Abstract
Background
Several patterns of grey and white matter changes have been separately described in young adults with first-episode psychosis. Concomitant investigation of grey and white matter densities in patients with first-episode psychosis without other psychiatric comorbidities that include all relevant imaging markers could provide clues to the neurodevelopmental hypothesis in schizophrenia.
Methods
We recruited patients with first-episode psychosis diagnosed according to the DSM-IV-TR and matched controls. All participants underwent magnetic resonance imaging (MRI). Voxel-based morphometry (VBM) analysis and mean diffusivity voxel-based analysis (VBA) were used for grey matter data. Fractional anisotropy and axial, radial and mean diffusivity were analyzed using tract-based spatial statistics (TBSS) for white matter data.
Results
We included 15 patients and 16 controls. The mean diffusivity VBA showed significantly greater mean diffusivity in the first-episode psychosis than in the control group in the lingual gyrus bilaterally, the occipital fusiform gyrus bilaterally, the right lateral occipital gyrus and the right inferior temporal gyrus. Moreover, the TBSS analysis revealed a lower fractional anisotropy in the first-episode psychosis than in the control group in the genu of the corpus callosum, minor forceps, corticospinal tract, right superior longitudinal fasciculus, left middle cerebellar peduncle, left inferior longitudinal fasciculus and the posterior part of the fronto-occipital fasciculus. This analysis also revealed greater radial diffusivity in the first-episode psychosis than in the control group in the right corticospinal tract, right superior longitudinal fasciculus and left middle cerebellar peduncle.
Limitations
The modest sample size and the absence of women in our series could limit the impact of our results.
Conclusion
Our results highlight the structural vulnerability of grey matter in posterior areas of the brain among young adult male patients with first-episode psychosis. Moreover, the concomitant greater radial diffusivity within several regions already revealed by the fractional anisotropy analysis supports the idea of a late myelination in patients with first-episode psychosis.
Introduction
Altered connectivity referring to abnormal functional integration between different brain regions has been considered a cardinal feature of schizophrenia.1 In line with the idea of an early default in brain development, it may reflect aberrant synaptic plasticity and wiring connections, excessive synaptic pruning or disruption of the intracortical myelination of white matter tracts.2 Although neurodevelopmental models have often been evoked to account for schizophrenia, some authors have also suggested the possible intervention of neurodegenerative processes.3 Some authors defended the idea that these alterations may be temporally close and possibly causally related to the emergence of the first episode of psychosis.4 To address this issue, numerous magnetic resonance imaging (MRI) studies have attempted to explore the presence of early neurodevelopmental deficits in patients with first-episode psychosis. Voxel-based morphometry (VBM), a fully automated image analysis of the whole brain that is free from a priori hypotheses, has yielded controversial data. Some studies reported less grey matter tissue globally in patients with first-episode psychosis compared with controls.5,6 Others reported less grey matter tissue confined to the frontal and temporal cortex in patients with first-episode psychosis.7,8 Contradictory data, such as a greater grey matter tissue in the frontal and temporal lobes in patients with first-episode psychosis have also been reported.9 The region of interest (ROI) analyses in such patients documented lower volume in the superior temporal gyrus,10 insula,11 cingulate cortex12 and at least 1 subdivision of the frontal lobe.13 Some studies revealed an enlargement of the cortical sulci in patients, as a greater global volume of cerebrospinal fluid (CSF) has been found in male patients with schizophrenia than in healthy controls,14 and a greater apparent diffusion coefficient within the bilateral insular cortex, hippocampus, temporal lobe and occipital area has been found in psychotic patients than in controls.15
Recently, the white matter changes in patients with first-episode psychosis have been investigated using diffusion tensor imaging (DTI). The voxel-based analysis (VBA) indicated a lower fractional anisotropy in patients than in controls that concerned both the interhemispheric tracts of the corpus collosum and long intrahemispheric tracts, such as the superior and inferior longitudinal fasciculus and inferior occipitofrontal fasciculus.16,17 Other areas of lower fractional anisotropy included the uncinate fasciculus, cingulum bundle, anterior thalamic radiation, corticospinal tract and cerebellum projections.16–19 Similarly, ROI studies reported a lower fractional anisotropy in the corpus callosum, uncinate fasciculus, superior longitudinal fasciculus and inferior longitudinal fasciculus in patients with first-episode psychosis.20,21 Axial, radial and mean diffusivity data were not conclusive in patients with first-episode psychosis.22,23 A recently introduced advanced analysis technique, tract-based spatial statistics (TBSS), that allows for reducing potential misregistrations by projecting all individual DTI parameters onto a group average white matter skeleton, has been used in 2 studies involving adolescents with first-episode psychosis.6,23 These studies reported lower fractional anisotropy that concerned a vast network of white matter tracts.
Several methodologic issues limited the impact of these studies. Most included patients with psychiatric comorbidities and did not control for a possible effect of antipsychotics and cannabis on brain structure. Most importantly, no concomitant assessment of grey and white matter alterations has been performed in patients with first-episode psychosis. To our knowledge, no study explored concomitant grey and white matter changes in young adults with first-episode psychosis using all of the relevant DTI markers (fractional anisotropy, and axial, radial and mean diffusivity). To address this issue, we performed a detailed investigation of grey matter (VBM, ROI analyses, mean diffusivity VBA) and white matter (TBSS analysis of DTI parameters) in young adults with first-episode psychosis who were free from major psychiatric comorbidities, controlling for medication and substance-use effects.
Methods
Participants
We recruited patients with first-episode psychosis from the specialized unit for early psychosis in the General Psychiatry Division of the Geneva University Hospitals, and we recruited healthy controls from the local community via advertisements. Patients had to present acute psychotic symptoms for no more than 1 year before evaluation and fulfill criteria of a psychotic disorder according to the DSM-IV-TR to receive a diagnosis of first-episode psychosis. For each patient, the diagnosis was established by combining information gathered from several sources (relatives, staff members and previous medical records when available) and using the Structured Clinical Interview for DSM disorders (SCID).24 Diagnoses were confirmed after a 6-month follow-up by 2 independent clinical psychiatrists. Patients were clinically rated for symptom severity using a 24-item Brief Psychiatric Rating Scale (BPRS).25 Exclusion criteria for both groups were history of major neurologic disorders (i.e., dementing conditions, tumours, neuroimmunological disorders), history of head trauma, presence of a current or a past DSM-IV psychiatric diagnosis (other than first-episode psychosis), current systemic medical disease requiring inpatient treatment, less than 4 years of formal education, and major hearing, vision or motor impairment. We also excluded those who regularly used stimulants and β-blockers, had substance abuse or dependence (other than cannabis or alcohol), had developmental difficulties (e.g., an IQ below 75, as reported in medical records) or had severe physical illness that precluded their participation in either phase of the project. After receiving formal approval of the study protocol from the local ethics committee, we obtained written informed consent from all participants before their inclusion in this study.
Patients underwent standardized neuropsychological assessment, including 5 tasks of the Tests of Attentional Performance:26 the alertness test for processing speed, the divided attention test for attentional capacity, the working memory test and the go/no go and the flexibility tests for executive functions. We performed group comparisons of demographic and clinical variables using the Mann–Whitney U test for independent samples. To standardize the medication load, we computed a chlorpromazine (CPZ) equivalent for each patient.27
MRI protocol
Magnetic resonance imaging was performed on a 1.5 T clinical whole-body system (Phillips Medical Systems). Structural imaging consisted of a 3-dimensional T1-weighted spoiled gradient echo sequence obtained with the following parameters: 124 coronal slices, slice thickness 1.5 mm, in plane resolution 0.94 × 0.94 mm2, echo time (TE) 6 ms, repetition time (TR) 35 ms, flip angle 45°, 1 average. Diffusion tensor imaging (DTI) used 6 noncollinear diffusion directions with b-factor 1000 s/mm2, 1 reference image with no diffusion weighting (b-factor 0), in plane resolution 1.95 × 1.95 mm2, slice thickness 5 mm, TE 70 ms, TR 3652 ms, 2 averages.
Voxel-based morphometry analysis
The VBM analyses were performed with the FSL software package (www.fmrib.ox.ac.uk/fsl/), version 4.1, according to standard processing steps.28 First, structural images were brain-extracted using BET (part of FSL). Then, tissue-type segmentation was carried out using FAST4 (part of FSL). Next, the alignment of the partial volume images into Montreal Neurological Institute (MNI) reference space was performed using the affine registration tool FLIRT followed by nonlinear registration using FNIRT. We then created a study-specific grey matter template, to which the native grey matter images were nonlinearly reregistered. Next, the modulated segmented images were smoothed with an isotropic Gaussian kernel with a sigma of 3 mm. Finally, we applied a mixed-effects model using permutation-based nonparametric testing (RANDOMISE, part of FSL), correcting for multiple comparisons by implementing threshold-free cluster enhancement (TFCE). The effect of group (first-episode psychosis v. controls) was tested with age, cannabis use, educational level and medication load as nonexplanatory coregressors. Fully TFCE-corrected p values < 0.05 were considered to be significant. The entire grey matter template included 256 365 voxels. To assess structural changes found in ROI studies, we repeated the VBM analyses with a unique grey matter mask of 65 233 voxels containing the superior, middle, inferior and medial frontal cortices; the orbitofrontal cortex; the superior, middle and inferior temporal cortices; the Heschl gyrus; the fusiform gyrus; the insular cortex; and the cingulate cortex.
Grey matter mean diffusivity voxel-based analysis
The mean diffusivity VBA was derived from the apparent diffusion coefficient–based morphometry analysis proposed by Ardekani and colleagues.15 This mean diffusivity VBA was performed in the FSL software package. In brief, the mean diffusivity images were corrected for eddy current and head motion. Then, these images were brain-extracted using BET. All participants’ mean diffusivity data were then aligned into the same MNI common space described for the VBM analysis using the nonlinear registration tool FNIRT. We applied a cortical grey matter mask including 156 460 voxels to the mean diffusivity data. Finally, the mixed-effects model used for the VBM analyses was also applied for this mean diffusivity VBA. Fully TFCE-corrected p values < 0.05 were considered to be significant.
White matter TBSS analysis of DTI data
The TBSS analysis of the DTI data was also performed in the FSL software package according to the standard procedure.29 In short, the images were corrected for eddy current and head motion. Then, fractional anisotropy images were created by fitting a tensor model to the raw diffusion data using FDT (part of FSL), and then brain-extracted using BET. All participants’ fractional anisotropy data were then aligned into the same MNI common space described for the VBM analysis using the nonlinear registration tool FNIRT. Next, the mean fractional anisotropy image was created and thinned to produce a mean fractional anisotropy skeleton representing the centres of all tracts common to the group. Our TBSS skeleton mask included 96 506 voxels. Each participant’s aligned fractional anisotropy data were then projected onto this skeleton. The other DTI-derived parameters (axial, radial and mean diffusivity) were analyzed in the same way by reusing the spatial transformation parameters that were estimated in the initial fractional anisotropy analysis. Voxelwise statistical analysis was performed with TFCE correction for multiple comparisons; we considered fully corrected p values < 0.05 to be significant. Once again, we applied a mixed-effects model with group (first-episode psychosis v. controls) as the main factor, with age, cannabis use, level of education and medication load as nonexplanatory coregressors.
Correlation analyses between MRI and clinical data
We conducted regression analysis within the first-episode psychosis group to assess correlations between symptoms and imaging data. More specifically, a positive symptom score and a negative symptom score were extracted from the BPRS scale using the method advocated by Ventura and colleagues.30 Concretely, the positive symptom score was the sum of the scores obtained in the bizarre behaviour, unusual thought content, disorientation, hallucinations and suspiciousness items of the BPRS, whereas the negative symptom score was the sum of the scores obtained in the blunted affect, motor retardation, emotional withdrawal and self-neglect items of the BPRS. Each of these 2 scores was next demeaned and separately used as a single explanatory regressor in either VBM, mean diffusivity VBA or TBSS data. Here again, we used age, level of education, cannabis and medication load as nonexplanatory coregressors. We considered results to be significant at p < 0.05, TFCE-corrected. We conducted equivalent analyses to examine the association between the neuropsychological tests within the first-episode psychosis group and either VBM, mean diffusivity VBA or TBSS data. More precisely, response latency of each neuropsychological test was demeaned and used as a single explanatory regressor in separate analyses with age, level of education, cannabis and medication load used as nonexplanatory coregressors.
Results
Demographic, clinical, pharmacological and cognitive data
We included 15 patients with first-episode psychosis and 16 healthy controls in our study. All participants were right-handed and male. There was no significant difference in age between patients and controls. However, the level of education was significantly lower and the cannabis consumption was significantly higher among patients than controls (Table 1). All participants had normal or corrected-to-normal visual acuity, and none reported a history of sustained head injury or neurologic disorders. Six patients fulfilled diagnostic criteria for paranoid schizophrenia, 6 for brief psychotic disorder, 2 for schizoaffective disorder and 1 for substance-induced psychotic disorder. All patients were treated with second-generation antipsychotics after admission to the specialized psychiatric unit. Patients were under antipsychotic treatment for 8 months at most before MRI. All patients received atypical neuroleptics, 13% received benzodiazepines at low dose and 6% received antidepressants at low dose. The cognitive performances of patients with first-episode psychosis for all of the 5 tasks were within the normal ranges (Table 2).
Table 1.
Demographic, clinical and pharmacological characteristics of patients with first-episode psychosis and healthy controls
| Group; mean (SD) | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | FEP, n = 15 | Control, n = 16 | z score | p value |
| Age, yr | 22 (2.2) | 22.5 (2.7) | −0.45 | 0.65 |
| Education, no. yr | 12 (1.6) | 15.2 (1.9) | −4.96 | < 0.001 |
| Cannabis consumption* | 1.9 (1.7) | 0.3 (0.7) | 3.49 | 0.001 |
| BPRS | 75.6 (22.8) | — | — | — |
| Positive symptoms | 17.9 (7.5) | — | — | — |
| Negative symptoms | 9.1 (4.8) | — | — | — |
| CPZ equivalents in mg† | 227 (145) | — | — | — |
BPRS = Brief Psychiatric Rating Scale;25 CPZ = chlorpromazine; FEP = first-episode psychosis; SD = standard deviation.
The cannabis consumption within 3 months preceding inclusion in the study was evaluated on a 5-point scale (0 = no consumption; 4 = high consumption).
The daily medication regimens for patients in the first-episode psychosis group were as follows: 100 mg clozapine (n = 2); 10 mg olanzapine (n = 2); 50 mg clozapine (n = 1); 75 mg clozapine (n = 1); 15 mg olanzapine (n = 1); 25 mg clozapine and 200 mg amisulpide (n = 1); 400 mg quetiapine (n = 2); 1 mg risperidone and 75 mg clozapine (n = 2); 10 mg olanzapine and 100 mg promazine (n = 1); 150 mg clozapine and 400 mg amisulpide (n = 1); 75 mg clozapine, 20 mg olanzapine and 100 mg promazine (n = 1).
Table 2.
Neuropsychological test results of patients with first-episode psychosis, n = 15*
| Cognitive domain; test measure | Mean (SD) score |
|---|---|
| Processing speed | |
| Alertness test | |
| Response latency, ms | 215 (27) |
| Anticipated response, % | 2 (2) |
| Attention | |
| Divided attention test | |
| Response latency, ms | 671 (78) |
| Correct response, % | 89 (8) |
| False-positive results, no. | 0.3 (0.5) |
| Working memory | |
| Working memory test | |
| Response latency, ms | 636 (161) |
| Correct response, % | 84 (14) |
| False-positive results, no. | 1.2 (1.4) |
| Executive function | |
| Go/no go test | |
| Response latency, ms | 580 (89) |
| Correct response, % | 94 (9) |
| False-positive results, no. | 1.5 (2.8) |
| Flexibility test | |
| Response latency, ms | 915 (322) |
| Correct response, % | 88 (8) |
SD = standard deviation.
Tests of Attentional Performance.2
Grey matter
The VBM analyses revealed no TFCE-corrected suprathreshold differences between patients with first-episode psychosis and controls when whole grey matter was considered. Similarly, the VBM analysis restricted to the frontal, temporal and cingulate cortices did not reveal significant group differences. However, the mean diffusivity VBA showed significantly higher mean diffusivity values in the first-episode psychosis group than the control group in one posterior region, including the lingual gyrus bilaterally, the occipital fusiform gyrus bilaterally, the right lateral occipital cortex and the right inferior temporal gyrus (Fig. 1, Table 3). The inverse comparison of the first-episode psychosis group with the control group yielded no significant voxels.
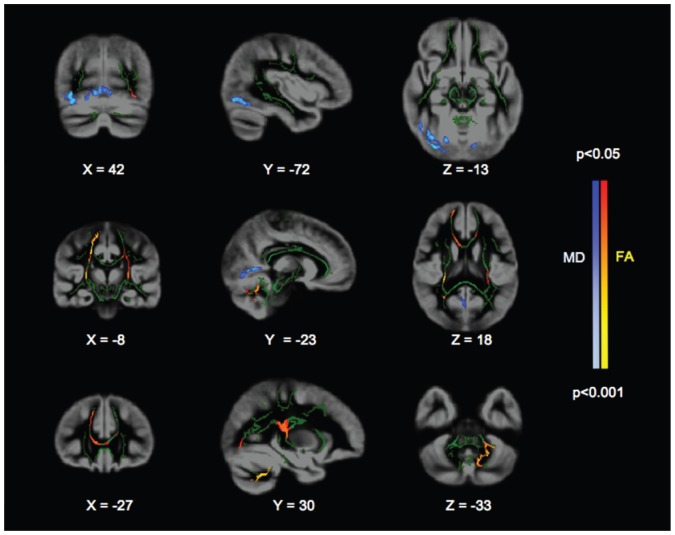
Fig. 1.
Mean diffusivity voxel-based analysis (VBA) and tract-based spatial statistics (TBSS) analysis of the patients with first-episode psychosis versus healthy controls shows the spatial distribution of threshold-free cluster enhancement–corrected significant differences between patients and controls. Grey areas denote the group average grey matter mask. Green areas denote the group average white matter skeleton. Blue and light blue areas denote significant voxels for the mean diffusivity VBA. Red and yellow areas denote significant voxels for the TBSS fractional anisotropy analysis. The image follows radiologic convention (left is right).
Table 3.
Grey matter mean diffusivity voxel-based analysis in patients with first-episode psychosis and healthy controls*
| Contrast; cluster index | Cluster size, mm3 | Maximum z score | Location of maximum z score† | Centre of gravity† | Side | Anatomic region‡ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| x | y | z | x | y | z | |||||
| FEP < control | ||||||||||
| No suprathreshold voxels | — | — | — | — | — | — | — | — | — | — |
| FEP > control | ||||||||||
| 1 | 6727 | 0.994 | 33 | −84 | −14 | 18.7 | −69.5 | −4.7 | Bilaterial Right | Lingual and occipital fusiform Lateral occipital and inferior temporal |
FEP = first-episode psychosis
Suprathreshold voxels after threshold-free cluster enhancement–correction for multiple comparisons for the voxel-based morphometry analysis. Significant threshold: z score ≥ 0.95.
Location provided in Montreal Neurological Institute space.
Label of the gyrus given for anatomic region.
White matter
Fractional anisotropy was significantly lower in the first-episode psychosis group than the control group in the genu of the corpus callosum, the right part of the minor forceps, the right part of the superior longitudinal fasciculus, the right corticospinal tract and the right posterior part of the inferior fronto-occiopital fasciculus (cluster 1, Table 4, Fig. 1). We observed additional lower fractional anisotropy values in the left middle cerebellar peduncles (cluster 2, Table 4, Fig. 1), the left corticospinal tract and genu of the corpus calosum (cluster 3, Table 4, Fig. 1), the left inferior longitudinal fasciculus and the left posterior inferior fronto-occipital fasciculus (cluster 4, Table 4, Fig. 1) in patients with first-episode psychosis.
Table 4.
Results of the white matter tract-based spatial statistics analysis; comparisons between patients with first-episode psychosis and healthy controls*
| Test; contrast; cluster index | Cluster size, mm3 | Maximum z score | Location of maximum z score† | Centre of gravity† | Side | Anatomic region | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| x | y | z | x | y | z | |||||
| Fractional anisotropy | ||||||||||
| FEP < control | ||||||||||
| 1 | 6031 | 0.998 | 23 | −33 | 43 | 19.7 | −12 | 36.2 | Right | Genu of the corpus callosum; superior longitudinal fasciculus; forceps minor; corticospinal tract; inferior fronto-occipital fasciculus |
| 2 | 2212 | 0.992 | −29 | −55 | −42 | −20.4 | −54.1 | −35.6 | Left | Middle cerebellar peduncle. |
| 3 | 1420 | 0.976 | −27 | −24 | 9 | −21.5 | −21.6 | 28.9 | Left | Genu of the corpus callosum; corticospinal tract |
| 4 | 682 | 0.966 | −35 | −73 | −10 | −37.6 | −64.2 | −4.5 | Left | Inferior longitudinal fasciculus, inferior fronto-occipital fasciculus |
| FEP > control | ||||||||||
| No suprathreshold voxels | — | — | — | — | — | — | — | — | — | — |
| Radial diffusivity | ||||||||||
| FEP < control | ||||||||||
| No suprathreshold voxels | — | — | — | — | — | — | — | — | — | — |
| FEP > control | ||||||||||
| 1 | 1072 | 0.985 | 23 | −33 | 44 | 14.5 | −31.5 | 34.2 | Right | Corticospinal tract; superior longitudinal fasciculus |
| 2 | 352 | 0.963 | −12 | −65 | −35 | −13.7 | −61.3 | −31.1 | Left | Middle cerebellar peduncle |
FEP = first-episode psychosis
Suprathreshold voxels after threshold-free cluster enhancement–correction for multiple comparisons for the voxel-based morphometry analysis. Significant threshold: z score ≥ 0.95.
Location provided in Montreal Neurological Institute space.
The radial diffusivity was greater in the first-episode psychosis group than the control group in the superior longitudinal fasciculus (cluster 1, Table 4) and the left middle cerebellar peduncle (cluster 2, Table 4). Of note, all the significant regions for radial diffusivity were also significant for fractional anisotropy. The analysis of mean diffusivity revealed no TFCE-corrected suprathreshold voxels but a trend within regions where radial diffusivity was significantly greater in patients with first-episode psychosis than controls. Finally, the analysis of axial diffusivity revealed no TFCE-corrected suprathreshold voxels.
Correlation analyses between MRI data, clinical data and neuropsychological tests
The correlation analysis for positive and negative symptoms yielded no TFCE-corrected suprathreshold voxels (VBM, mean diffusivity VBA, fractional anisotropy TBSS, radial diffusivity TBSS). We found no significant correlations between MRI data and the response latency of the different neuropsychological tests.
Discussion
Grey matter changes in first-episode psychosis
When the entire brain was considered, no significant differences in the amount of grey matter tissue were found between patients with first-episode psychosis and healthy controls in classical VBM analyses. Moreover, the restricted analysis encompassing the fronto-temporal lobe and the cingulate gyrus (including 65 233 voxels) did not reveal any significant differences either. In contrast, the analysis of the whole grey matter with mean diffusivity VBA (including 156 460 voxels) showed a significantly greater mean diffusivity in the first-episode psychosis group than in the control group within the medial occipital cortex bilaterally, and the right lateral occipital and right inferior temporal cortices. Interestingly, Ardekani and colleagues,15 using a similar index, found a greater apparent diffusion coefficient in patients with schizophrenia than controls in regions including the temporal and occipital areas. In agreement with Narr and colleagues,14 our results suggest that the presence of a subtle enlargement of the cortical sulci in posterior areas rather than lower cortical grey matter tissue is a robust neuro-anatomical correlate of first-episode psychosis.
White matter changes in first-episode psychosis
Our results strongly support the previously published VBM analyses of DTI data. We found lower fractional anisotropy in our first-episode psychosis group than in the control group in the genu of the corpus callosum and in the right minor forceps (as reported by Perez-Iglesias and colleagues16), in the right superior longitudinal fasciculus and the right corticospinal tract (as reported by Kyriakopoulos and colleagues17), and in the left middle cerebellar peduncle (as reported by Kyriakopoulos and colleagues19). Compared to ROI studies, our findings are congruent with the lower fractional anisotropy reported in the genu of the corpus callosum, right superior longitudinal fasciculus and left inferior longitudinal fasciculus in patients with first-episode psychosis.20,21 However, our results are discordant with the lower fractional anisotropy reported in the splenium of the corpus callosum, uncinate fasciculus and left superior longitudinal fasciculus.20,21 Overall, our results were similar to those reported in 2 recent TBSS studies involving adolescents with first-episode psychosis with respect to the lower fractional anisotropy among a vast network of fibre tracts.6,23 However, in contrast to Douaud and colleagues,23 we also found greater radial diffusivity in a network of fibre tracts similar to those highlighted in the fractional anisotropy analysis.
Our findings of a widespread lower fractional anisotropy in several corticocortical tracts give additional support to the disconnection hypothesis in first-episode psychosis. It is thus likely that neuronal interactions could be impaired by microstructural abnormalities in the geometry of axonal branches, the caliber of axons and/or the spatial distribution of synapses.31 Although the type of structural alterations underlying the change of the different DTI indexes is still debated,32 lower fractional anisotropy combined with greater radial diffusivity has often been associated with a lack of myelin.33 Thus, the lower fractional anisotropy and the concomitant greater radial diffusivity within several regions already highlighted in the fractional anisotropy analysis in our series would support the view of tardy myelination in patients with schizophrenia, possibly due to a delay in ongoing brain maturation.34
Impact of pharmacological treatment
Importantly, these differences could not have been due to the treatment of all patients with antipsychotic medications. We controlled for the influence of medication in the present study, as medication load was entered as a nonexplanatory coregressor both in the mean diffusivity VBA and TBSS analyses. It is highly likely that the period of time between the introduction of the pharmacological treatment and MRI (< 8 mo) was probably too short to have a significant impact on grey matter integrity. When medication load was entered as a unique regressor, we did not find any significant correlation between medication load and mean diffusivity VBA or TBSS parameters.
Grey versus white matter changes in first-episode psychosis
To our knowledge, 3 cross-sectional studies have concomitantly analyzed grey and white matter in patients with first-episode psychosis. James and colleagues6 and Douaud and colleagues23 studied both grey matter VBM and white matter TBSS in adolescent patients, whereas Moriya and colleagues35 studied grey matter VBM, and fractional anisotropy and mean diffusivity VBA in patients with first-episode psychosis between the ages of 13 and 52 (mean 30) years. James and colleagues6 found globally less grey matter tissue and lower fractional anisotropy in a vast network of white matter tracts in patients with first-episode psychosis than in controls. Douaud and colleagues23 found less grey matter tissue in the Heschl gyrus, parietal operculum and the supplementary motor area and lower fractional anisotropy in a vast network of white matter tracts in patients with first-episode psychosis than in controls. Finally, Moriya and colleagues35 did not find any significant difference in the amount of grey matter tissue or fractional anisotropy between patients with first-episode psychosis and controls. In line with the findings reported in these 3 studies, our results suggest that both grey and white matter changes are more pronounced in patients with first-episode psychosis with younger age at onset than in those with older age at onset. In any case, these combined results, as well as the drastic reduction of the grey and white matter alterations in adolescent patients with first-episode psychosis after 1 year follow-up reported by Haller and colleagues,36 argue against the classical neurodegenerative scenario.
Our results suggest that VBM analysis of grey matter could be less sensitive than TBSS analysis of fractional anisotropy. The restricted VBM analysis (including only 65 233 voxels) failed to show any significant results, whereas the TBSS analysis (including 96 506 voxels) of the fractional anisotropy within all the white matter tracts yielded significant results. This observation is consistent with several combined VBM and TBSS studies.36,37
Correlation between clinical or cognitive measures and grey or white matter data
In this series, we found no significant correlations between grey and white matter data and clinical or cognitive measures. Previous studies showed that at least some of the clinical parameters, such as delusion severity, may be associated with grey and white matter changes in patients with first-episode psychosis.38,39 Similarly, some previous contributions pointed to the positive correlation between executive function and working memory performances and white matter changes in these patients.21,40 Similar observations were made by Minatogawa-Chang and colleagues41 with respect to grey matter volumes in prefrontal and temporoparietal areas. We cannot exclude that the limited number of highly selected patients with first-episode psychosis and the use of 6-direction DTI with 5 mm slice thickness may have prevented us from detecting subtle correlations between clinical, cognitive and MRI data. However, this is an unlikely scenario given the positive data obtained in group comparisons with the same patient sample size. Alternatively, this absence of a relation may reflect the fact that grey and white matter changes are trait markers of the disease progression that remain independent of the clinical (or cognitive) status. Future studies in cohorts with first-episode psychosis are clearly needed to further explore this issue.
Limitations
Strengths of this study include the use of various MRI analyses (VBM, mean diffusivity VBA and TBSS), the high reliability of the diagnosis owing to the 6 months of follow-up by 2 independent psychiatrists, the careful exclusion of psychiatric comorbidities that could impact on the structural imaging data and our control for pharmacological treatment effect on MRI parameters. However, several limitations should also be taken into account. First, similar to previous studies involving patients with first-episode psychosis, our sample size was relatively small. Second, we only included male patients in our series. Third, the use of only 6 gradients of direction limited the precision of fractional anisotropy values. Fourth, since our study focused on the identification of subtle MRI changes in patients with first-episode psychosis and their possible cognitive repercussions, we did not administer the same cognitive tasks in our control group. Thus, we are not able to definitively rule out the possibility that the neural differences we found between patients and controls are owing to a difference in cognitive performances between these 2 groups. However, several arguments did not support this hypothesis. First, our study patients obtained cognitive performances well within the age-related norms, and we did not find any significant correlations between cognitive tests and neuroimaging data in the patient group. Second, we did not find any significant correlations between cognitive tests (including processing speed, attention, working memory and executive function tests) and neuroimaging data (including grey matter and mean diffusivity VBA and fractional anisotropy TBSS) within a group of 16 young healthy controls between the ages of 20 and 34 years in a subset of data from among our previous studies.42 Finally, a detailed history of cannabis use (including duration and quantity of consumption) for our patients was missing.
Conclusion
Future longitudinal studies addressing these limitations in community-dwelling series are needed to explore whether early grey and white matter changes may represent a predictive marker of evolution of first-episode psychosis.
Acknowledgements
This work was performed in collaboration with the Centre of biomedical imagery (CIBM) in Geneva, Switzerland.
Footnotes
Competing interests: None declared.
Contributors: A. Ruef, L. Curtis, F. Lazeyras, K. Lövblad, A. Malafosse, P. Giannakopoulos and M. Merlo designed the study. A. Ruef, L. Curtis, S. Bessero, M. Badan Bâ, F. Lazeyras and K. Lövblad acquired the data, which A. Ruef, L. Curtis, G. Moy, S. Bessero, F. Lazeyras, S. Haller and P. Giannakopoulos analyzed. A. Ruef, L. Curtis, G. Moy, S. Bessero and P. Giannakopoulos wrote the article, which A. Ruef, L. Curtis, G. Moy, M. Badan Bâ, F. Lazeyras, K. Lövblad, S. Haller, A. Malafosse, P. Giannakipoulos and M. Merlo reviewed. All authors approved its publication.
References
- 1.Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–27. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitford TJ, Ford JM, Mathalon DH, et al. Schizophrenia, myelination, and delayed corollary discharges: a hypothesis. Schizophr Bull. doi: 10.1093/schbul/sbq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barztokis G. Schizophrenia: breakdown in the well-regulated lifelong process of brain development and maturation. Neuropsychopharma-cology. 2002;27:672–83. doi: 10.1016/S0893-133X(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 4.Whitford TJ, Grieve SM, Farrow TF, et al. Progressive grey matter atrophy over the first 2–3 years of illness in first-episode schizophrenia: a tensor-based morphometry study. Neuroimage. 2006;32:511–9. doi: 10.1016/j.neuroimage.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 5.Crespo-Facorro B, Roiz-Santianez R, Perez-Iglesias R, et al. Global and regional cortical thinning in first-episode psychosis patients: relationships with clinical and cognitive features. Psychol Med. 2011;41:1449–60. doi: 10.1017/S003329171000200X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James A, Hough M, James S, et al. Greater white and grey matter changes associated with early cannabis use in adolescent-onset schizophrenia (AOS) Schizophr Res. 2011;128:91–7. doi: 10.1016/j.schres.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Bergé D, Carmona S, Rovira M, et al. Gray matter volume deficits and correlation with insight and negative symptoms in first-psychotic-episode subjects. Acta Psychiatr Scand. 2011;123:431–9. doi: 10.1111/j.1600-0447.2010.01635.x. [DOI] [PubMed] [Google Scholar]
- 8.Paillère-Martinot M, Caclin A, Artiges E, et al. Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophr Res. 2001;50:19–26. doi: 10.1016/s0920-9964(00)00137-7. [DOI] [PubMed] [Google Scholar]
- 9.Salgado-Pineda P, Baeza I, Perez-Gomez M, et al. Sustained attention impairment correlates to gray matter decreases in first episode neuroleptic-naive schizophrenic patients. Neuroimage. 2003;19:365–75. doi: 10.1016/s1053-8119(03)00094-6. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi T, Wood SJ, Yung AR, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366–76. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- 11.Kasai K, Shenton ME, Salisbury DF, et al. Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. Arch Gen Psychiatry. 2003;60:1069–77. doi: 10.1001/archpsyc.60.11.1069. [DOI] [PubMed] [Google Scholar]
- 12.Hirayasu Y, Shenton ME, Salisbury DF, et al. Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry. 1999;156:1091–3. doi: 10.1176/ajp.156.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirayasu Y, Tanaka S, Shenton ME, et al. Prefrontal gray matter volume reduction in first episode schizophrenia. Cereb Cortex. 2001;11:374–81. doi: 10.1093/cercor/11.4.374. [DOI] [PubMed] [Google Scholar]
- 14.Narr KL, Sharma T, Woods RP, et al. Increases in regional sub-arachnoid CSF without apparent cortical gray matter deficit in schizophrenia: modulating effects of sex and age. Am J Psychiatry. 2003;160:2169–80. doi: 10.1176/appi.ajp.160.12.2169. [DOI] [PubMed] [Google Scholar]
- 15.Ardekani BA, Bappal A, D’Angelo D, et al. Brain morphometry using diffusion-weighted magnetic resonance imaging: application to schizophrenia. Neuroreport. 2005;16:1455–9. doi: 10.1097/01.wnr.0000177001.27569.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pérez-Iglesias R, Tordesillas-Gutierrez D, Barker GJ, et al. White matter defects in first episode psychosis patients: a voxelwise analysis of diffusion tensor imaging. Neuroimage. 2010;49:199–204. doi: 10.1016/j.neuroimage.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Kyriakopoulos M, Perez-Iglesias R, Woolley JB, et al. Effect of age at onset of schizophrenia on white matter abnormalities. Br J Psychiatry. 2009;195:346–53. doi: 10.1192/bjp.bp.108.055376. [DOI] [PubMed] [Google Scholar]
- 18.Kumra S, Ashtari M, Cervellione KL, et al. White matter abnormalities in early-onset schizophrenia: a voxel-based diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2005;44:934–41. doi: 10.1097/01.chi.0000170553.15798.94. [DOI] [PubMed] [Google Scholar]
- 19.Kyriakopoulos M, Vyas NS, Barker GJ, et al. A diffusion tensor imaging study of white matter in early-onset schizophrenia. Biol Psychiatry. 2008;63:519–23. doi: 10.1016/j.biopsych.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter DM, Tang CY, Friedman JI, et al. Temporal characteristics of tract-specific anisotropy abnormalities in schizophrenia. Neuroreport. 2008;19:1369–72. doi: 10.1097/WNR.0b013e32830abc35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsgodt KH, van Erp TG, Poldrack RA, et al. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry. 2008;63:512–8. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Friedman JI, Tang C, Carpenter D, et al. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2008;165:1024–32. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- 23.Douaud G, Smith S, Jenkinson M, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–86. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 25.Lukoff D, Liberman RP. Symptom monitoring in the rehabilitation of schizophrenic patients. Schizophr Bull. 1986;12:578–602. doi: 10.1093/schbul/12.4.578. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann P, Fimm B. Tests d’évaluation de l’attention (TEA) Würselen (Germany): Psytest; 1994. [Google Scholar]
- 27.Andreasen NC, Pressler M, Nopoulos P, et al. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–62. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mechelli A, Price CJ, Friston KJ, et al. Voxel-based morphometry of the human brain: methods and applications. Curr Med Imaging Rev. 2005;1:105–13. [Google Scholar]
- 29.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Ventura J, Nuechterlein KH, Subotnik KL, et al. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Res. 2000;97:129–35. doi: 10.1016/s0165-1781(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 31.Friston KJ, Frith CD. Schizophrenia: A disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 32.Wheeler-Kingshott CA, Cercignani A. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61:1255–60. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Sun SW, Naismith RT, et al. Assessing optic nerve pathology with diffusion MRI: from mouse to human. NMR Biomed. 2008;21:928–40. doi: 10.1002/nbm.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis KL, Stewart DG, Friedman JI, et al. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–56. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 35.Moriya J, Kakeda S, Abe O, et al. Gray and white matter volumetric and diffusion tensor imaging (DTI) analyses in the early stage of first-episode schizophrenia. Schizophr Res. 2010;116:196–203. doi: 10.1016/j.schres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Haller S, Xekardaki A, Delaloye C, et al. Combined analysis of grey matter voxel-based morphometry and white matter tract-based spatial statistics in late-life bipolar disorder. J Psychiatry Neurosci. 2011;36:391–401. doi: 10.1503/jpn.100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bodini B, Khaleeli Z, Cercignani M, et al. Exploring the relationship between white matter and gray matter damage in early primary progressive multiple sclerosis: an in vivo study with TBSS and VBM. Hum Brain Mapp. 2009;30:2852–61. doi: 10.1002/hbm.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashtari M, Cottone J, Ardekani BA, et al. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch Gen Psychiatry. 2007;64:1270–80. doi: 10.1001/archpsyc.64.11.1270. [DOI] [PubMed] [Google Scholar]
- 39.Whitford TJ, Farrow TF, Williams LM, et al. Delusions and dorsomedial frontal cortex volume in first-episode schizophrenia: a voxel-based morphometry study. Psychiatry Res. 2009;172:175–9. doi: 10.1016/j.pscychresns.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Pérez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, et al. White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry. 2010;167:451–8. doi: 10.1176/appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- 41.Minatogawa-Chang TM, Schaufelberger MS, Ayres AM, et al. Cognitive performance is related to cortical grey matter volumes in early stages of schizophrenia: a population-based study of firstepisode psychosis. Schizophr Res. 2009;113:200–9. doi: 10.1016/j.schres.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moy G, Millet P, Haller S, et al. Magnetic resonance imaging determinants of intraindividual variability in the elderly: combined analysis of grey and white matter. Neuroscience. 2011;186:88–93. doi: 10.1016/j.neuroscience.2011.04.028. [DOI] [PubMed] [Google Scholar]