1 mL) at room temperature. TCEP·HCl (tris(2–carboxyethyl)phosphine hydrochloride, 2 equiv.) was added to the reaction mixture for preventing undesired oxidation of the thiol group and pH was adjusted to 7.0–7.5 using aqueous NaHCO3. The condensation reaction was quenched by adding 0.05 M aqueous HCl and the conversion yield in the reaction was estimated by analytical HPLC. As shown in Fig. S2, 18F–4 was converted to 18F–6 in more than 95% yield within 1 min. Given that 18F–4 was fully converted to 18F–6 in a few minutes at room temperature, the condensation reaction should be appropriate for 18F–labeling of biomolecules.
18F–labeling of a dimeric cRGD peptide
We selected a dimeric cRGD peptide for our labeling because cRGD peptides have been shown to target αvβ3 integrin expression in tumors.24–25 A number of 18F prosthetic groups have been employed for labeling cRGD, some of which are currently in clinical trials such as [18F]Galacto–RGD26 and [18F]FPPRGD2.27–28 However, most of these 18F labeling chemistries require multi–step syntheses that resulted in low radiochemical yield and could be challenging to routinely produce for clinic use26–30 Since 18F–CBT could be easily obtained by direct 18F/tosylate substitution and the condensation reaction between 18F–CBT and cRGD proceeds readily at room temperature, this novel 18F–prosthetic group could provide more benefits over those previously reported. In detail, N–terminal cysteine was introduced to a cRGD dimer 7 using N–Boc–Cys(Trt) succinimidyl ester in 2 steps (Scheme 2). The cRGD derivative 8 was then reacted with 18F–4 in anhydrous DMF at room temperature. The conversion yield reached 92% after incubating 20 min with the 18F–labeling agent at room temperature as determined by analytical radio–HPLC (Fig. S3). After the labeling reaction was finished, the crude product was purified by semi–preparative HPLC. The observed RCY of the 18F–labeled product 9 was 80% (decay–corrected to end of synthesis) and the radiochemical purity was more than 99%. The final product [18F]CBTRGD2 9 was obtained in an isolated overall yield of 7.5 ± 2.7% (decay–uncorrected) with a total synthesis time of 100 min (Table 1). Specific radioactivity of the final product was 1.3 ± 0.15 Ci/μmol. It is important to note that, during the HPLC purification of crude [18F]CBTRGD2, all the impurity, reagent and starting material have distinct retention time from the product (> 10 min) and no other peaks appeared around the product peak on the HPLC chromatograph at UV 254 nm (Fig. S4). In comparison to [18F]FPPRGD2 as previously reported in clinical studies, two impurities were identified to have retention times close to the final product (< 1 min) and are difficult to remove.27 Therefore, CBT–based 18F labeling chemistry could offer the following advantages: 1) higher RCY; 2) fewer synthetic steps and shorter synthesis time; and 3) easier HPLC purification.
Scheme 2.
18F–labeling of cRGD2 peptide analog using 18F–4a
aReagents and conditions: (a) N–Boc–Cys(Trt) succinimidyl ester, (b) TFA, triisopropylsilane, DCM, 79% over 2 steps, (c) 18F–4, DIPEA, TCEP·HCl, room temperature, 20 min, 80% (decay corrected to end of synthesis, isolated yield).
Table 1.
Comparison of radiochemical procedure
| [18F]FPPRGD2 | [18F]CBTRGD2 | |
|---|---|---|
| Radiosynthetic steps | 4 | 2 |
| Total synthesis time | 180 min | 100 min |
| RCY [a] (n = 3) | 3.5 ± 1.8% | 7.5 ± 2.7% |
| Specific radioactivity | 0.97 ± 0.06 Ci/μmol | 1.3 ± 0.15 Ci/μmol |
decay–uncorrected, isolated yield
In vivo evaluation of [18F]CBTRGD2 with U87MG tumor xenograft model
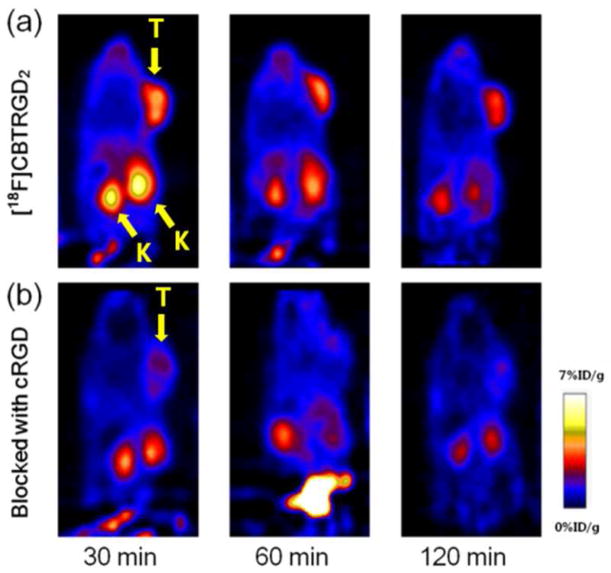
Tumor targeting efficiency of [18F]CBTRGD2 9 was evaluated in U87MG tumor bearing nude mice by static small animal imaging PET scans (Fig. 2). PET imaging results showed that the tumor site was clearly visualized with good tumor–to–background contrast within 30 min and the clearance of tracer uptake was observed at 120 min (Fig. 2a). PET quantification results demonstrated that tumor-to-background (or muscle) ratios were 4.5±0.83 (30 min), 5.4±0.72 (60 min) and 6.3±1.1 (120 min). The tumor uptake of [18F]CBTRGD2 was 5.62 ± 1.15, 4.25 ± 0.82, 3.35 ± 0.71% at 30, 60, 120 min, respectively (Table 2). A blocking experiment where the tracer was co–injected with cRGD dimer (20 mg/kg) showed significantly reduced tumor uptake of the tracer (1.62 ± 0.10, 1.26 ± 0.23, 0.81 ± 0.04% at 30, 60, 120 min, respectively), suggesting that [18F]CBTRGD2 specifically bound integrin αvβ3 receptor (Fig. 2b). We also carried out a comparison experiment with tracer 9 and [18F]FPPRGD2 in the same mice. PET imaging results showed that the specific tumor uptake of [18F]CBTRGD2 was 50% higher than that of [18F]FPPRGD2 at 30 min (Fig S5, Table S1). [18F]CBTRGD2 also showed higher uptakes in organs such as liver and kidney than [18F]FPPRGD2, likely due to the increased lipophilicity from the aromatic structure in 18F–CBT.
Figure 2.
Representative small–animal PET images of U87MG tumor–bearing (right shoulder) mice. (a) Whole body coronal images at 30 min, 60 min and 120 min after i.v. injection of [18F]CBTRGD2. (b) Whole body coronal images at 30 min, 60 min and 120 min after co–injection of [18F]CBTRGD2 with cRGD2 peptide (blocking). (T: U87MG tumor, K: kidney)
Table 2.
Uptake of [18F]CBTRGD2 in U87MG tumor, kidney and liver derived from PET quantification (%ID/g, n = 3)
| Organ | Time | [18F]CBTRGD2 | Blocked with cRGD2 |
|---|---|---|---|
| Tumor | 30 min | 5.62 ± 1.15 | 1.62 ± 0.10 |
| 60 min | 4.25 ± 0.82 | 1.26 ± 0.13 | |
| 120 min | 3.35 ± 0.71 | 0.49 ± 0.02 | |
| Kidney | 30 min | 5.46 ± 1.10 | 2.76 ± 0.85 |
| 60 min | 3.99 ± 1.08 | 1.26 ± 0.23 | |
| 120 min | 2.26 ± 0.67 | 0.81 ± 0.04 | |
| Liver | 30 min | 3.64 ± 0.76 | 1.34 ± 0.06 |
| 60 min | 2.84 ± 0.76 | 0.70 ± 0.08 | |
| 120 min | 2.15 ± 0.53 | 0.32 ± 0.01 |
Recently the [4+2] inverse electron demand Diels–Alder reaction was applied to 18F–labeling of a cRGD peptide.31 The rate of this conjugation method was faster than the other reactions described, but this ligation unfortunately created a mixture of isomers. Moreover, the ligation product from the reaction between tetrazine and trans–cyclooctene was large and hydrophobic, and thus large amounts of radioactivity could be detected in normal organs. Considering that hydrophobic derivatization of isotope labeled tracers often spoils their desired in vivo pharmacokinetics properties, a relatively small hydrophobic part used in the CBT–cysteine reaction may have less adverse effect on the biodistribution of the final tracer.
Site–specific 18F–labeling of N–terminal cysteine bearing RLuc8
We next investigated site–specific 18F–labeling of a protein using 18F–4. Several 18F–prosthetic groups targeting lysine8–9, 32–34 and cysteine35–37 residues have been applied to protein labeling by means of amidation, conjugate addition and so on. But these methods normally result in a mixture of randomly labeled proteins. Moreover, non site–specific labeling of proteins often resulted in decreased biological activity. For example, 18F–labeled anti–carcinoembryonic agent diabody using N–succinimidyl–4–[18F]fluorobenzote ([18F]SFB) showed lower immunoreactivity compared with non–labeled protein.33 Therefore, site–specific labeling of an amino acid residue away from the active site of the protein is highly desirable. Additionally, an efficient reaction under mild conditions is preferred for 18F–labeling of protein.
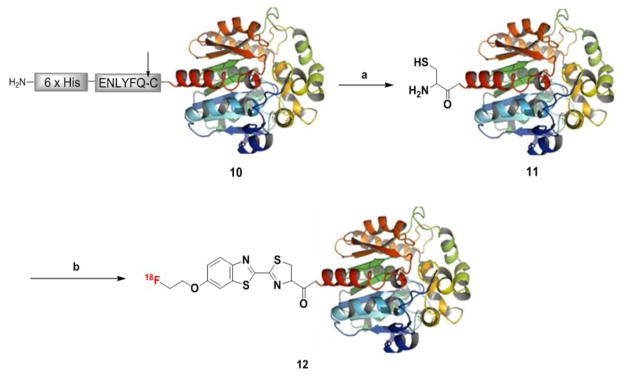
For this study, the bioluminescent protein Renilla luciferase (RLuc8) was used as a model protein. The peptide substrate of tobacco etch virus (TEV) protease was fused at the N–terminus region of RLuc8 10 to generate N–terminal cysteine (Scheme 3). TEV protease was added to the purified fusion protein to cleave the peptide substrate. After the reaction, it was purified with Ni–NTA agarose by using 6xHis tag in front of the TEV protease sequence to provide the N–terminal cysteine RLuc (Cys–RLuc) 11. During this procedure, uncleaved RLuc8 10 and TEV protease with 6xHis tag could be separated from the product. Cys–RLuc 11 was used in next step without furthur purification. In the 18F–labeling reaction, 18F–4 was added to Cys–RLuc 11 (5 nmol) in phosphate buffer saline (PBS, pH = 7.5 with 2 mM TECP), and then the labeling reaction proceeded at 37 °C for 30 min. The purification was accomplished by size exclusion chromatography (NAP–10 column) eluted with PBS (pH = 7.4) to afford [18F]CBT–RLuc 12 with 262 mCi/μmol of specific activity. The observed RCY was 12 ± 0.7% (n = 3, decay–corrected to end of synthesis, isolated yield) and radiochemical purity was more than 99% as determined by radio–TLC analysis (Fig. S6). In comparison, the same labeling reaction with RLuc8 10, which did not contain the required N–terminal cysteine group for coupling, provided no 18F–labeled product. Therefore we can conclude that 18F–labeling of 11 with 18F–4 is site–specific. After the labeling reaction, the bioluminescent property of RLuc8 remained unchanged. The observed bioluminescent intensities of Cys–RLuc 11 and the purified product from the 18F–labeling reaction were nearly the same (Fig. S7).
Scheme 3.
18F–labeling of Cys–RLuc8 11 using 18F–4a
a Reagents and conditions: (a) TEV protease (100 μg of protein/10 units of enzyme), 30 °C, 6 h, (b) 18F–4, 37 °C, 30 min, 12 ± 0.7% (decay–corrected to end of synthesis, isolated yield).
To evaluate our new imaging probe in a living subject, we then carried out a PET imaging experiment by injecting [18F]CBT–RLuc 12 (ca. 100 μCi) in non–tumor bearing mice. PET images of different time points (20, 60 and 100 min) showed that primary radioactivity uptake was observed in the renal system within 20 min of injection and then subsequently cleared into bladder by 100 min (Fig. 3). Based on PET quantification of the collected images, non–specific liver accumulation was not observed (Table 3). These results were correlated well with a previous biodistribution study using 124I–labeled RLuc8.38
Figure 3.
Whole body coronal images at 20 min, 60 min and 100 min after i.v. injection of [18F]CBT–RLuc8. (K: kidney)
Table 3.
Uptake of [18F]CBT–RLuc8 in kidney and liver derived from PET quantification (%ID/g, n = 3)
| Organ | Time | [18F]CBT–RLuc8 |
|---|---|---|
| Kidney | 20 min | 17.3 ± 1.28 |
| 60 min | 7.94 ± 1.07 | |
| 100 min | 2.98 ± 0.32 | |
| Liver | 20 min | 7.18 ± 0.94 |
| 60 min | 3.15 ± 0.30 | |
| 100 min | 1.61 ± 0.19 | |
| Muscle | 20 min | 1.24 ± 0.03 |
| 60 min | 1.02 ± 0.02 | |
| 100 min | 0.50 ± 0.04 |
Among various 18F–precursors, 18F–fluorobenzaldehyde ([18F]FBA)39–40 targeting an aminooxy group and 18F–maleimide containing prosthetic groups41–42 reacting with a thiol have been developed as site–specific labeling agents for proteins. They normally provided good 18F–labeling results in terms of RCY and purity for each target protein. But the labeling reaction using [18F]FBA has to be done under aqueous acidic condition (pH < 4.5) that would potentially abolish the bioactivity of pH sensitive enzymes or proteins. In case of maleimide–containing prosthetic groups, their synthesis always require an additional coupling step because maleimide structure is quite labile under the required basic 18F–labeling conditions that often results in lengthy synthesis times and decreased RCYs. Moreover, these site–specific methods involved relatively complicated chemical ligation reactions or protein engineering procedures along with a couple of purification steps to generate a single reactive functional group on the protein. Compared with these methods, the N–terminal cysteine residue can be easily produced by a simple enzymatic cleavage with TEV protease and additional modification and purification steps are unnecessary for the 18F–labeling reaction. Using this procedure, an N–terminal cysteine group can be readily prepared in a fusion protein. Therefore, our method has just three steps in total for protein labeling: 1) protein modification with TEV protease, 2) radio–synthesis of 18F–4, and 3) a condensation reaction between 18F–4 and N–terminal cysteine protein under neutral condition, and it provides a useful and general protocol for efficient 18F–labeling of various biomolecules.
CONCLUSIONS
This study demonstrated a highly efficient 18F–labeling strategy of biomolecules such as peptide ligands and proteins based on a rapid condensation reaction of 18F–4. The tosylated CBT derivative used for preparing 18F–4 can be easily synthesized from a commercially available precursor in two simple steps. The fast and specific reactivity of 18F–4 toward a free cysteine residue provides excellent RCYs under ambient temperature. The fast and specific reactivity of 18F–4 toward a free cysteine residue provides excellent RCYs under ambient temperature. Depending on the cysteine-containing molecules, the labeling can proceed efficiently in a number of different solvent systems, as demonstrated in DMF, MeOH/water and PBS buffer solutions. An N–terminal cysteine could be introduced into the target molecules by chemical synthesis or enzymatic processing of specific peptide sequence fused to the target protein. Using this convenient labeling method, [18F]CBTRGD2 has been prepared in much shorter synthesis time with a higher RCY compared to the PET tracer currently under clinical trial, [18F]FPPRGD2, and has shown better tumor targeting efficiency in mice than [18F]FPPRGD2 prepared by a different prosthetic group, 4–nitrophenyl 2–18F–fluoropropionate (18F–NFP). The increased accumulation of [18F]CBTRGD2 in livers may be due to the lipophilic structure of the CBT group, which should be ameliorated with modifications of the CBT structure that increase its hydrophilicity. On the other hand, when 18F–4 was used for site–specific 18F–labeling of RLuc8, the 18F–labeled protein showed little alteration in its biodistribution as revealed by PET imaging in healthy mice. We anticipate that this method could be generally applied to afford efficient and site–specific 18F–labeling of peptides, proteins or antibodies that contain an N–terminal cysteine.
Supplementary Material
Acknowledgments
This work was supported in part by an IDEA award from Department of Defense Breast Cancer Research Program (W81XWH–09–1–0057) and the NCI ICMIC@Stanford (1P50CA114747–06).
Footnotes
Synthesis and characterization of compounds, stability test of 2-cyanobenzothiazole (CBT) structures, radiochemical procedures for peptide and protein labeling and in vivo microPET imaging. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 2.Wester HJ, Schottelius M, Scheidhauer K, Meisetschläger G, Herz M, Rau FC, Reubi JC, Schwaiger M. PET imaging of somatostatin receptors: design, synthesis and preclinical evaluation of a novel 18F-labelled, carbohydrated analogue of octreotide. Eur J Nucl Med Mol Imaging. 2003;30:117–122. doi: 10.1007/s00259-002-1012-1. [DOI] [PubMed] [Google Scholar]
- 3.Stoeklin G, Wester HJ. Positron emission tomography: A critical assessment of recent trends. Kluwer Academic Publishers; the Netherlands: 1998. pp. 57–90. [Google Scholar]
- 4.Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3 targeted radiotracers for tumor imaging. Mol Pharm. 2006;3:472–487. doi: 10.1021/mp060049x. [DOI] [PubMed] [Google Scholar]
- 5.Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew Chem Int Ed. 2008;47:8998–9033. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- 6.Thumshirn G, Hersel U, Goodman SL, Kessler H. Multimeric cyclic RGD peptides as potential tools for tumor targeting: solid-phase peptide synthesis and chemoselective oxime ligation. Chem Eur J. 2003;9:2717–2725. doi: 10.1002/chem.200204304. [DOI] [PubMed] [Google Scholar]
- 7.Poethko T, Schottelius M, Thumshirn G, Hersel U, Herz M, Henriksen G, Kessler H, Schwaiger M, Wester HJ. Two-step methodology for high-yield routine radiohalogenation of peptides: 18F-labeled RGD and octreotide analogs. J Nucl Med. 2004;45:892–902. [PubMed] [Google Scholar]
- 8.Wüst F, Hultsch C, Bergmann R, Johannsen B, Henle T. Radiolabelling of isopeptide Nε-(γ-glutamyl)-L-lysine by conjugation with N-succinimidyl-4-[18F]fluorobenzoate. Appl Radiat Isot. 2003;59:43–48. doi: 10.1016/s0969-8043(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 9.Wester HJ, Hamacher K, Stöcklin G. A comparative study of N.C.A. Fluorine-18 labeling of proteins via acylation and photochemical conjugation. Nucl Med Biol. 1996;23:365–372. doi: 10.1016/0969-8051(96)00017-0. [DOI] [PubMed] [Google Scholar]
- 10.Cai W, Zhang X, Wu Y, Chen X. A thiol-reactive 18F-labeling agent, N-[2-(4-18F-fluorobenzamido) ethyl]maleimide, and synthesis of RGD peptide-based tracer for PET imaging of αvβ3 integrin expression. J Nucl Med. 2006;47:1172–1180. [PMC free article] [PubMed] [Google Scholar]
- 11.de Bruin B, Kuhnast B, Hinnen F, Yaouancq L, Amessou M, Johannes L, Samson A, Boisgard R, Tavitian B, Dollé F. 1-[3-(2-[18F]Fluoropyridin-3-yloxy)propyl]pyrrole-2,5-dione: design, synthesis, and radiosynthesis of a new [18F]fluoropyridine-based maleimide reagent for the labeling of peptides and proteins. Bioconjugate Chem. 2005;16:406–420. doi: 10.1021/bc0497463. [DOI] [PubMed] [Google Scholar]
- 12.Gill HS, Marik J. Preparation of 18F-labeled peptides using the copper(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition. Nat Protoc. 2011;6:1718–1725. doi: 10.1038/nprot.2011.390. [DOI] [PubMed] [Google Scholar]
- 13.Maschauer S, Einsiedel J, Haubner R, Hocke C, Ocker M, Hübner H, Kuwert T, Gmeiner P, Prante O. Labeling and glycosylation of peptides using Click chemistry: a general approach to 18F-glycopeptides as effective imaging probes for positron emission tomography. Angew Chem Int Ed. 2010;49:976–979. doi: 10.1002/anie.200904137. [DOI] [PubMed] [Google Scholar]
- 14.Glaser M, Årstad E. “Click labeling” with 2-[18F]fluoroethylazide for positron emission tomography. Bioconjugate Chem. 2007;18:989–993. doi: 10.1021/bc060301j. [DOI] [PubMed] [Google Scholar]
- 15.Li ZB, Wu Z, Chen K, Chin FT, Chen X. Click chemistry for 18F-labeling of RGD peptides and microPET imaging of tumor integrin αvβ3 expression. Bioconjugate Chem. 2007;18:1987–1994. doi: 10.1021/bc700226v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marik J, Sutcliffe JL. Click for PET: rapid preparation of [18F]fluoropeptides using CuI catalyzed 1,3-dipolar cycloaddition. Tetrahedron Lett. 2006;47:6681–6684. [Google Scholar]
- 17.Campbell–Verduyn LS, Mirfeizi L, Schoonen AK, Dierckx RA, Elsinga PH, Feringa BL. Strain-promoted copper-Free “Click” chemistry for 18F radiolabeling of bombesin. Angew Chem Int Ed. 2011;50:11117–11120. doi: 10.1002/anie.201105547. [DOI] [PubMed] [Google Scholar]
- 18.Evans HL, Slade RL, Carroll L, Smith G, Nguyen Q, Iddon L, Kamaly N, Stöckmann H, Leeper FJ, Aboagye EO, Spivey AC. Copper-free click–a promising tool for pre-targeted PET imaging. Chem Commun. 2012;48:991–993. doi: 10.1039/c1cc16220a. [DOI] [PubMed] [Google Scholar]
- 19.Li ZB, Cai H, Hassink M, Blackman ML, Brown RCD, Conti PS, Fox JM. Tetrazine-trans-cyclooctene ligation for the rapid construction of 18F labeled probes. Chem Commun. 2010;46:8043–8045. doi: 10.1039/c0cc03078c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keliher EJ, Reiner T, Turetsky A, Hilderbrand SA, Weissleder R. High-yielding, two-step 18F labeling strategy for 18F-PARP1 inhibitors. ChemMedChem. 2011;6:424–427. doi: 10.1002/cmdc.201000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiner T, Keliher EJ, Earley S, Marinelli B, Weissleder R. Synthesis and in vivo imaging of a 18F-labeled PARP1 inhibitor using a chemically orthogonal scavenger-assisted high-performance method. Angew Chem Int Ed. 2011;50:1922–1925. doi: 10.1002/anie.201006579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren H, Xiao F, Zhan K, Kim Y–P, Xie H, Xia Z, Rao J. A biocompatible condensation reaction for the labeling of terminal cysteine residues on proteins. Angew Chem Int Ed. 2009;48:9658–9662. doi: 10.1002/anie.200903627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang G, Ren H, Rao J. A biocompatible condensation reaction for controlled assembly of nanostructures in living cells. Nature Chem. 2010;2:54–60. doi: 10.1038/nchem.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Tohme M, Park R, Hou Y, Bading JR, Conti PS. Micro-PET imaging of αvβ3-integrin expression with 18F-labeled dimeric RGD peptide. Mol Imaging. 2004;3:96–104. doi: 10.1162/15353500200404109. [DOI] [PubMed] [Google Scholar]
- 25.Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin αvβ3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 26.Haubner R, Kuhnast B, Mang C, Weber WA, Kessler H, Wester HJ, Schwaiger M. [18F]Galacto-RGD: synthesis, radiolabeling, metabolic stability, and radiation dose estimates. Bioconjugate Chem. 2004;15:61–69. doi: 10.1021/bc034170n. [DOI] [PubMed] [Google Scholar]
- 27.Chin FT, Shen B, Liu S, Berganos RA, Chang E, Mittra E, Chen X, Gambhir SS. First experience with clinical-grade [18F]FPP(RGD)2: an automated multi-step radiosynthesis for clinical PET studies. Mol Imaging Biol. 2012;14:88–95. doi: 10.1007/s11307-011-0477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittra ES, Golis ML, Iagaru AH, Kardan A, Burton L, Berganos R, Chang E, Liu S, Shen B, Chin FT, Chen X, Gambihr SS. Pilot pharmacokinetic and dosimetric studies of 18F-FPPRGD2: a PET radiopharmaceutical agent for imaging αvβ3 integrin levels. Radiology. 2011;260:182–191. doi: 10.1148/radiol.11101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poethko T, Schottelius M, Thumshirn G, Herz M, Haubner R, Henriksen G, Kessler H, Schwaiger M, Wester HJ. Chemoselective pre-conjugate radiohalogenation of unprotected mono- and multimeric peptides via oxime formation. Radiochim Acta. 2004;92:317–328. [Google Scholar]
- 30.Liu S, Liu Z, Chen K, Yan Y, Watzlowik P, Wester HJ, Chin FT, Chen X. 18F-labeled galacto and PEGylated RGD dimers for PET Imaging of αvβ3 integrin expression. Mol Imaging Biol. 2010;12:530–538. doi: 10.1007/s11307-009-0284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selvaraj R, Liu S, Hassink M, Huang C, Yap L, Park R, Fox JM, Li ZB, Conti PS. Tetrazine-trans-cyclooctene ligation for the rapid construction of integrin αvβ3 targeted PET tracer based on a cyclic RGD peptide. Bioorg Med Chem Lett. 2011;21:5011–5014. doi: 10.1016/j.bmcl.2011.04.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kostikov AP, Chin J, Orchowski K, Niedermoser S, Kovacevic MM, Aliaga A, Jurkschat K, Wängler B, Wängler C, Wester HJ, Schirrmacher R. Oxalic acid supported Si–18F-radiofluorination: one-step radiosynthesis of N-succinimidyl 3-(Di-tert-butyl[18F]fluorosilyl)benzoate ([18F]SiFB) for protein labeling. Bioconjugate Chem. 2012;23:106–114. doi: 10.1021/bc200525x. [DOI] [PubMed] [Google Scholar]
- 33.Cai W, Olafsen T, Zhang X, Cao Q, Gambhir SS, Williams LE, Wu AM, Chen X. PET imaging of colorectal cancer in xenograft-bearing mice by use of an 18F-labeled T84.66 anti–carcinoembryonic antigen diabody. J Nucl Med. 2007;48:304–310. [PubMed] [Google Scholar]
- 34.Grierson JR, Yagle KJ, Eary JF, Tait JF, Gibson DF, Lewellen B, Link JM, Krohn KA. Production of [F-18]fluoroannexin for imaging apoptosis with PET. Bioconjugate Chem. 2004;15:373–379. doi: 10.1021/bc0300394. [DOI] [PubMed] [Google Scholar]
- 35.Berndt M, Pietzsch J, Wuest F. Labeling of low-density lipoproteins using the 18F-labeled thiol-reactive reagent N-[6-(4-[18F]fluorobenzylidene)aminooxyhexyl]maleimide. Nucl Med Biol. 2007;34:5–15. doi: 10.1016/j.nucmedbio.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Toyokuni T, Walsh JC, Dominguez A, Phelps ME, Barrio JR, Gambhir SS, Satyamurthy N. Synthesis of a new heterobifunctional linker, N-[4-(aminooxy)butyl]maleimide, for facile access to a thiol-reactive 18F-labeling agent. Bioconjugate Chem. 2003;14:1253–1259. doi: 10.1021/bc034107y. [DOI] [PubMed] [Google Scholar]
- 37.Wuest F, Berndt M, Bergmann R, van den Hoff J, Pietzsch J. Synthesis and application of [18F]FDG-maleimidehexyloxime ([18F]FDG-MHO): a [18F]FDG-based prosthetic group for the chemoselective 18F-labeling of peptides and proteins. Bioconjugate Chem. 2008;19:1202–1210. doi: 10.1021/bc8000112. [DOI] [PubMed] [Google Scholar]
- 38.Venisnik KM, Olafsen T, Loening AM, Iyer M, Gambhir SS, Wu AM. Bifunctional antibody-Renilla luciferase fusion protein for in vivo optical detection of tumors. Protein Eng Des Sel. 2006;19:453–460. doi: 10.1093/protein/gzl030. [DOI] [PubMed] [Google Scholar]
- 39.Flavell RR, Kothari P, Bar–Dagan M, Synan M, Vallabhajosula S, Friedman JM, Muir TW, Ceccarini G. Site-specific 18F-labeling of the protein hormone leptin using a general two-Step ligation procedure. J Am Chem Soc. 2008;130:9106–9112. doi: 10.1021/ja801666z. [DOI] [PubMed] [Google Scholar]
- 40.Namavari M, De Jesus OP, Cheng Z, De A, Kovacs E, Levi J, Zhang R, Hoerner JK, Grade H, Syud FA, Gambhir SS. Direct site-specific radiolabeling of an affibody protein with 4-[18F]fluorobenzaldehyde via oxime chemistry. Mol Imaging Biol. 2008;10:177–181. doi: 10.1007/s11307-008-0142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Link JM, Stekhova S, Yagle KJ, Smith C, Krohn KA, Tait JF. Site-specific labeling of Annexin V with F-18 for apoptosis imaging. Bioconjugate Chem. 2008;19:1684–1688. doi: 10.1021/bc800164d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gill HS, Tinianow JN, Ogasawara A, Flores JE, Vanderbilt AN, Raab H, Scheer JM, Vandlen R, Williams S, Marik J. A modular platform for the rapid site-specific radiolabeling of proteins with 18F exemplified by quantitative positron emission tomography of human epidermal growth factor receptor 2. J Med Chem. 2009;52:5816–5825. doi: 10.1021/jm900420c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.