Abstract
Background
Preserved insight into illness has been suggested to be predictive of outcome in patients with schizophrenia. We aimed to investigate the functional substrate underlying preserved insight in these patients.
Methods
We recruited patients with paranoid schizophrenia and healthy controls matched for age and sex. Patients were grouped according to preserved or impaired insight into illness using the Scale to assess Unawareness of Mental Disorder (SUMD). Whole-brain technetium-99m ethyl cysteinate dimer single photon emission computed tomography regional cerebral blood flow was compared at the voxel level between the 2 groups using a statistical parametric map (voxel-level significance of p < 0.001, uncorrected; cluster level significance of p < 0.05, uncorrected).
Results
We enrolled 31 right-handed patients with schizophrenia and 18 controls in our study. Twenty-one (67.7%) patients had preserved insight. The 2 groups did not differ significantly in demographic and clinical characteristics or in treatment. Compared with controls, the whole group of patients showed bilateral frontotemporal hypoperfusions, with no statistical difference between patients with preserved or impaired insight for these areas. Patients with preserved insight showed significantly increased perfusion of the bilateral precuneus relative to those with impaired insight.
Limitations
Patients with subtypes other than paranoid schizophrenia have to be investigated to assess whether involvement of the precuneus in patients with preserved insight can be identified across the full spectrum of subtypes and symptoms of schizophrenia. Moreover, our study concerned only the central dimension (awareness of mental disorder) of 1 scale (SUMD); other dimensions of insight could be studied.
Conclusion
Our results show that schizophrenia with preserved insight is associated with greater perfusion of the precuneus, a brain area known to be involved in self-consciousness, suggesting a compensatory mechanism of fronto-temporal impairment.
Introduction
Lack of insight, particularly unawareness of mental disorder, affects 30%–50% of patients with schizophrenia.1–4 Resulting from metacognitive capacities, insight can be conceptualized as a self-representation: that is, not simply possessing a piece of knowledge, but rather, making narrative sense of one’s condition.5 As such, awareness of a mental disorder also includes awareness of the need to take medication and of the social consequences of the mental disorder.6
Impaired insight has been associated with disease severity,7 cognitive deficits8,9 and with social and vocational functioning impairments.8,9 On the other hand, preserved insight has been suggested to be predictive of treatment outcome in patients with schizophrenia.3,10,11 Preserved insight is linked in particular with improved adherence to treatment12 and reduced risk of relapse and readmission to hospital.13 Because improving awareness is a major objective of treating schizophrenia, understanding the neurobiological substrate underlying insight may serve to develop oriented neuropsychiatric treatment.14–16 So far, studies have mainly used structural imaging,15,17–23 and more rarely functional imaging.17,24 Lack of insight in patients with schizophrenia frequently has been associated with impairment of the prefrontal cortex,14,18–20 temporal areas15–17 and precuneus.15,19 Interestingly, it has been suggested that a network associating these regions is involved in experiencing consciousness and self-awareness in healthy individuals.25,26 Yet, studies on schizophrenia have specifically focused on impaired insight in correlation analyses14 or in comparison studies between patients with impaired insight and healthy controls.18,20–22 Scarce data are available regarding the functional neural substrate of preserved insight in patients with schizophrenia. One functional magnetic resonance imaging (fMRI) study has reported a correlation between improved insight and activation of the left medial prefrontal cortex after recovering from an acute episode.27
Technetium-99m ethyl cysteinate dimer single photon emission computed tomography (99mTc ECD-SPECT) is a valuable functional brain imaging tool to study regional cerebral blood flow (rCBF) in patients with a variety of psychiatric disorders,23 including schizophrenia.25–28 In this study, we investigated the functional substrate underlying preserved insight in patients with schizophrenia in comparison with those with impaired insight and psychiatrically healthy controls using whole-brain 99mTc ECD-SPECT voxel-based statistical analysis of rCBF. Without any regional a priori hypothesis, differences between these distinct groups may either correspond to more functional brain abnormalities in patients with impaired insight, or possibly to functional compensation in patients with preserved insight.
Methods
Participants
We retrospectively collected data for patients with schizophrenia. The inclusion criteria were age older than 18 years, French native language, diagnosis of paranoid schizophrenia according to the DSM-IV-TR criteria confirmed through administration of a Structured Clinical Interview,29 stable disease (i.e., no need for hospital admission at inclusion, no major change in the patients’ condition for 2 months before inclusion)30 and outpatient status. The last 2 criteria were selected to include patients with mild disease severity, thus facilitating the recruitment of patients with preserved insight. To increase the homogeneity of our sample, we decided to include patients with paranoid schizophrenia only. Exclusion criteria were psychiatric diagnosis other than paranoid schizophrenia on Axis I of the DSM-IV-TR, decompensated organic disease and mental retardation.
We also included psychiatrically healthy controls similar to patients in age and sex. Controls had to be free of neurologic/psychiatric disease and cognitive complaints, and they had to have a normal brain MRI scan. They were screened for anxiety and depression using the Anxiety and Depression Hospital Scale (subscores < 8).31
The local ethics committee approved investigations (registration number of clinical trial: NCT00484523). Our research was conducted in accordance with the Declaration of Helsinki and French good clinical practices.32,33 In particular, both patients and controls received an explanation of the study and gave written, informed consent after a standardized and structured clinical interview.
Assessment of insight
We assessed insight into illness using the Scale to assess Unawareness of Mental Disorder (SUMD)–shortened version.12,34 We focused on the 3 central items of insight16 involved in the dimension “awareness of mental disorder:”35 having a mental disorder, the need to take medication and the consequences of the mental disorder. Each of these items is rated on a 3-point scale: 1 = aware, 2 = somewhat aware/somewhat unaware and 3 = severely unaware. The dimension score is obtained by summing the 3 items.36 Scores range from 3, which indicates the greatest insight (i.e., aware for each subscore) to 9, which indicates the least insight (i.e., severely unaware for each subscore).
To confirm the validity of using 1 dimension of insight, we performed 2 types of analysis. First, a factor analysis using principal component analysis (PCA) and varimax rotation was used to explore the instrument’s structure (structural validity) based on the correlation matrix. Principal component analysis is a suitable technique to identify underlying dimensions as it reduces multiple variables into a smaller number of variables that describe the data most efficiently.37 In our study, the PCA yielded 1 dimension explaining 75.3% of the variance. Moreover, when items are used to form a dimension, they need to have internal consistency. The items should all measure the same thing, so they should be correlated with one another.38 A useful coefficient for assessing internal consistency is Cronbach α.39 In our study, the internal reliability (Cronbach α) was at an acceptable level of 0.83 (greater than the results of a previous study34).
Patients were then grouped by preserved insight (score = 3) or impaired insight (score > 3).
Data collection
The following data were collected:
Sociodemographic information: sex, age and level of education.
Clinical characteristics: duration of illness and psychotic symptoms based on the Positive and Negative Syndrome Scale (PANSS).40 The PANSS was decomposed into 5 factors (positive, negative, hostility–excitation, cognitive and depression factors), which are of specific interest for our study because of their potential link to insight.41
Drug information: antidepressants and antipsychotic medication (first- [FGAs] and second-generation antipsychotics [SGAs]). Cumulative antipsychotic dose was measured by converting the dose of each drug into chlorpromazine equivalents (mg/d), and adding these together (equiv_cmz).42
Single photon emission computed tomography protocol
We performed brain SPECT in all participants using the same camera under the same conditions.43 For patients with schizophrenia, this procedure took place after but on the same day as insight measurement. The participants received an injection of 740 MBq of 99mTc ECD and rested for 1 hour in quiet surroundings with their eyes closed. Single photon emission computed tomography image acquisition was performed using a double-headed rotating γ camera (e.cam; Siemens) equipped with a fan beam collimator. Thirty-two 40-second projections per participant were collected in 128 × 128 format. Tomographic 3-dimensional reconstruction was performed using a filtered back projection algorithm. A voxel × voxel group study was then performed using SPM5 (Wellcome Trust Centre for Neuroimaging) running on MATLAB (Mathworks Inc.)
Images were initially converted from the DICOM to the Analyze format using MRIcro (www.mricro.com) and transferred to SPM. Data were then standardized with the Montreal Neurological Institute (MNI) atlas using a 12-parameter affine transformation followed by nonlinear transformations and trilinear interpolation. Dimensions of resulting voxels were 2 × 2 × 2 mm. Standardized data were then smoothed with a Gaussian filter (full-width at half-maximum 12 mm) to blur individual variations in gyral anatomy and to increase signal-to-noise ratio. We performed subgroup analyses using the comparison of the effect of interest with the between-subject variance, which compares the effect of interest with the between-subject variance. We considered age, sex, level of education (elementary v. high school), disease duration and treatment (equiv_cmz; antipsychotic [typical v. atypical]; antidepressants [yes v. no]) to be nuisance variables. We used the “proportional scaling” routine to check for individual variations in global brain perfusion. We obtained the SPM (T) maps at a height threshold voxel-level significance of p < 0.001, uncorrected and a height threshold cluster-level significance of p < 0.05, uncorrected.44 Normalized perfusion values of significant clusters were then extracted. Finally, MNI coordinates were converted into Talairach coordinates, and brain structures were identified using the Talairach Daemon database (www.talairach.org/daemon.html).
Statistical analysis
First, we determined functional brain abnormalities by comparing the whole group of patients (preserved and impaired insight) with age- and sex-matched controls. The sociodemographic and clinical characteristics, medication information and brain SPECT perfusions were secondarily compared between the 2 patient groups (preserved v. impaired insight) using the Mann–Whitney U test for continuous variables, a χ2 test or Fisher exact test for categorical variables and the SPM routine for brain imaging.45
All statistical tests were 2-sided. We considered results to be significant at p < 0.05 (except for brain imaging analysis, see SPECT protocol). Statistical analyses were performed using the SPSS version 17.0 software package (SPSS Inc.).
Results
Patient characteristics
Thirty-one right-handed patients (mean age 32.2 [standard deviation (SD) 10.5] yr, 22 men) participated in this study. We also included 18 healthy, right-handed controls (mean age 32.3 [SD 7.0] yr, 13 men). Table 1 shows the characteristics of the 31 patients included. The mean duration of illness was 11.3 (SD 8.3) years. As expected, disease severity was mild, with a mean total PANSS score of 63.6 (SD 14.9). Second-generation antipsychotics were prescribed to 90.3% and antidepressants to 25.8% of the patients.
Table 1.
Sociodemographic and clinical characteristics of patients with schizophrenia with preserved or impaired insight
| Characteristic | Group; mean (SD)* | Preserved v. impaired p value | ||
|---|---|---|---|---|
|
| ||||
| All patients, n = 31 | Preserved insight, n = 21 | Impaired insight, n = 10 | ||
| Male sex, no. (%) | 22 (71.0) | 13 (61.9) | 9 (90.0) | 0.20 |
| Age, yr | 32.2 (10.5) | 32.6 (10.2) | 31.5 (11.6) | 0.80 |
| High school–level education, no. (%) | 11 (35.5) | 7 (33.3) | 4 (40.0) | 0.72 |
| Duration of illness, yr | 11.3 (8.3) | 12.0 (7.6) | 9.9 (9.8) | 0.53 |
| PANSS, total score | 63.6 (14.9) | 60.9 (15.7) | 70.0 (10.8) | 0.12 |
| Positive factor | 15.1 (6.2) | 14.3 (4.9) | 16.9 (8.3) | 0.28 |
| Negative factor | 23.3 (9.0) | 23.0 (8.8) | 24.1 (10.0) | 0.75 |
| Hostility–excitation factor | 7.2 (2.4) | 7.3 (2.1) | 6.9 (3.1) | 0.65 |
| Depressive factor | 9.6 (3.6) | 9.8 (3.4) | 9.4 (4.2) | 0.80 |
| Cognitive factor | 6.3 (2.8) | 6.5 (2.8) | 5.7 (3.0) | 0.46 |
| Antipsychotics | ||||
| Second-generation antipsychotics, no. (%) | 28 (90.3) | 18 (85.7) | 10 (100.0) | 0.53 |
| Chlorpromazine equivalent daily dose | 431.7 (342.5) | 473.5 (370.8) | 343.8 (269.7) | 0.33 |
| Antidepressants, no. (%) | 8 (25.8) | 7 (33.3) | 1 (10.0) | 0.22 |
PANSS = Positive and Negative Syndrome Scale;40 SD = standard deviation.
Unless otherwise indicated.
In comparison to healthy controls, the whole group of patients exhibited hypoperfusions within bilateral frontotemporal areas, including the bilateral superior and left medial frontal gyrus, bilateral superior temporal sulcus and particularly in the left middle and right superior temporal gyrus (voxel-level significance of p < 0.001, uncorrected; cluster-level significance of p < 0.05, uncorrected; Fig. 1A, Table 2). No significant hyperperfusion was found in patients relative to healthy controls.
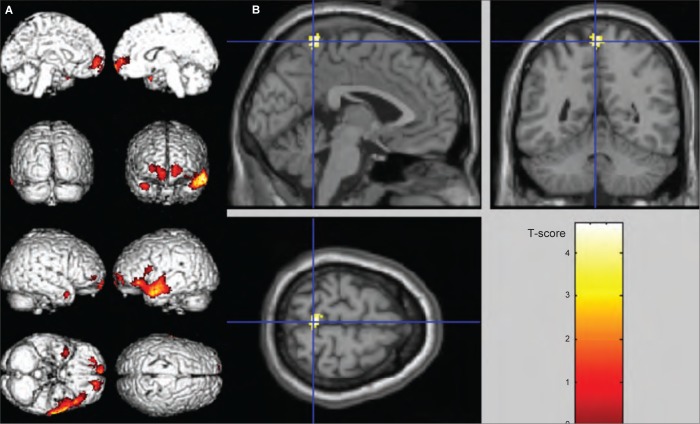
Fig. 1.
Anatomic localization of significant single photon emission computed tomography findings (voxel-level significance of p < 0.001, uncorrected; cluster-level significance of p < 0.05, uncorrected). (A) Hypoperfusions of the whole group of patients in comparison to healthy controls on SPM surface rendered in the bilateral superior frontal gyrus, the left medial frontal gyrus, the left middle and right superior temporal gyrus. (B) In comparison with patients with altered insight, those with preserved insight showed significant increased perfusion in the bilateral precuneus, projected onto sections of a normal magnetic resonance imaging scan set spatially normalized and smoothed into the standard SPM template.
Table 2.
Talairach coordinates of significant* single photon emission computed tomography findings in patients with schizophrenia with preserved or impaired insight and healthy controls
| Contrast | k | p value, cluster | T-score | p value, voxel | Talairach coordinate, mm | Brain region | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| x | y | z | ||||||
| Patients < healthy controls | 1964 | < 0.001 | 5.15 | < 0.001 | −61 | −2 | −10 | Left middle temporal gyrus |
| 455 | 0.001 | 4.39 | < 0.001 | 24 | 49 | 1 | Right superior frontal gyrus | |
| 162 | 0.026 | 4.35 | < 0.001 | 36 | 5 | −24 | Right superior temporal gyrus | |
| 327 | 0.003 | 4.11 | < 0.001 | −16 | 54 | −4 | Left superior frontal gyrus | |
| 3.71 | < 0.001 | −12 | 58 | 3 | Left medial frontal gyrus | |||
| Altered insight < preserved insight | 105 | 0.029 | 4.68 | < 0.001 | −2 | −45 | 65 | Precuneus |
Voxel-level significance of p < 0.001, uncorrected; cluster-level significance of p < 0.05, uncorrected.
Results from SPM5 are listed in decreasing order of peak T-score value within each cluster. The k value represents the number of significant voxels in the particular cluster.
Comparison of patients with preserved and impaired insight
Among the 31 patients with schizophrenia, 21 patients (67.7%) had preserved insight. Patients with preserved or impaired insight did not differ significantly in sociodemographic (age, sex, level of education), clinical (illness duration, PANSS) or medication data (antipsychotic medication, equiv_cmz, anti-depressants; Table 1).
In comparison to patients with impaired insight, those with preserved insight showed significantly increased perfusion in the bilateral precuneus (voxel-level significance of p < 0.001, uncorrected; cluster-level significance of p < 0.05, uncorrected; Fig. 1B, Table 2). No additional difference was found in SPECT perfusion between patients with preserved and impaired insight, particularly within frontotemporal areas.
Perfusion values of the bilateral precuneus cluster were extracted and are presented in Figure 2.
Fig. 2.
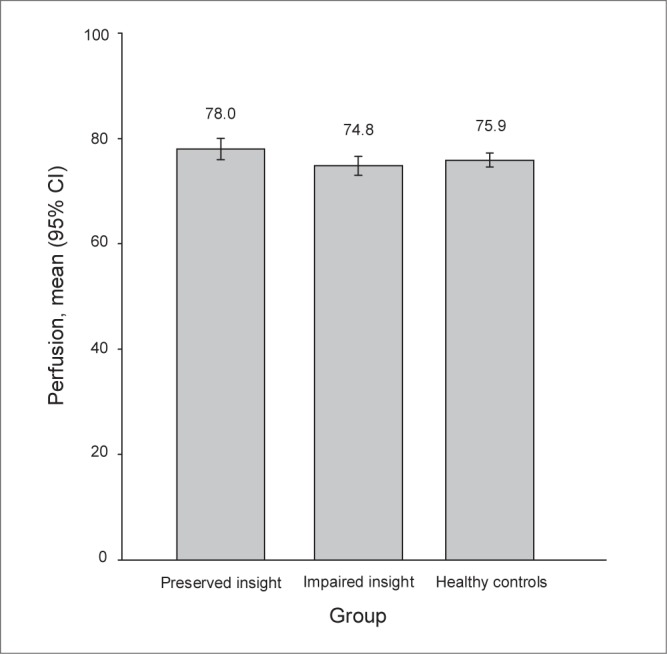
Precuneus single photon emission computed tomography perfusion in patients with schizophrenia with either preserved or impaired insight and in healthy controls. CI = confidence interval.
Discussion
This whole-brain voxel-based study specifically investigated the neural substrate underlying preserved insight in patients with schizophrenia. Because impaired insight is associated with schizophrenia severity, we focused on a sample of clinically stable outpatients characterized by mild disease severity to increase the proportion of patients in our sample with preserved insight.7 Our results show that patients with preserved insight exhibit greater precuneus perfusion than patients with impaired insight and similar hypoperfusion of frontotemporal areas to patients with impaired insight. Our findings may help to better understand the functional neural basis of preserved awareness in patients with schizophrenia and contribute to the development of new treatments targeting insight and related follow-up strategies.
Our findings are in support of those from previous studies in patients with schizophrenia that have reported the impairment of a complex neural network14,15 linked to unawareness of mental disorder involving the frontal, temporal and parietal brain regions.18–21,46,47 The relation between frontal regions and insight has been revealed in structural imaging studies.18–20,46–48 In particular, the dorsolateral prefrontal cortex has been involved in illness unawareness by interfering with self-monitoring and conceptual organization.46,48 Previous studies have also reported an association between temporal lobe regions and insight.15,21 These areas, more precisely the superior temporal sulcus, have been described in mental processes related to self-awareness and awareness of others.49,50 More recently, impairment of the superior temporal sulcus has been implicated in the functional substrate underlying quality of life,51 which is closely associated to insight in patients with schizophrenia.52 Located in the medial parietal lobe, the precuneus has been mainly involved in self-consciousness.14,21 In healthy controls, the precuneus is engaged in self-related mental representations: at rest (through the default mode network53) and during tasks about reflection on one’s own personality traits and physical appearance54 or about comparison between self- to non–self representations.55 The precuneus may be seen as a nodal structure in self-representation,53,56 functionally connected to frontal, temporal and other parietal regions.25,26,28,57 On the whole, these findings suggest that the multidimensional construct of insight relies on the fronto–temporo–parietal network.14
Our findings may contribute to clarifying the specific role of the precuneus in the neural network underlying preserved insight. Within this fronto–temporo–parietal network, the greater precuneus perfusion in patients with preserved versus impaired insight observed in our study may reflect the solicitation of additional neural resources to accomplish conscious self-representation in patients with frontotemporal impairment. Interestingly, a previous study has reported a correlation between improved insight and fMRI activation of the left medial prefrontal cortex after recovering from an acute episode;24 this area is involved in the same network as the precuneus.58 From a cognitive perspective, a recent study has identified memory acquisition as the only significant predictor of knowledge outcome involved in insight.59 On the other hand, insight, conceptualized as a self-representation,5 particularly draws on episodic memory in patients with schizophrenia.60 Moreover, it has been suggested that precuneus perfusion is correlated with linking new information with prior information, with an important role in retrieval of episodic memory.17 We can thus hypothesize that the precuneus involvement might reflect a better binding ability and retrieval/reintegration of episodic memory61 and thereby underlie preserved insight. In addition, the precuneus has also been shown to be activated in emotional functions, especially in the evaluation of emotion in the self and others.53 In a recent study, the precuneus has been involved in awareness of one’s own emotional state by integrating interoceptive information with information about the environmental and contextual situation.62 On the other hand, decreased emotional awareness has also been associated with attenuation of precuneus activation.57 These findings could indicate that in patients with preserved insight the precuneus plays an integrative role in the general binding ability of information to generate self-emotional or mental-states evaluations.
Because preserved insight is associated with favourable prognosis and is an important therapeutic goal in patients with schizophrenia, precuneus-mediated cognitive functions should be considered in the treatment and follow-up of patients with impaired insight. Significant gains in insight may result by focusing cognitive remediation on memory training tasks and episodic encoding strategies. Interestingly, Fiszdon and colleagues63 and Gsottschneider and colleagues64 showed the effectiveness of such training for improving memory performance in patients with schizophrenia. Moreover, a recent study60 has shown that improvement of insight and self-awareness in cognitive behaviour therapy is associated with increased precuneus activation during distorted conditions. In the same way, individual psychotherapy could be used to develop metacognitive capacity for self-reflection, especially by seeking to sharpen awareness.65,66 On the other hand, repetitive transcranial magnetic stimulation (rTMS) applied to the precuneus in healthy individuals has been found to enhance performance in memory67 and visuospatial information.68 Clearly, more research is needed to determine the effects of precuneus rTMS on insight in patients with schizophrenia. Finally, the potential clinical value of brain SPECT deserves further attention to improve the evaluation of insight and to assess the effect of therapy.69
Limitations
Some limitations of this study have to be carefully considered. First, our homogeneous sample may not be representative of the entire population of patients with schizophrenia. Patients in our study all had paranoid schizophrenia and were middle-aged men with mild disease severity and more than 5 years of illness duration. Confirmation is therefore needed in more diverse and larger groups of patients. Patients with subtypes other than paranoid schizophrenia, particularly those with serious negative or cognitive symptoms, have to be investigated to assess whether involvement of the precuneus in patients with preserved insight can be identified across the full spectrum of subtypes and symptoms of schizophrenia, especially since awareness of different kinds of symptoms may require different kinds of cognitive activities. On the other hand, our study concerned only 1 instrument (SUMD), which is a researcher-rated method of insight assessment. Although SUMD is valid and reliable for assessing insight,6,70 previous research has established that a moderate correlation exists between researcher-rated and self-report insight scales because each of these instruments may be measuring a different aspect of insight.71 It would be interesting to know whether our findings can be replicated with other insight instruments. In the same way, our study concerned only 1 central dimension —“awareness of mental disorder” — of the SUMD, as in several previous studies,16,19,72 and other dimensions of insight should be studied. Moreover, the 3 insight items of SUMD could be studied separately to facilitate comparisons with other neuro-imaging reports.
Conclusion
Our results show that schizophrenia with preserved insight is associated with an increased perfusion of the precuneus, a brain area involved in self-consciousness compared with impaired insight. This precuneus involvement may be a compensatory mechanism of frontotemporal impairment. This hypothesis, which should be strengthened by further connectivity studies within the prefrontal–superior temporal sulcus–precuneus network, may have important implications for the development of insight-oriented neuropsychiatric treatment and related follow-up.
Acknowledgments
This work was supported by the Institut national de la santé et de la recherche médicale (INSERM; Centre d’Investigation Clinique, Hôpital de la Conception, Marseille) and by Assistance Publique — Hôpitaux de Marseille (AP-HM; PHRC 2007/09).
Footnotes
Competing interests: None declared.
Contributors: C. Faget-Agius, R. Richieri, O. Mundler, C. Lançon and E. Guedj designed the study. C. Faget-Agius, R. Padovani, R. Richieri, O. Mundler and E. Guedj acquired the data. L. Boyer and E. Guedj analyzed the data. C. Faget-Agius, L. Boyer and E. Guedj wrote the article. C. Faget-Agius, R. Padovani, R. Richieri, O. Mundler, C. Lançon and E. Guedj reviewed the article. All authors approved its publication.
References
- 1.Mutsatsa SH, Joyce EM, Hutton SB, et al. Relationship between insight, cognitive function, social function and symptomatology in schizophrenia: the West London first episode study. Eur Arch Psychiatry Clin Neurosci. 2006;256:356–63. doi: 10.1007/s00406-006-0645-7. [DOI] [PubMed] [Google Scholar]
- 2.Thompson KN, McGorry PD, Harrigan SM. Reduced awareness of illness in first-episode psychosis. Compr Psychiatry. 2001;42:498–503. doi: 10.1053/comp.2001.27900. [DOI] [PubMed] [Google Scholar]
- 3.David AS. Insight and psychosis. Br J Psychiatry. 1990;156:798–808. doi: 10.1192/bjp.156.6.798. [DOI] [PubMed] [Google Scholar]
- 4.Baier M. Insight in schizophrenia: a review. Curr Psychiatry Rep. 2010;12:356–61. doi: 10.1007/s11920-010-0125-7. [DOI] [PubMed] [Google Scholar]
- 5.Lysaker PH, Buck KD, Salvatore G, et al. Lack of awareness of illness in schizophrenia: conceptualizations, correlates and treatment approaches. Expert Rev Neurother. 2009;9:1035–43. doi: 10.1586/ern.09.55. [DOI] [PubMed] [Google Scholar]
- 6.Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150:873–9. doi: 10.1176/ajp.150.6.873. [DOI] [PubMed] [Google Scholar]
- 7.Mintz AR, Dobson KS, Romney DM. Insight in schizophrenia: a meta-analysis. Schizophr Res. 2003;61:75–88. doi: 10.1016/s0920-9964(02)00316-x. [DOI] [PubMed] [Google Scholar]
- 8.Erickson M, Jaafari N, Lysaker P. Insight and negative symptoms as predictors of functioning in a work setting in patients with schizophrenia. Psychiatry Res. 2011;189:161–5. doi: 10.1016/j.psychres.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Saeedi H, Addington J, Addington D. The association of insight with psychotic symptoms, depression, and cognition in early psychosis: a 3-year follow-up. Schizophr Res. 2007;89:123–8. doi: 10.1016/j.schres.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Amador XF, Flaum M, Andreasen NC, et al. Awareness of illness in schizophrenia and schizoaffective and mood disorders. Arch Gen Psychiatry. 1994;51:826–36. doi: 10.1001/archpsyc.1994.03950100074007. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz RC, Cohen BN, Grubaugh A. Does insight affect long-term impatient treatment outcome in chronic schizophrenia? Compr Psychiatry. 1997;38:283–8. doi: 10.1016/s0010-440x(97)90061-4. [DOI] [PubMed] [Google Scholar]
- 12.Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21:129–41. doi: 10.2165/00023210-200721020-00004. [DOI] [PubMed] [Google Scholar]
- 13.Drake RJ, Dunn G, Tarrier N, et al. Insight as a predictor of the outcome of first-episode nonaffective psychosis in a prospective cohort study in England. J Clin Psychiatry. 2007;68:81–6. doi: 10.4088/jcp.v68n0111. [DOI] [PubMed] [Google Scholar]
- 14.Antonius D, Prudent V, Rebani Y, et al. White matter integrity and lack of insight in schizophrenia and schizoaffective disorder. Schizophr Res. 2011;128:76–82. doi: 10.1016/j.schres.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchy L, Ad-Dab’bagh Y, Malla A, et al. Cortical thickness is associated with poor insight in first-episode psychosis. J Psychiatr Res. 2011;45:781–7. doi: 10.1016/j.jpsychires.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Lysaker PH, Dimaggio G, Buck KD, et al. Poor insight in schizophrenia: links between different forms of metacognition with awareness of symptoms, treatment need, and consequences of illness. Compr Psychiatry. 2011;52:253–60. doi: 10.1016/j.comppsych.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Lou HC, Luber B, Crupain M, et al. Parietal cortex and representation of the mental self. Proc Natl Acad Sci U S A. 2004;101:6827–32. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laroi F, Fannemel M, Ronneberg U, et al. Unawareness of illness in chronic schizophrenia and its relationship to structural brain measures and neuropsychological tests. Psychiatry Res. 2000;100:49–58. doi: 10.1016/s0925-4927(00)00063-9. [DOI] [PubMed] [Google Scholar]
- 19.Shad MU, Muddasani S, Prasad K, et al. Insight and prefrontal cortex in first-episode schizophrenia. Neuroimage. 2004;22:1315–20. doi: 10.1016/j.neuroimage.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Sapara A, Cooke M, Fannon D, et al. Prefrontal cortex and insight in schizophrenia: a volumetric MRI study. Schizophr Res. 2007;89:22–34. doi: 10.1016/j.schres.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Cooke MA, Fannon D, Kuipers E, et al. Neurological basis of poor insight in psychosis: a voxel-based MRI study. Schizophr Res. 2008;103:40–51. doi: 10.1016/j.schres.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan KD, Dazzan P, Morgan C, et al. Insight, grey matter and cognitive function in first-onset psychosis. Br J Psychiatry. 2010;197:141–8. doi: 10.1192/bjp.bp.109.070888. [DOI] [PubMed] [Google Scholar]
- 23.Richieri R, Boyer L, Lançon C, et al. Predict the outcome of depression after rTMS using neuroimaging: Issue of response or non-response? Brain Stimul. 2012 Feb. doi: 10.1016/j.brs.2011.12.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Lee KH, Brown WH, Egleston PN, et al. A functional magnetic resonance imaging study of social cognition in schizophrenia during an acute episode and after recovery. Am J Psychiatry. 2006;163:1926–33. doi: 10.1176/ajp.2006.163.11.1926. [DOI] [PubMed] [Google Scholar]
- 25.Mauri MC, Gaietta M, Dragogna F, et al. Hallucinatory disorder, an original clinical picture? Clinical and imaging data. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:523–30. doi: 10.1016/j.pnpbp.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Shi S, Shu L. A review of SPECT studies in psychiatry in China. Neuropsychiatr Dis Treat. 2006;2:43–51. [PMC free article] [PubMed] [Google Scholar]
- 27.Ertugrul A, Volkan-Salanci B, Basar K, et al. The effect of clozapine on regional cerebral blood flow and brain metabolite ratios in schizophrenia: relationship with treatment response. Psychiatry Res. 2009;174:121–9. doi: 10.1016/j.pscychresns.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Kohno T, Shiga T, Kusumi I, et al. Left temporal perfusion associated with suspiciousness score on the Brief Psychiatric Rating Scale in schizophrenia. Psychiatry Res. 2006;147:163–71. doi: 10.1016/j.pscychresns.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 2000. text revised. [Google Scholar]
- 30.Appelberg B, Tuisku K, Joffe G. Is it worth while changing clinically stable schizophrenic out-patients with mild to moderate residual symptoms and/or side effects from conventional to atypical antipsychotics? A prospective, randomised study with olanzapine. Eur Psychiatry. 2004;19:516–8. doi: 10.1016/j.eurpsy.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 31.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 32.Act n°78-17 of 6 January 1978 on Data Processing, Data Files and Individual Liberties (amended by the Act of 6 August 2004 relating to the protection of individuals with regard to the processing of personal data) Paris: Commission Nationale de l’Informatique et des Libertés; 2004. [Google Scholar]
- 33.World Medical Association. Declaration of Helsinki, Ethical Principles for Medical Research Involving Human Subjects. Seoul (Korea): The Association; 2008. [Google Scholar]
- 34.Boyer L, Aghababian V, Richieri R, et al. Insight into illness, neuro-cognition and quality of life in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:271–6. doi: 10.1016/j.pnpbp.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Tharyan A, Saravanan B. Insight and psychopathology in schizophrenia. Indian J Psychiatry. 2000;42:421–6. [PMC free article] [PubMed] [Google Scholar]
- 36.Paillot CM, Ingrand P, Jaafari N Insight Study Group. What is the value of a single global score of insight into mental disorders? Ann Med Psychol (Paris) 2011;169:459–60. [Google Scholar]
- 37.Tabachnick BG, Fidell LS. Using multivariate statistics. 5th ed. Boston (MA): Pearson/Allyn & Bacon; 2007. [Google Scholar]
- 38.Bland JM, Altman DG. Cronbach’s alpha. BMJ. 1997;314:572. doi: 10.1136/bmj.314.7080.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- 40.Kay SR, Opler LA, Fiszbein A. Significance of positive and negative syndromes in chronic schizophrenia. Br J Psychiatry. 1986;149:439–48. doi: 10.1192/bjp.149.4.439. [DOI] [PubMed] [Google Scholar]
- 41.Lançon C, Auquier P, Nayt G, et al. Stability of the five-factor structure of the Positive and Negative Syndrome Scale (PANSS) Schizophr Res. 2000;42:231–9. doi: 10.1016/s0920-9964(99)00129-2. [DOI] [PubMed] [Google Scholar]
- 42.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 43.Richieri R, Boyer L, Farisse J, et al. Predictive value of brain perfusion SPECT for rTMS response in pharmacoresistant depression. Eur J Nucl Med Mol Imaging. 2011;38:1715–22. doi: 10.1007/s00259-011-1850-9. [DOI] [PubMed] [Google Scholar]
- 44.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guedj E, Barbeau EJ, Didic M, et al. Identification of subgroups in amnestic mild cognitive impairment. Neurology. 2006;67:356–8. doi: 10.1212/01.wnl.0000225076.73312.d4. [DOI] [PubMed] [Google Scholar]
- 46.Shad MU, Muddasani S, Keshavan MS. Prefrontal subregions and dimensions of insight in first-episode schizophrenia — a pilot study. Psychiatry Res. 2006;146:35–42. doi: 10.1016/j.pscychresns.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Shad MU, Tamminga CA, Cullum M, et al. Insight and frontal cortical function in schizophrenia: a review. Schizophr Res. 2006;86:54–70. doi: 10.1016/j.schres.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Flashman LA, McAllister TW, Johnson SC, et al. Specific frontal lobe subregions correlated with unawareness of illness in schizophrenia: a preliminary study. J Neuropsychiatry Clin Neurosci. 2001;13:255–7. doi: 10.1176/jnp.13.2.255. [DOI] [PubMed] [Google Scholar]
- 49.Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 50.Materna S, Dicke PW, Thier P. The posterior superior temporal sulcus is involved in social communication not specific for the eyes. Neuropsychologia. 2008;46:2759–65. doi: 10.1016/j.neuropsychologia.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 51.Boyer L, Richieri R, Faget C, et al. Impairment of superior temporal sulcus in schizophrenia: insight for involvement of metacognition in quality of life of patients. Psychiatry Res. doi: 10.1016/j.pscychresns.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 52.Aghababian V, Auquier P, Baumstarck-Barrau K, et al. Relationship between insight and self-reported quality of life among shizophrenic patients [Article in French] Encéphale. 2011;37:162–71. doi: 10.1016/j.encep.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 54.Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage. 2002;17:1080–6. [PubMed] [Google Scholar]
- 55.Kircher TT, Senior C, Phillips ML, et al. Towards a functional neuroanatomy of self processing: effects of faces and words. Brain Res Cogn Brain Res. 2000;10:133–44. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- 56.Vogeley K, May M, Ritzl A, et al. Neural correlates of first-person perspective as one constituent of human self-consciousness. J Cogn Neurosci. 2004;16:817–27. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- 57.Swart M, Bruggeman R, Laroi F, et al. COMT Val158Met polymorphism, verbalizing of emotion and activation of affective brain systems. Neuroimage. 2011;55:338–44. doi: 10.1016/j.neuroimage.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 58.Zhang S, Li CS. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2011 Nov. doi: 10.1016/j.neuroimage.2011.11.023. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jahn T, Pitschel-Walz G, Gsottschneider A, et al. Neurocognitive prediction of illness knowledge after psychoeducation in schizophrenia: results from the Munich COGPIP study. Psychol Med. 2011;41:533–44. doi: 10.1017/S0033291710001029. [DOI] [PubMed] [Google Scholar]
- 60.Kumari V, Antonova E, Fannon D, et al. Beyond dopamine: functional MRI predictors of responsiveness to cognitive behaviour therapy for psychosis. Front Behav Neurosci. 2010;4:4. doi: 10.3389/neuro.08.004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waters FA, Maybery MT, Badcock JC, et al. Context memory and binding in schizophrenia. Schizophr Res. 2004;68:119–25. doi: 10.1016/S0920-9964(03)00221-4. [DOI] [PubMed] [Google Scholar]
- 62.Terasawa Y, Fukushima H, Umeda S. How does interoceptive awareness interact with the subjective experience of emotion? An fMRI Study. Hum Brain Mapp. 2011 Nov. doi: 10.1002/hbm.21458. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fiszdon JM, Whelahan H, Bryson GJ, et al. Cognitive training of verbal memory using a dichotic listening paradigm: impact on symptoms and cognition. Acta Psychiatr Scand. 2005;112:187–93. doi: 10.1111/j.1600-0447.2005.00565.x. [DOI] [PubMed] [Google Scholar]
- 64.Gsottschneider A, Keller Z, Pitschel-Walz G, et al. The role of encoding strategies in the verbal memory performance in patients with schizophrenia. J Neuropsychol. 2011;5(Pt 1):56–72. doi: 10.1348/174866410X497382. [DOI] [PubMed] [Google Scholar]
- 65.Lysaker PH, Buck KD, Carcione A, et al. Addressing metacognitive capacity for self reflection in the psychotherapy for schizophrenia: a conceptual model of the key tasks and processes. Psychol Psychother. 2010 Aug. doi: 10.1348/147608310X520436. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Moritz S, Veckenstedt R, Randjbar S, et al. Antipsychotic treatment beyond antipsychotics: metacognitive intervention for schizophrenia patients improves delusional symptoms. Psychol Med. 2011;41:1823–32. doi: 10.1017/S0033291710002618. [DOI] [PubMed] [Google Scholar]
- 67.Luber B, Kinnunen LH, Rakitin BC, et al. Facilitation of performance in a working memory task with rTMS stimulation of the precuneus: frequency- and time-dependent effects. Brain Res. 2007;1128:120–9. doi: 10.1016/j.brainres.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 68.Oshio R, Tanaka S, Sadato N, et al. Differential effect of double-pulse TMS applied to dorsal premotor cortex and precuneus during internal operation of visuospatial information. Neuroimage. 2010;49:1108–15. doi: 10.1016/j.neuroimage.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 69.Guedj E, Cammilleri S, Colavolpe C, et al. Follow-up of pain processing recovery after ketamine in hyperalgesic fibromyalgia patients using brain perfusion ECD-SPECT. Eur J Nucl Med Mol Imaging. 2007;34:2115–9. doi: 10.1007/s00259-007-0589-9. [DOI] [PubMed] [Google Scholar]
- 70.Amador XF, Strauss DH. The Scale to Assess Unawareness of Mental Disorder. New York: Columbia University and New York Psychiatric Institute; 1990. [Google Scholar]
- 71.Young DA, Campbell Z, Zakzanis KK, et al. A comparison between an interview and a self-report method of insight assessment in chronic schizophrenia. Schizophr Res. 2003;63:103–9. doi: 10.1016/s0920-9964(02)00378-x. [DOI] [PubMed] [Google Scholar]
- 72.Ha TH, Youn T, Ha KS, et al. Gray matter abnormalities in paranoid schizophrenia and their clinical correlations. Psychiatry Res. 2004;132:251–60. doi: 10.1016/j.pscychresns.2004.05.001. [DOI] [PubMed] [Google Scholar]