Abstract
Background
Mismatch negativity (MMN) and P3a are event-related potentials that index deviance detection and the orienting response, respectively. We have previously shown that the MMN/P3a complex is impaired in patients with schizophrenia and affective spectrum psychoses, which suggests that it may index a common pathophysiology and argues against the purported specificity in schizophrenia. Further research is warranted to determine whether patients with bipolar-spectrum disorders show similar impairments in these biomarkers.
Methods
We assessed patients aged 15–30 years with early schizophrenia-spectrum disorders (schizophrenia, schizoaffective disorder, schizophreniform disorder), early bipolar-spectrum disorders (bipolar I or II, with and without psychotic features) and healthy, matched controls. We acquired MMN/P3a amplitudes during a 2-tone, auditory paradigm with 8% duration deviants. Clinical, psychosocial and neuropsychological assessments were also undertaken.
Results
We included 20 patients with schizophrenia-spectrum disorders, 20 with bipolar-spectrum disorders and 20 controls in our study. Both patient groups showed significantly reduced frontocentral MMN and central P3a amplitudes. The schizophrenia-spectrum group had additional impairments in left temporal MMN and frontal P3a. Both patient groups performed worse than controls across psychosocial and clinical measures; however, only the schizophrenia-spectrum group performed significantly worse than controls for cognitive measures. Correlational analyses between patient groups revealed associations between frontocentral or left temporal MMN and psychiatric symptomatology or quality of life measures.
Limitations
Limitations to our study include the modest sample size and the lack of control with regards to the effects of other (i.e., nonantipsychotic) psychotropic medications.
Conclusion
Compared with patients in early stages of schizophrenia-spectrum disorders, those in the early stages of bipolar-spectrum disorders are similarly impaired in established biomarkers for schizophrenia. These findings support a shared diathesis model for psychotic and bipolar disorders. Furthermore, MMN/P3a may be a biomarker for a broader pathophysiology that overlaps traditional diagnostic clusters.
Introduction
A current topic of controversy in psychiatry is whether or not bipolar-spectrum disorders should be considered as part of the psychosis cluster in the upcoming DSM-V. Currently, bipolar-spectrum disorders are classified as mood disorders in line with the Kraeplinian dichotomy; however, recent literature suggests that there may be more overlap in the underlying neurobiology in these major psychiatric illnesses than once thought.1 It is not unusual for patients with similar phenotypic presentation and common symptomatology to receive a diagnosis of either a bipolar-spectrum or a schizophrenia-spectrum disorder, particularly in the early stages of illness. Thus, investigation of the underlying neurobiology of these 2 major psychiatric groups is crucial to our understanding of their pathophysiological overlap. Shared genetic vulnerabilities in individuals with bipolar disorder and schizophrenia have been documented and describe how some symptoms overlap and how some are unique.2–5 In support of the shared genetic basis of these disorders, there is evidence that first-degree relatives of probands with bipolar disorder or schizophrenia are at increased risk for either disorder, irrespective of the phenotype present in their relative.6
Mismatch negativity (MMN) is an event-related potential (ERP) that has been touted as a biomarker of schizophrenia,7 with more than 40 studies showing that it is impaired in this patient group.8 Similarly, P300 has also been posited to be a biomarker for schizophrenia,7,9 as is its methodological variant, P3a.9,10 Mismatch negativity is a negative going component that represents neural mismatch detection processes between stimuli stored in short-term memory.11 The positive going P3a component is putatively reported to reflect frontocentral orienting processes12,13 elicited following the detection of the deviant stimuli.14 Friedman and colleagues13 describe the neurophysiological “handover” from MMN to P3a, where the former is a preattentive index of deviance detection and the latter reflects the involuntary capture of attention. There is accumulating evidence, however, suggesting that these biomarkers may not be specific to schizophrenia; rather, they reflect a common or overlapping pathophysiology in psychotic (and possibly other psychiatric) disorders. Our group15,16 has previously reported that both MMN and P3a are impaired in first-episode patients with a diagnosed disorder on the psychotic spectrum (i.e., schizophrenia, schizoaffective and schizophreniform disorders) and in those with an affective-spectrum disorder (i.e., bipolar disorder, major depressive disorder with psychotic features). In contrast to research that specifically focuses on schizophrenia, very few studies have investigated MMN (and concomitant P3a) in patients with bipolar disorder.
Studies have reported MMN impairments being exclusive to patients with schizophrenia, but not bipolar disorder.17–19 On closer inspection of these studies, there are methodologic considerations that warrant caution. First, in the study by Catts and colleagues,17 patients with bipolar disorder were, on average, 6 years older and had a different control group than the patients with schizophrenia. Furthermore, all of the patients with bipolar disorder were in remission, whereas most of those with schizophrenia were hospitalized at the time of testing. In the study by Umbricht and colleagues,18 the bipolar group was much smaller than the schizophrenia group (16 v. 26 patients), and these numbers were compounded by the fact that one-quarter of the patients with bipolar disorder were in remission. Moreover, in both studies,17,18 symptom severity was substantially greater in the schizophrenia than the bipolar group. The study by Salisbury and colleagues19 compared younger patients with first-episode “psychotic bipolar disorder” and those with first-episode schizophrenia. While there were no differences between both patient groups and controls at baseline, at the 18-month follow-up, patients with first-episode schizophrenia were the only group who showed MMN reductions (which was associated with a reduction in left Heschl gyrus grey matter). It should also be noted that these 3 studies differed in terms of their use of the deviant stimulus type. One study used duration deviants only,17 one employed both duration and frequency deviants18 and one used only frequency deviants.19 There is some evidence to suggest that the frequency deviant type may be more sensitive to illness progression in psychosis,20,21 whereas duration deviants have been shown to be impaired in early and later stages of illness.15,22–24
Hall and colleagues25 assessed MMN and P300 (separately) in patients with psychotic bipolar disorder. Whereas MMN was found to be normal, P300 was significantly reduced and delayed in patients compared with controls. The authors suggested that P300, but not MMN, was a valid endophenotype, albeit with limited specificity. In contrast, Andersson and colleagues26 found that patients with bipolar II disorder had decreased frontal MMN in the absence of a P300 impairment (although they reported a delayed P3a for female patients). Given such contrasting findings and questions surrounding the specificity of MMN and P300 (and P3a), there is a need to further explore these biomarkers in patients with bipolar disorder.
Our study aimed to expand our previous findings in schizophrenia and affective-spectrum psychoses by evaluating the MMN/P3a complex in young outpatients in the early stages of either schizophrenia-spectrum or bipolar-spectrum (with and without psychotic features) disorders. Neuropsychological and psychosocial functioning in these groups were also measured and examined for association with the ERP components.
Methods
Participants
We recruited patients aged 15–30 years from a specialized youth mental health service;27 participants were required to have either first- or second-episode schizophrenia-spectrum psychosis, bipolar disorder or bipolar disorder with psychotic features. Diagnoses were determined by a psychiatrist using DSM-IV criteria. Case reviews by a clinical psychologist (R.B.) using the psychosis and mood disorders section of the Structured Clinical Interview for DSM-IV28 confirmed diagnoses within the schizophrenia or bipolar spectra. The Brief Psychiatric Rating Scale (BPRS)29 and the Hamilton Rating Scale for Depression (HAM-D)30 were used to quantify current symptoms. We recruited healthy controls from the community.
Exclusion criteria for all participants were major medical illness, history of neurologic disease, intellectual and/or developmental disability, insufficient English and current substance dependence. Participants abstained from drug and alcohol use for 48 hours before testing. To verify recent abstinence, participants underwent an alcohol breathalyzer and saliva drug screening for amphetamines, cannabis, opioids, benzodiazepines and cocaine. All participants were rated by a psychiatrist or research psychologist using the Social and Occupational Functioning Assessment Scale (SOFAS).31 All participants completed the self-report Depression Anxiety and Stress Scales (DASS)32 and World Health Organization Quality of Life (WHOQOL-BREF) questionnaire,33 which measures 4 domains (physical, psychological, social and environmental) of quality of life. The study was approved by the University of Sydney Human Research Ethics Committee, and all participants gave written informed consent.
Neurophysiological testing
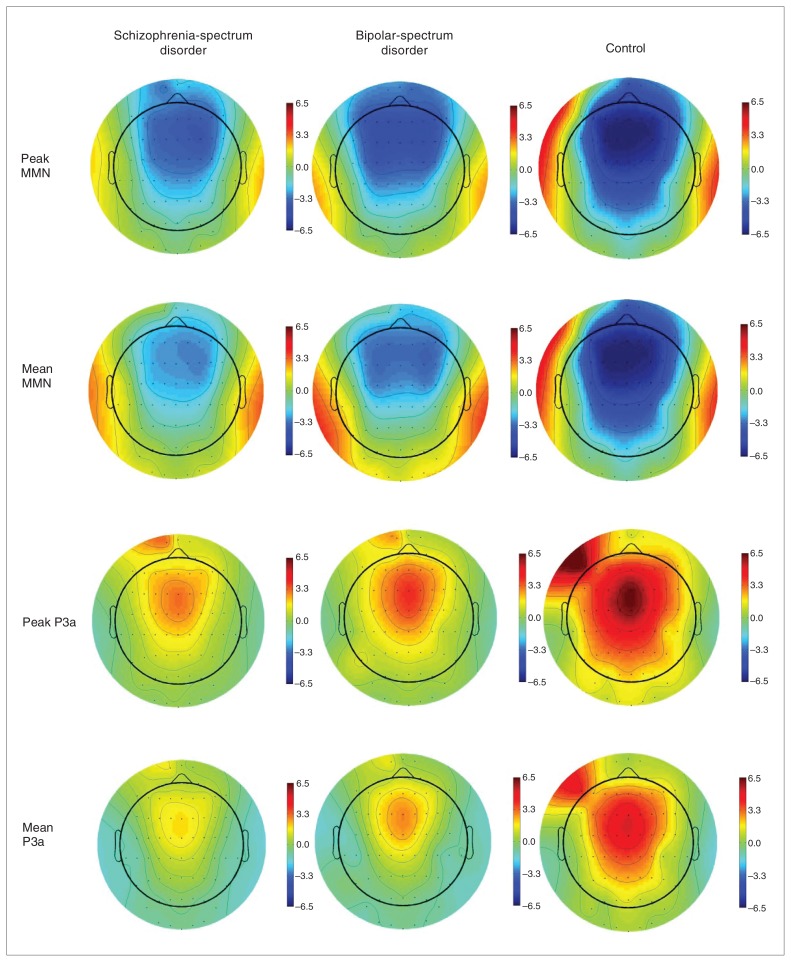
After preparation for electroencephalography (EEG) recording, participants were presented (via headphones) with 2500 binaural pure tones (1000 Hz, 75 dB SPL, 10 ms rise/fall) at a 500 ms stimulus onset asynchrony; this comprised a pseudorandom sequence of 2300 (92%) 50 ms standard tones and 200 (8%) 100 ms deviant tones. Tones were presented while participants watched a silent video. Participants were then asked to report back the storyline of the movie at the end of the task. A 64-channel Quik-cap (Neuroscan) acquired EEG data from sites according to the standard 10–20 international system (including mastoids). Data were referenced to a nose electrode. Vertical and horizontal electro-oculograms34 (EOG) were assessed for eye-blink artifacts, and we corrected eye blink–contaminated data using established algorithms.35 Scalp and EOG potentials were amplified and digitized continuously at 500 Hz (SynAmps2, Scan 4.3.1 software). Offline signal processing and analyses were performed using Neuroscan Scan 4.3.1 (Compumedics) software. Data were filtered using a bandpass filter (0.15–20 Hz), and we rejected epochs of the EEG recordings that were contaminated by movement artifacts (± 100 μV). Analysis of variance (ANOVA) confirmed that there were no differences in the number of accepted epochs between the groups for each stimulus type (p = 0.22). We resolved mismatch difference waveforms by subtracting ERP waveforms elicited by the deviant stimuli from those of the standard stimuli. The mean amplitude, peak amplitude and peak latency were determined for MMN and P3a according to established epoch windows of 135–205 ms and 250–300 ms, respectively.14 The head maps (Fig. 1) were derived using MATLAB scripts, as implemented by the EEGLAB software program.36 We obtained MMN measures at 4 sites: midline frontocentral (Fz, Cz) and temporal (left and right mastoid; M1 and M2, respectively), whereas P3a was processed at frontocentral sites only (P3a is not elicited at temporal sites).
Fig. 1.
Head maps depicting the peak and mean amplitudes for mismatched negativity (MMN; top rows) and P3a (bottom rows) recorded across scalp sites for schizophrenia-spectrum disorder, bipolar-spectrum disorder and control groups.
Neuropsychological assessment
Premorbid intelligence (“predicted IQ”) was estimated based on performance on the Wechsler Test of Adult Reading.37 We assessed processing speed using the Trail-Making Test, part A (TMT A),38 with attention-switching assessed by part B (TMT B).38 Verbal learning and memory were assessed via the Rey Auditory Verbal Learning Test (RAVLT);38 variables assessed were immediate recall (sum of trials 1–5; RAVLT A1–A5) and 20-minute delayed recall (trial 7; RAVLT A7).38
Statistical analyses
Differences in clinical, psychosocial, neuropsychological and neurophysiological measures across the 3 groups were assessed using 1-way ANOVA, with results of p < 0.05 considered to be significant. We used the Levene test to determine homogeneity of variance. We calculated a Welch statistic and reported corrected degrees of freedom and p values when this assumption was violated. We used the Scheffé test to determine post hoc pair-wise comparison statistics. The Pearson product moment correlation coefficient or Spearman Rho (if data remained non-normal after transformation) was calculated for the entire patient sample and was restricted to relations between MMN and P3a peak amplitudes and key clinical, psychosocial and neuropsychological variables. To further minimize the likelihood of type I errors, only correlations at p < 0.01 were considered to be significant.
Results
Participants
We included 40 patients in our study: 20 with either first- or second-episode schizophrenia-spectrum psychosis and 20 with either bipolar disorder or bipolar disorder with psychotic features. A subsample of these patients (15 from the schizophrenia-spectrum group and 6 from from the bipolar-spectrum group) have been included in a previous study.16 Diagnoses in the schizophrenia-spectrum group were schizophrenia (n = 7), schizoaffective disorder (n = 5) and schizophreniform disorder (n = 8); those in the bipolar-spectrum group were bipolar I disorder (n = 6), bipolar I disorder with psychotic features (n = 9), bipolar II disorder (n = 2) and bipolar II disorder with psychotic features (n = 3). The control group included 20 healthy participants. All but 4 patients were taking psychotropic medication at the time of testing, the status of which is summarized in Appendix 1, available at cma.ca/jpn.
Demographic, clinical and social functioning findings
Matching for age and sex among the groups was achieved (Table 1). As expected, both patient groups performed significantly worse than controls across all of the clinical and psychosocial measures (all p < 0.05), except for the positive symptoms, depression and mania subscores of the BPRS and social subscore of the WHOQOL-BREF (only the bipolar-spectrum group performed worse than controls) and for the negative symptoms subscore of the BPRS (only the schizophrenia-spectrum group performed worse than controls; Table 1). The pair-wise comparisons revealed that the bipolar-spectrum group had significantly worse current depressive symptoms (p = 0.032) measured by total HAM-D score, and worse current manic symptoms (p = 0.014) measured by BPRS mania sub-score than the schizophrenia-spectrum group.
Table 1.
Clinical and psychosocial variables in bipolar-spectrum disorder, schizophrenia-spectrum disorder and control groups
| Group; mean (SD)* | Post hoc pair-wise comparison, p value‡ | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Variable | BSD | SSD | Control | Degrees of freedom | Between-group test† | SSD v. BSD | SSD v. control | BSD v. control |
| Sex, female:male | 12:8 | 8:12 | 10:10 | 2 | 0.4 | — | — | — |
| Age, yr | 21.0 (4.1) | 22.3 (3.7) | 23.1 (3.7) | 2, 57 | 1.5 | — | — | — |
| SOFAS | 61.6 (12.5) | 58.6 (14.1) | 92.1 (3.8) | 2, 29 | 97.0§ | — | < 0.001 | < 0.001 |
| HAM-D total | 10.4 (5.5) | 6.2 (7.4) | 1.8 (2.4) | 2, 31.5 | 22.1§ | 0.032 | 0.040 | < 0.001 |
| BPRS | ||||||||
| Total | 42.2 (13.2) | 34.8 (10.2) | 25.9 (1.8) | 2, 26.6 | 14.3§ | — | 0.019 | < 0.001 |
| Positive | 13.7 (6.3) | 10.0 (4.0) | 7.6 (1.0) | 2, 26.0 | 12.0§ | — | — | < 0.001 |
| Negative | 6.7 (2.0) | 7.1 (2.8) | 5.1 (0.2) | 2, 26.3 | 11.8§ | — | 0.002 | — |
| Depress | 11.2 (3.2) | 8.8 (4.3) | 7.1 (1.2) | 2, 28.5 | 14.7§ | — | — | < 0.001 |
| Mania | 11.1 (5.6) | 9.2 (2.1) | 7.1 (0.3) | 2, 27.0 | 14.0¶ | 0.014 | — | < 0.001 |
| WHOQOL-BREF | ||||||||
| Physical | 24.2 (4.7) | 23.8 (5.2) | 30.8 (2.6) | 2, 32.3 | 23.1§ | — | < 0.001 | < 0.001 |
| Psychological | 17.2 (4.5) | 18.3 (5.2) | 23.5 (2.9) | 2, 55 | 11.8§ | — | 0.002 | < 0.001 |
| Social | 9.0 (2.7) | 9.4 (2.5) | 11.4 (2.6) | 2, 55 | 4.8** | — | — | 0.024 |
| Environmental | 28.8 (4.7) | 28.6 (5.2) | 33.4 (4.6) | 2, 55 | 6.1¶ | — | 0.011 | 0.021 |
BPRS = Brief Psychiatric Rating Scale;30 BSD = bipolar-spectrum disorder; HAM-D = Hamilton Rating Scale for Depression;31 SD = standard deviation; SOFAS = Social and Occupational Functioning Assessment Scale;32 SSD = schizophrenia-spectrum disorder; WHOQOL-BREF = World Health Organization Quality of Life questionnaire.34
Unless otherwise indicated.
χ2 or analysis of variance.
Scheffé test.
p < 0.001.
p < 0.01.
p < 0.05.
Neurophysiological findings
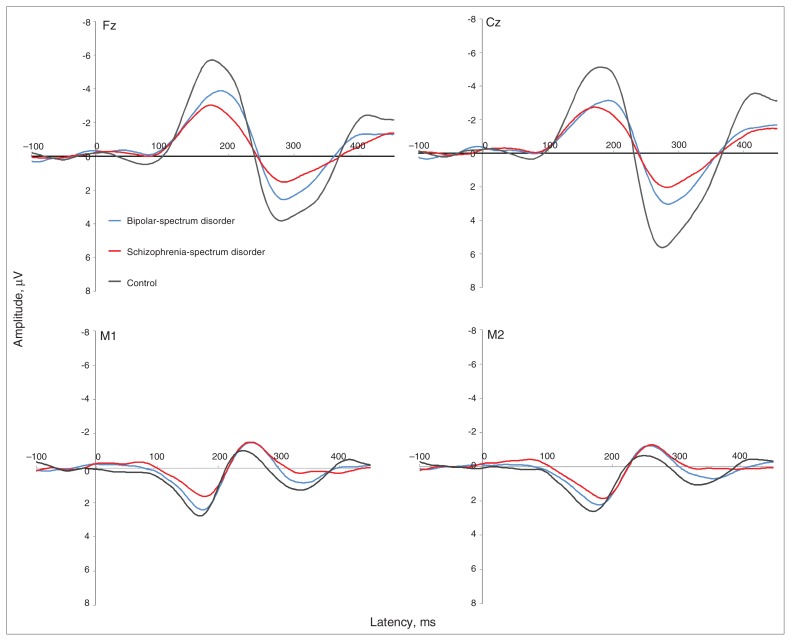
Average ERP waveforms at frontal, central and temporal sites are provided in Figure 2. There were no differences in the overall pattern for mean and peak amplitudes (Fig. 1); therefore, for brevity only the peak amplitude statistics are reported. For MMN peak amplitudes, post hoc pair-wise comparisons revealed significant differences between both the patient groups and controls at Fz, Cz and M1 (Table 2). Both patient groups had reduced amplitudes compared with controls at frontal and central sites (schizophrenia-spectrum group: p < 0.001 at Fz and Cz; bipolar-spectrum group: p = 0.004 at Cz and p = 0.012 at Fz). The left temporal MMN peak amplitude was significantly reduced only in the schizophrenia-spectrum group (p = 0.032). There were no significant differences for the right temporal MMN amplitude. Across all sites, the patient groups did not significantly differ from each other in MMN peak amplitude. There were no significant differences among the 3 groups for MMN peak latency aside from the schizophrenia-spectrum group showing a significantly delayed latency compared with controls at the right temporal site (p = 0.012).
Fig. 2.
Grand average event-related potentials for early bipolar-spectrum disorder (dashed line), early schizophrenia-spectrum disorder (grey line) and control (black line) groups at (clockwise, from top left) frontal (Fz), central (Cz), right temporal (M2) and left temporal (M1) sites. The schizophrenia-spectrum disorder group showed reduced frontocentral (Fz, Cz) and temporal (M1, M2) mismatch negativity (MMN; 135–205 ms) and frontocentral (Fz, Cz) P3a (250–300 ms) amplitudes (μV) at frontocentral sites (Fz, Cz) compared with controls. The bipolar-spectrum disorder group showed reduced frontocentral (Fz, Cz) MMN (135–205 ms) and central (Cz) P3a (250–300 ms) amplitudes (μV) compared with controls. Note that M1 and M2 waveforms are reversed in polarity owing to the nose-referenced recording.
Table 2.
Mismatch negativity recorded at Fz, Cz, M1, M2 and P3a recorded at Fz and Cz in bipolar-spectrum disorder, schizophrenia-spectrum disorder and control groups
| Event-related potential | Group; mean peak amplitude (SD) | ANOVA, F2,57 | Post hoc pair-wise comparison, p value* | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| BSD | SSD | Control | SSD v. BSD | SSD v. controls | BSD v. controls | ||
| MMN | |||||||
| Fz | −4.3 (1.9) | −3.4 (2.0) | −6.3 (2.1) | 10.6† | — | < 0.001 | 0.012 |
| Cz | −3.8 (1.9) | −3.3 (1.8) | −6.0 (2.2) | 10.4† | — | < 0.001 | 0.004 |
| M1 | 2.6 (1.4) | 1.9 (1.3) | 3.0 (1.3) | 3.7§ | — | 0.032 | — |
| M2 | 2.4 (1.1) | 2.1 (1.1) | 2.9 (1.2) | 2.8 | — | — | — |
| P3a | |||||||
| Fz | 3.1 (2.7) | 2.2 (1.7) | 4.6 (2.1) | 6.0‡ | — | 0.005 | — |
| Cz | 3.7 (2.1) | 2.8 (2.0) | 6.2 (2.7) | 12.1† | — | < 0.001 | 0.004 |
ANOVA = analysis of variance; BSD = bipolar-spectrum disorder; MMN = mismatch negativity; SD = standard deviation; SSD = schizophrenia-spectrum disorder.
Scheffé test.
p < 0.001.
p < 0.01.
p < 0.05.
Similarly, there were significant post hoc pair-wise comparison differences between both patient groups and controls for P3a peak amplitudes. Both patient groups showed reduced central P3a amplitudes compared with controls (p < 0.001 for the schizophrenia-spectrum group and p = 0.004 for the bipolar-spectrum group). For P3a amplitudes at the frontal site, only the schizophrenia-spectrum group showed a reduction compared with controls (p = 0.005). There were no significant group differences for P3a latency.
Neuropsychological findings
There were significant differences between the groups for all the neuropsychological variables (Table 3). Post hoc pair-wise comparisons revealed that only the schizophrenia-spectrum group significantly differed from controls across these variables (p = 0.034 for predicted IQ and TMT B, p = 0.018 for TMT A, p < 0.001 for RAVLT A1–A5 and RAVLT A7). There were no significant differences between the bipolar-spectrum group and controls. Furthermore, both patient groups were found to significantly differ on performance of TMT A and RAVLT A1–A5, with the schizophrenia-spectrum group performing worse than the bipolar-spectrum group (p = 0.042 and p = 0.010, respectively).
Table 3.
Neuropsychological variables in bipolar-spectrum disorder, schizophrenia-spectrum disorder and control groups
| Group; mean (SD) | Post hoc pair-wise comparison, p value* | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Variable | BSD | SSD | Control | Degrees of freedom | ANOVA, F | SSD v. BSD | SSD v. controls | BSD v. controls |
| Predicted IQ | 103.1 (9.6) | 99.2 (10.1) | 106.9 (7.5) | 2, 59 | 3.6§ | — | 0.034 | — |
| TMT A | 25.2 (9.6) | 32.4 (10.7) | 22.8 (4.3) | 2, 31.3 | 6.8‡ | 0.042 | 0.004 | — |
| TMT B | 63.5 (25.6) | 77.3 (30.3) | 54.3 (15.1) | 2, 32.7 | 4.6§ | — | 0.018 | — |
| RAVLT A1–A5 | 55.4 (10.3) | 46.0 (11.2) | 59.1 (5.2) | 2, 31.6 | 10.8† | 0.010 | < 0.001 | — |
| RAVLT A7 | 11.5 (3.7) | 9.6 (3.3) | 13.2 (1.1) | 2, 27.8 | 11.5† | — | < 0.001 | — |
Correlation findings
We found a highly significant (p < 0.001) correlation for the whole patient group (i.e., n = 40; schizophrenia-spectrum plus bipolar-spectrum) that involved MMN peak amplitude at Fz for the WHOQOL-BREF psychological domain (r = 0.42). We observed 5 other correlations of moderate significance (i.e., p < 0.01). The HAM-D total score (r = −0.35) was associated with MMN peak amplitude at Fz. Furthermore, MMN peak amplitude at M1 (left temporal) was associated with 4 variables: WHOQOL-BREF psychological (r = −0.35), WHOQOL-BREF social (r = −0.38), BPRS total (r = 0.43) and BPRS positive symptoms (r = 0.40; Table 4). For all of these significant correlations, reduced MMN amplitude was associated with worse symptoms/psychosocial functioning. It is interesting to note that there were no significant correlations with any of the cognitive measures, nor were there any for the P3a amplitudes.
Table 4.
Pearson correlation coefficients between key neurophysiological variables (MMN peak amplitude at Fz, Cz, M1 and M2; P3a peak amplitude at Fz and Cz) and social functioning, quality of life, depressive and neuropsychological variables in bipolar-spectrum disorder and schizophrenia-spectrum disorder groups, n = 40
| MMN peak amplitude | P3a peak amplitude | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variable | Fz | Cz | M1 | M2 | Fz | Cz |
| Age | −0.12 | −0.06 | 0.07 | 0.06 | 0.18 | 0.15 |
| SOFAS | 0.09 | 0.07 | −0.02 | 0.06 | −0.02 | 0.00 |
| WHOQOL-BREF | ||||||
| Physical | 0.22 | −0.13 | −0.31 | −0.13 | −0.19 | −0.15 |
| Psychological | 0.42‡ | 0.24 | −0.35† | −0.11 | −0.17 | −0.09 |
| Social | 0.12 | −0.05 | −0.38† | −0.20 | −0.17 | −0.13 |
| Environmental | 0.05 | −0.05 | −0.25 | −0.08 | −0.13 | −0.09 |
| HAM-D total | −0.35† | −0.24 | 0.24 | −0.03 | 0.16 | 0.06 |
| BPRS | ||||||
| Total | −0.17 | −0.01 | 0.43† | 0.04 | 0.23 | 0.26 |
| Positive symptoms* | −0.10 | 0.04 | 0.40† | 0.17 | −0.05 | 0.02 |
| Negative symptoms* | 0.06 | 0.13 | 0.17 | −0.06 | −0.05 | 0.00 |
| TMT A | 0.23 | 0.17 | −0.05 | −0.08 | 0.03 | 0.09 |
| TMT B | 0.12 | 0.20 | 0.15 | 0.23 | −0.08 | −0.06 |
| RAVLT A1–A5 | −0.26 | −0.14 | 0.01 | 0.01 | −0.05 | 0.04 |
| RAVLT A7 | −0.13 | −0.20 | 0.07 | 0.15 | 0.08 | 0.12 |
BPRS = Brief Psychiatric Rating Scale;30 HAM-D = Hamilton Rating Scale for Depression;31 SOFAS = Social and Occupational Functioning Assessment Scale;32 TMT A and B = Trail–Making Test, part A or B;39 RAVLT = Rey Auditory Verbal Learning Test;39 WHOQOL-BREF = World Health Organization Quality of Life questionnaire.34
Spearman Rho correlation coefficients were used to calculate psychotic symptom variables.
p < 0.01.
p < 0.001
Discussion
To our knowledge, our study is the first to report deficits across several neurophysiological biomarkers (known to be impaired in individuals with chronic schizophrenia) in young people in the early stages of bipolar-spectrum disorder and in those in the early stages of schizophrenia-spectrum disorder. In comparison with controls, both patient groups showed frontocentral MMN/P3a deficits and corresponding significant associations with clinical and psychosocial measures. Interestingly, whereas both patient groups showed similar impairments in psychosocial functioning, only the schizophrenia-spectrum group showed significant impairments in cognitive measures. This finding suggests that for young people with a diagnosed bipolar-spectrum disorder, neurophysiological impairments may precede any notable changes in their attention and verbal learning/memory.
Overall, in terms of MMN/P3a impairment in psychosis, the results of the present study are in keeping with our previous findings15,16 and with those reported in the literature.39 Having said this, a novel aspect of the present study is that there was a significant reduction in the left temporal MMN for the schizophrenia-spectrum group only. Whereas this finding has been reported previously, it has been observed only in samples with chronic schizophrenia.40,41 It is important to note, however, that it is likely that impairments at mastoid/temporal sites have often not been reported owing to limitations in recording techniques (e.g., the mastoids or linked ears were used as the reference, thus precluding a viable analysis of ERPs at or near the mastoid sites).7,42,43 Contrary to previous understanding, our finding suggests that there is a disruption of temporal neural networks in the early stages of schizophrenia-spectrum psychoses in addition to the commonly reported frontocentral impairment.
In their recent review, Näätänen and Kahkonen8 suggested that a dampened frontocentral MMN response may contribute to negative symptoms (including social withdrawal) by diminishing one’s ability to switch attention to socially relevant auditory cues. In contrast, abnormalities in auditory perception and discrimination are thought to be more associated with a dampened temporal MMN. Broadly, both of these associations are reflected in the present study. There was an association between frontal MMN and current depressive symptoms (HAM-D total score), which is consistent with other evidence showing a link between MMN and depression.44,45 Our study also revealed an association between left temporal MMN and positive symptoms (i.e., the smaller the MMN amplitude, the higher the positive symptoms sub-score), which is in keeping with previous findings in samples with chronic schizophrenia.40,41
Limitations
Our findings need to be considered in light of some limitations. First, this study had a modest sample size, and future studies with larger numbers are needed to determine the robustness of the MMN/P3a impairments and their associated functional measures in these major psychiatric subgroups. Second, although we chose to represent bipolar-spectrum disorders as a whole, it will be helpful to divide this group further into patients with bipolar disorder with versus those without psychotic features for subsequent comparisons. Similarly, the schizophrenia-spectrum group can be further divided. More broadly, the diagnostic stability of early psychotic and bipolar disorders is something that needs to be addressed in future studies; this is particularly important for diagnoses, such as schizophreniform disorder, that are determined within narrower time frames. To illustrate this point, all 8 patients who had schizophreniform disorder at the time of MMN assessment were followed up for a minimum of 14 months after MMN acquisition. Five of these patients retained the diagnosis; however, at follow-up 2 were determined to have schizophrenia (because their symptoms persisted beyond 6 mo) and 1 was determined to have schizoaffective disorder. Thus, the present study is limited by its cross-sectional design; our interpretation of the findings must be treated with caution since, by their very nature, diagnoses of an early-stage schizophrenia-spectrum or bipolar-spectrum disorder can be difficult to distinguish and may very likely fluctuate with time. Third, the generalization of our findings within the broader literature may be limited by our use of a duration deviant stimuli alone. Whereas numerous studies have shown that this deviant type reveals a reduction in psychosis,17,23,46,47 there are also studies that have shown that the frequency deviant type may be more sensitive to stage of illness in those with a psychotic disorder.21,48 Future studies that examine patients in the early stages of bipolar disorders (and compare them with those who have schizophrenia-spectrum disorders) should consider using multifeature MMN paradigms (i.e., including duration and frequency deviant stimuli) to probe for stage of illness markers. In addition, owing to multiple comparisons, the correlational analyses should be treated as exploratory. Despite this, the associations found here are consistent with the literature, suggesting that their inclusion in larger, better powered studies would be warranted. Finally, the potential effect of medication is another limitation that needs consideration. Whereas antipsychotics have not been shown to affect the neurophysiological components investigated in the present study, less is known about the effects of mood stabilizers and antidepressants.49–53
Conclusion
Despite the limitations, our overall findings suggest that an impaired MMN/P3a complex is apparent in the early stages of bipolar-spectrum and schizophrenia-spectrum disorders, providing evidence for shared psychopathological processes in patient groups that may be better understood in terms of a neurobiological continuum rather than separate disorders.
Acknowledgements
This work was supported in part by the National Health and Medical Research Council (NHMRC) of Australia via a fellowship (ID 511921) and a program grant (ID 566529).
Footnotes
Competing interests: None declared for M. Kaur, R.A. Battisti, J. Lagopoulos and P.B. Ward. As above for I.B. Hickie and D.F. Hermens; their institution also receives grant support from the Mental Health & Drug & Alcohol Office, NSW Department of Health. I.B. Hickie declares board membership with Headspace, consulting for Bupa Australia, the National Council on Mental Healthy and Access to Allied Psychological Services.
Contributors: M. Kaur, I.B. Hickie and D.F. Hermens designed the study. M. Kaur, R.A. Battisti and D.F. Hermens acquired the data, which M. Kaur, R.A. Battisti, J. Lagopoulos, P.B. Ward and D.F. Hermens analyzed. M. Kaur and D.F. Hermens wrote the article. All authors reviewed the article and approved its publication.
References
- 1.Goldberg DP, Andrews G, Hobbs MJ. Where should bipolar disorder appear in the meta-structure? Psychol Med. 2009;39:2071–81. doi: 10.1017/S0033291709990304. [DOI] [PubMed] [Google Scholar]
- 2.Boks MPM, Leask S, Vermunt JK, et al. The structure of psychosis revisited: the role of mood symptoms. Schizophr Res. 2007;93:178–85. doi: 10.1016/j.schres.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Berrettini WH. Susceptibility loci for bipolar disorder: overlap with inherited vulnerability to schizophrenia. Biol Psychiatry. 2000;47:245–51. doi: 10.1016/s0006-3223(99)00226-7. [DOI] [PubMed] [Google Scholar]
- 4.Lewis CM, Levinson DF, Wise LH, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: schizophrenia. Am J Hum Genet. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burmeister M, McInnis MG, Zollner S. Psychiatric genetics: progress amid controversy. Nat Rev Genet. 2008;9:527–40. doi: 10.1038/nrg2381. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein P, Yip BH, Bjork C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–9. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Javitt DC, Spencer KM, Thaker GK, et al. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Näätänen R, Kahkonen S. Central auditory dysfunction in schizophrenia as revealed by the mismatch negativity (MMN) and its magnetic equivalent MMNm: a review. Int J Neuropsychopharmacol. 2009;12:125–35. doi: 10.1017/S1461145708009322. [DOI] [PubMed] [Google Scholar]
- 9.Turetsky BI, Bilker WB, Siegel SJ, et al. Profile of auditory information-processing deficits in schizophrenia. Psychiatry Res. 2009;165:27–37. doi: 10.1016/j.psychres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher DJ, Labelle A, Knott VJ. Auditory hallucinations and the P3a: attention-switching to speech in schizophrenia. Biol Psychol. 2010;85:417–23. doi: 10.1016/j.biopsycho.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Näätänen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behav Brain Sci. 1990;13:201–88. [Google Scholar]
- 12.Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci Biobehav Rev. 2001;25:355–73. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- 14.Light GA, Swerdlow NR, Braff DL. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J Cogn Neurosci. 2007;19:1624–32. doi: 10.1162/jocn.2007.19.10.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermens DF, Ward PB, Redoblado Hodge MA, et al. Impaired MMN/P3a complex in first episode psychosis: cognitive and psychosocial associations. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:822–9. doi: 10.1016/j.pnpbp.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Kaur M, Battisti RA, Ward PB, et al. MMN/P3a deficits in first episode psychosis: comparing schizophrenia-spectrum and affective-spectrum subgroups. Schizophr Res. 2011;130:203–9. doi: 10.1016/j.schres.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Catts SV, Shelley AM, Ward PB, et al. Brain potential evidence for an auditory sensory memory deficit in schizophrenia. Am J Psychiatry. 1995;152:213–9. doi: 10.1176/ajp.152.2.213. [DOI] [PubMed] [Google Scholar]
- 18.Umbricht D, Koller R, Schmid L, et al. How specific are deficits in mismatch negativity generation to schizophrenia? Biol Psychiatry. 2003;53:1120–31. doi: 10.1016/s0006-3223(02)01642-6. [DOI] [PubMed] [Google Scholar]
- 19.Salisbury DF, Kuroki N, Kasai K, et al. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–9. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasser PE, Schall U, Todd J, et al. Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophr Bull. 2011;37:131–40. doi: 10.1093/schbul/sbp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Todd J, Michie PT, Schall U, et al. Deviant matters: duration, frequency, and intensity deviants reveal different patterns of mismatch negativity reduction in early and late schizophrenia. Biol Psychiatry. 2008;63:58–64. doi: 10.1016/j.biopsych.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Shelley AM, Ward PB, Catts SV, et al. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol Psychiatry. 1991;30:1059–62. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- 23.Baldeweg T, Klugman A, Gruzelier J, et al. Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophr Res. 2004;69:203–17. doi: 10.1016/j.schres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Baldeweg T, Klugman A, Gruzelier JH, et al. Impairment in frontal but not temporal components of mismatch negativity in schizophrenia. Int J Psychophysiol. 2002;43:111–22. doi: 10.1016/s0167-8760(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 25.Hall MH, Schulze K, Rijsdijk F, et al. Are auditory P300 and duration MMN heritable and putative endophenotypes of psychotic bipolar disorder? A Maudsley Bipolar Twin and Family Study. Psychol Med. 2009;39:1277–87. doi: 10.1017/S0033291709005261. [DOI] [PubMed] [Google Scholar]
- 26.Andersson S, Barder HE, Hellvin T, et al. Neuropsychological and electrophysiological indices of neurocognitive dysfunction in bipolar II disorder. Bipolar Disord. 2008;10:888–99. doi: 10.1111/j.1399-5618.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 27.Scott E, Naismith S, Whitwell B, et al. Delivering youth-specific mental health services: the advantages of a collaborative, multi-disciplinary system. Australas Psychiatry. 2009;17:189–94. doi: 10.1080/10398560802657322. [DOI] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2007. [Google Scholar]
- 29.Ventura J, Green MF, Shaner A, et al. Training and quality assurance with the Brief Psychiatric Rating Scale: “the drift busters. Int J Methods Psychiatr Res. 1993;3:221–4. [Google Scholar]
- 30.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 31.Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry. 1992;149:1148–56. doi: 10.1176/ajp.149.9.1148. [DOI] [PubMed] [Google Scholar]
- 32.Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33:335–43. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- 33.Skevington SM, Lotfy M, O’Connell KA. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A Report from the WHOQOL Group. Qual Life Res. 2004;13:299–310. doi: 10.1023/B:QURE.0000018486.91360.00. [DOI] [PubMed] [Google Scholar]
- 34.Kim SJ, Lyoo IK, Hwang J, et al. Frontal glucose hypometabolism in abstinent methamphetamine users. Neuropsychopharmacology. 2005;30:1383–91. doi: 10.1038/sj.npp.1300699. [DOI] [PubMed] [Google Scholar]
- 35.Semlitsch HV, Anderer P, Schuster P, et al. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 36.Delorme A. EEGLAB: an open source toolbox for analysis of single trial EEG dynamics. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Wechsler D. Wechsler Test of Adult Reading. San Antonio (TX): Psychological Corporation; 2002. [Google Scholar]
- 38.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- 39.Näätänen R, Kujala T, Winkler I. Auditory processing that leads to conscious perception: a unique window to central auditory processing opened by the mismatch negativity and related responses. Psychophysiology. 2011;48:4–22. doi: 10.1111/j.1469-8986.2010.01114.x. [DOI] [PubMed] [Google Scholar]
- 40.Thönnessen H, Zvyagintsev M, Harke KC, et al. Optimized mismatch negativity paradigm reflects deficits in schizophrenia patients. A combined EEG and MEG study. Biol Psychol. 2008;77:205–16. doi: 10.1016/j.biopsycho.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Youn T, Park H-J, Kim J-J, et al. Altered hemispheric asymmetry and positive symptoms in schizophrenia: equivalent current dipole of auditory mismatch negativity. Schizophr Res. 2003;59:253–60. doi: 10.1016/s0920-9964(02)00154-8. [DOI] [PubMed] [Google Scholar]
- 42.Michie PT. What has MMN revealed about the auditory system in schizophrenia? Int J Psychophysiol. 2001;42:177–94. doi: 10.1016/s0167-8760(01)00166-0. [DOI] [PubMed] [Google Scholar]
- 43.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Takei Y, Kumano S, Hattori S, et al. Preattentive dysfunction in major depression: a magnetoencephalography study using auditory mismatch negativity. Psychophysiology. 2009;46:52–61. doi: 10.1111/j.1469-8986.2008.00748.x. [DOI] [PubMed] [Google Scholar]
- 45.Bar-Haim Y, Marshall PJ, Fox NA, et al. Mismatch negativity in socially withdrawn children. Biol Psychiatry. 2003;54:17–24. doi: 10.1016/s0006-3223(03)00175-6. [DOI] [PubMed] [Google Scholar]
- 46.Light GA, Braff DL. Stability of mismatch negativity deficits and their relationship to functional impairments in chronic schizophrenia. Am J Psychiatry. 2005;162:1741–3. doi: 10.1176/appi.ajp.162.9.1741. [DOI] [PubMed] [Google Scholar]
- 47.Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005;62:127–36. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- 48.Rasser PE, Schall U, Todd J, et al. Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophr Bull. 2011;37:131–40. doi: 10.1093/schbul/sbp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korostenskaja M, Dapsys K, Siurkute A, et al. Effects of olanzapine on auditory P300 and mismatch negativity (MMN) in schizophrenia spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:543–8. doi: 10.1016/j.pnpbp.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 50.Umbricht D, Javitt D, Novak G, et al. Effects of clozapine on auditory event-related potentials in schizophrenia. Biol Psychiatry. 1998;44:716–25. doi: 10.1016/s0006-3223(97)00524-6. [DOI] [PubMed] [Google Scholar]
- 51.Umbricht D, Javitt D, Novak G, et al. Effects of risperidone on auditory event-related potentials in schizophrenia. Int J Neuropsychopharmacol. 1999;2:299–304. doi: 10.1017/S1461145799001595. [DOI] [PubMed] [Google Scholar]
- 52.Pekkone E, Hirvonen J, Ahveninen J, et al. Memory-based comparison process not attenuated by haloperidol: a combined MEG and EEG study. Neuroreport. 2002;13:177–81. doi: 10.1097/00001756-200201210-00040. [DOI] [PubMed] [Google Scholar]
- 53.Leung S, Croft R, Baldeweg T, et al. Acute dopamine D(1) and D(2) receptor stimulation does not modulate mismatch negativity (MMN) in healthy human subjects. Psychopharmacology (Berl) 2007;194:443–51. doi: 10.1007/s00213-007-0865-1. [DOI] [PubMed] [Google Scholar]