Abstract
Background
Previous studies have provided evidence of food motivation circuitry dysfunction in individuals with anorexia nervosa. However, methodological limitations present challenges to the development of a cohesive neurobiological model of anorexia nervosa. Our goal was to investigate the neural circuitry of appetite dysregulation across states of hunger and satiety in active and weight-restored phases of anorexia nervosa using robust methodology to advance our understanding of potential neural circuitry abnormalities related to hedonic and nonhedonic state and trait.
Methods
We scanned women with active anorexia nervosa, weight-restored women with anorexia nervosa and healthy-weight controls on a 3-T Siemens magnetic resonance scanner while they viewed images of high- and low-calorie foods and objects before (premeal) and after (postmeal) eating a 400 kcal meal.
Results
We enrolled 12 women with active disease, 10 weight-restored women with anorexia nervosa and 11 controls in our study. Compared with controls, both weight-restored women and those with active disease demonstrated hypoactivity premeal in the hypothalamus, amygdala and anterior insula in response to high-calorie foods (v. objects). Postmeal, hypoactivation in the anterior insula persisted in women with active disease. Percent signal change in the anterior insula was positively correlated with food stimuli ratings and hedonic and nonhedonic appetite ratings in controls, but not women with active disease.
Limitations
Our findings are limited by a relatively small sample size, which prevented the use of an analysis of variance model and exploration of interaction effects, although our substantial effect sizes of between-group differences suggest adequate power for our statistical analysis approach. Participants taking psychotropic medications were included.
Conclusion
Our data provide evidence of potential state and trait hypoactivations in food motivation regions involved in the assessment of food’s reward value and integration of these with interoceptive signalling of one’s internal state of well-being, with important relations between brain activity and homeostatic and hedonic aspects of appetite. Our findings give novel evidence of disruption in neurobiological circuits and stress the importance of examining both state and trait characteristics in the investigation of brain phenotypes in individuals with anorexia nervosa.
Introduction
Thus far, findings on the pathophysiology of anorexia nervosa have failed to affect prognosis.1 Prediction of long-term out-comes has consistently identified weight gain during treatment after initial symptom onset as critical to better prognosis,2,3 highlighting the need to increase food intake early during treatment. However, efforts at weight restoration are confounded by the complex phenotype in individuals with anorexia nervosa, which involves intense motivation to lose weight. Accurate measurement of appetite in this population is challenging, as subjective ratings of hunger (a homeostatic aspect of appetite) and desire to eat preferred foods (reflecting hedonic drive) are lower in patients with anorexia nervosa than controls,4,5 suggesting either denial of appetitive drive6 or marked inability to process internal hunger and satiation cues.7 Elucidation of the mechanisms behind this discrepancy between self-reported lack of hunger and objective state of starvation represents a significant treatment barrier and may benefit from investigation of the neural pathways involved in hedonic and nonhedonic aspects of food intake in individuals with anorexia nervosa.
Neurobiological mechanisms governing appetite in humans are highly complex. Beyond the established roles of hypothalamic nuclei,8 neuroimaging research on individuals with healthy weight has identified regions involved in appetite regulation, including the nucleus accumbens, amygdala, hippocampus, orbitofrontal cortex, anterior cingulate cortex and insula.9–11 In general, activation in these regions increases in response to food stimuli (especially foods of high caloric content and reward value) during hunger, with resolution following consumption of a satiating meal.9,10 Recent functional magnetic resonance imaging (fMRI) studies have shed light on neural circuitry deficits in women with anorexia nervosa. Of the 9 studies12–20 specifically related to hunger, satiation and processing of food stimuli, 6 studies12–17 examined patients with active anorexia nervosa (active) in comparison with healthy-weight controls (unless otherwise noted, effects described in the text that follows in individuals with anorexia nervosa are in comparison to healthy-weight controls). The earliest of these reports12 described hyperactivation in response to high-calorie food stimuli in the insula, anterior cingulate and amygdala–hippocampal region in 6 women with active anorexia nervosa. In a later study of 16 women with mixed subtype anorexia nervosa, food stimuli elicited hyperactivation in the ventromedial prefrontal cortex and lingual gyrus and hypoactivation in the inferior parietal lobule and cerebellum.13 In this same study, a subgroup of 9 women with restricting type anorexia nervosa demonstrated hyperactivation in the medial prefrontal cortex.13
Using a design that incorporated variable states of appetitive motivation, Santel and colleagues14 found hypoactivation in response to food pictures in 13 women with restricting type anorexia nervosa in the lingual gyrus after a 12-hour fast, which was associated with self-report measures of dietary restraint and disinhibition, and in the inferior parietal lobe during satiety. In a recent report on 12 women with restrictive type anorexia nervosa using a similar paradigm, patients displayed hyperactivation in response to high-calorie foods in the posterior cingulate cortex and hypoactivation in the anterior cingulate cortex after a 6-hour fast.15 Following consumption of a small standardized meal, women with anorexia nervosa exhibited hypoactivation in the lateral prefrontal cortex, with differences lateralized in the insula: hypoactivation in the right and hyperactivation in the left insula.15
In response to food images after a 4-hour fast in 11 women with restrictive type anorexia nervosa, Joos and colleagues16 reported hyperactivity in patients with anorexia nervosa in the amygdala, which was negatively related to disgust ratings of the images viewed. Healthy controls, on the other hand, displayed greater activation in the middle/posterior cingulate cortex. Finally, focusing on gustatory processing in active anorexia nervosa, a sample of 12 women with restricting type disease were administered sips of chocolate milk and water during hunger (8-h fast) and satiety (after intake of variable amounts of food).17 In response to the taste of chocolate milk (v. water) during hunger, women with active anorexia nervosa showed increased activity in the amygdala and decreased activity in the insula.
The remaining 3 reports18–20 focused on potential brain activity phenotypes associated with state (i.e., active anorexia nervosa) versus trait (i.e., ranging from improvement in eating pathology severity and weight-restoration to full recovery from anorexia nervosa) characteristics. In the only fMRI study thus far to directly compare women with chronic anorexia nervosa (n = 8), weight-recovered women with anorexia nervosa (n = 9) and healthy controls (n = 9), hyperactivation in response to food stimuli in weight-recovered women was found primarily in the prefrontal cortex and anterior cingulate cortex in separate comparisons with anorexia nervosa and controls, with hypoactivation in visual areas.18 In an additional fMRI study investigating the trait neural response to gustatory stimuli in 16 weight-recovered women with anorexia nervosa, results revealed significant reduction in the blood oxygen level–dependent response to water and sucrose in the insula, caudate, putamen and anterior cingulate.19 Finally, using a multimodal paradigm involving rewarding and aversive visual and gustatory food stimuli, Cowdrey and colleagues20 recently described hyperactivation in weight-restored women with anorexia nervosa in the ventral striatum in response to rewarding gustatory stimuli, in the insula following aversive gustatory taste, and in the anterior cingulate cortex and caudate in response to aversive visual food stimuli.
These 9 published reports12–20 revealed considerable inconsistencies, with the possible exception of insula12,15 and amygdala12,16,17 hyperactivation and inferior parietal lobule hypoactivation13,14 in women with active anorexia nervosa. There were no consistent findings in weight-restored women. Although abnormalities in the anterior cingulate, lingual gyrus and prefrontal cortex are present in other studies, the directions of effects are inconsistent. These discrepancies likely result from substantial variability among studies, including issues related to sample (e.g., small size, wide age range/duration of illness, mixed subgroups, inclusion of only active or weight-restored women), study design (e.g., variable fasting duration/prescan caloric intake, nonstandardized meals, no control for menstrual status where applicable, variable food stimuli reward value within a single food block) and statistical and technical approaches (e.g., low magnetic resonance field strength, failure to collect field mapping for correction of geometrical distortion, whole-brain analysis, insufficient unique stimuli to achieve adequate power, uncorrected p values, insufficient statistical detail). Hence, although previous studies have provided initial evidence of food motivation circuitry dysfunction in women with anorexia nervosa, methodological limitations present challenges to the development of a cohesive neurobiological model and inhibit progress toward successful treatment approaches.
In the present study, we used a neuroanatomical approach (targeting specific regions in our circuitry of interest) with a robust fMRI paradigm in weight-restored women with anorexia nervosa, those with active disease and healthy controls to determine whether women with anorexia nervosa demonstrate abnormal patterns of activation in food motivation circuitry during processing of food cues in the context of high and low motivation, and whether these abnormal patterns resolve following recovery. Given the substantial inconsistencies in methodology and results in the anorexia nervosa neuroimaging literature, we based our rationale for region-of-interest (ROI) selection and predicted group differences on well-established brain activity patterns in healthy-weight control samples9,10 and behavioural evidence of altered appetite regulation in women with anorexia nervosa.4,5 Specifically, we focused on regions associated with homeostatic (hypothalamus) and nonhomeostatic (amygdala, hippocampus, anterior cingulate, orbitofrontal cortex) food motivation. We hypothesized that compared with controls, women with active anorexia nervosa would exhibit hypoactivation in these regions in response to high-calorie food stimuli, indicating deficits in the neural circuitry controlling appetite regulation. We further predicted that these regional hypoactivities in women with anorexia nervosa would be present in response to stimuli that typically produce the most robust neural responses in healthy-weight individuals (i.e., high-calorie foods).21 With respect to appetite manipulation, we expected women with active anorexia nervosa to demonstrate hypoactivation both after a prolonged fast and after consuming a satiating meal, reflecting the persistent state of starvation and appetite dysregulation in this disorder, despite discrete periods of food intake.
Weight-restored women often display incomplete normalization of eating behaviour and other core diagnostic features after weight recovery,22,23 suggesting persistent pathology despite weight gain. We therefore expected that results for weight-recovered women would fall between those for women with active disease and healthy controls. In weight-restored women compared with controls, we hypothesized similar hypoactivation in food motivation regions before eating, suggesting dysregulation of food reward circuitry that could be associated with trait (rather than state) during hunger. Finally, we predicted no differences between weight-restored women and controls in activation in these regions after eating, indicating restoration of functioning in these regions in response to food intake, which may be related to successful weight recovery.
Methods
Participants
We recruited women with anorexia nervosa–restricting type (active group) and weight-restored women with a history of anorexia nervosa–restricting type or binge-eating/purging type (weight-restored group) between the ages of 19 and 28 years from surrounding treatment centres and from the community through advertisements. For inclusion in the study, they were required to meet diagnostic criteria for anorexia nervosa according to the DSM-IV,24 either present or past. Diagnoses were made using the Structured Clinical Interview25 for DSM-IV, administered by a trained psychiatric nurse practitioner or doctoral-level clinical psychologist and supervised by a senior psychiatrist with extensive experience in the diagnosis and treatment of eating disorders. Weight recovery was defined as maintenance of 90%–110% ideal body weight (which takes into account height, weight and frame size) for at least 6 months.26 Exclusion criteria included use of hormones, history of psychosis, objective binging/purging behaviours more than once a month within the last 3 months, history of diabetes mellitus, active substance abuse, contraindication to MRI and past gastrointestinal tract surgery. We recruited healthy control women with regular menses, no pubertal delay and 90%–110% ideal body weight from the community. Exclusion criteria were the same as those for the anorexia nervosa groups with the following exceptions: history of objective binging/purging behaviours, amenorrhea, excessive exercise within the last 3 months and any psychiatric disorder. After complete description of the study, we obtained written informed consent from all participants. The study was approved by the Partners HealthCare institutional review board.
Procedures
Participants arrived at the Massachusetts General Hospital Clinical Research Center having fasted for 12 hours. Healthy controls and weight-restored women presented during the follicular phase of the menstrual cycle (day 1–10). The first (premeal) fMRI scanning session occurred at 8:00 am. Participants were then asked to consume a 400 kcal mixed meal standardized for micro- and macronutrient content (18% calories from protein, 23% from fat and 59% from carbohydrates) over the course of 15 minutes. On meal completion, bionutrition staff weighed the portion of the meal that remained to determine exact caloric intake. The second (postmeal) fMRI scanning session began at about 9:15 am. Immediately before and after each fMRI scanning session, participants rated their appetite (hunger, desire to eat favourite food) using visual analogue scales and their current anxiety level using the State-Trait Anxiety Inventory.29 After each fMRI scanning session, participants rated a selection of the food and nonfood stimuli on valence (highly unappetizing/unpleasant to highly appetizing/pleasant) using visual analogue scales. After the postmeal scan, participants completed study questionnaires (Spielberger State-Trait Anxiety Inventory,29 Eating Disorder Examination Questionnaire,27 Beck Depression Inventory-228).
Functional MRI paradigm
We performed fMRI scanning while participants viewed 100 high-calorie (sweet [cake, doughnuts] and savoury [pizza, chips]) and 100 low-calorie (fruit, vegetables, grilled fish) food stimuli, 100 nonfood stimuli (household objects) and 100 fixation stimuli in a block design with the block order pseudorandomized and counterbalanced. Five 4-minute runs (5 images/block; 16 blocks/run) were presented. Full colour stimuli, matched for luminance, were presented for 3 seconds; each image was presented 1 time only to each participant. To ensure attention to the stimuli, we instructed participants to look at each image closely and to press a button when pictures changed. To create the fixation slides, food and nonfood stimuli were Fourier transformed to create images with the same physical properties but without recognizable content. A Dell Latitude D820 computer running Presentation software (Neurobehavioural Systems) projected visual stimuli onto a screen at the rear of the magnet bore; participants viewed the stimuli through a head coil–mounted mirror. This and similar fMRI paradigms have been shown to elicit robust activation in our ROIs in previous studies in healthy individuals.9–11,21
Functional MRI scanning parameters
Whole-brain functional imaging was performed on a Siemens 3-T Trio (8-channel head coil) scanner using a gradient-echo echo planar imaging (EPI) pulse sequence (33 oblique-axial slices, 4 mm thick, repetition time [TR] 2000 ms, echo time [TE] 30 ms, flip angle 90°, field of view [FOV] 200 × 200 mm, 120 images per run). Immediately before EPI data acquisition, a magnetic (B0) fieldmap (magnitude and phase images with the same slice prescription, number and thickness as the EPI scans) was collected for later use in distortion correction of the EPI scans. A T1-weighted 3-dimensional spoiled gradient recalled scan was also acquired (128 sagittal slices, 1.33 mm thick, TR 2350 ms, TE 3.39 ms, flip angle 7°, FOV 256 × 256 mm).
Data analysis
We preprocessed fMRI data using Statistical Parametric Mapping (SPM8; Wellcome Trust Center for Neuroimaging) and custom routines in MATLAB (Mathworks Inc.). Processing began with realignment and geometric unwarping of EPI images using magnetic fieldmaps, correction for bulk-head motion, nonlinear volume-based spatial normalization using the standard Montreal Neurological Institute (MNI) brain template and spatial smoothing with a Gaussian filter (6 mm full-width at half-maximum). Correction of geometric distortions in regions with high susceptibility artifact, such as the frontal and temporal lobes,30–32 improves registration between functional and anatomic data sets, allowing for better estimation of anatomic localization of functional activation in group analyses.33,34 We used well-established artifact detection tools (http://web.mit.edu.ezp-prod1.hul.harvard.edu/swg/software.htm) to identify and exclude outliers in the global mean image time series and movement parameters. After preprocessing, statistical analysis was performed at the single-subject level. Specific comparisons of interest (high-calorie foods v. objects, separately for premeal and postmeal) were tested using linear contrasts, and SPM maps were created based on these contrasts.
Results from the single-subject level were submitted to second-level random effects analyses. We used independent sample t tests to compare the size of a particular effect between groups. Given hypotheses about specific brain regions, we used an approach in SPM8 that limits voxel-wise analyses to voxels within a priori ROIs. Anatomically defined ROIs included the hypothalamus, nucleus accumbens, amygdala, hippocampus, orbitofrontal cortex, anterior cingulate cortex and anterior insula. Anatomic borders of hypothesized regions were defined using a manually segmented MNI-152 brain. A priori ROIs were segmented and parcellated as individual structures.35–39 All structures were segmented using a contour line and manual editing, producing core files for subcortical grey matter and cortical parcellation units that could be overlaid on the SPM8 canonical brain using the Wake Forest University PickAtlas40 toolbox for SPM to localize foci meeting significance and cluster thresholds. We performed small volume correction to identify significant clusters (voxel-wise p < 0.05, uncorrected; extent threshold = 2 voxels in the hypothalamus and nucleus accumbens, given their small volumes, and 4 voxels for all other ROIs), as per the standard SPM8 statistical thresholding approach combining height (p value) and size (number of voxels) thresholds, determined by Gaussian random field theory.41 This conjoint thresholding provides p values that are corrected for the entire volume.41 From these identified clusters, we report results for ROIs significant at p < 0.1 (corrected for multiple comparisons within the search volume using family-wise error [FWE] correction). We considered results to be significant if they reached a voxel-wise significance of p < 0.05, FWE-corrected.
Although the main focus of this investigation was on between-group differences, within-group analyses of the high-calorie versus object contrast (separately for premeal, postmeal and for premeal v. postmeal) for each group were also conducted and are reported as supplementary data (Appendix 1, available at cma.ca/jpn). Beyond a priori ROIs, regions reaching a stricter threshold at the whole-brain level for the main between-group analyses (voxel-wise p < 0.05, uncorrected; extent threshold = 10 voxels) are also reported as supplementary data (Appendix 1).
Between-group random-effects analyses were then repeated using % ideal body weight as a confounding variable in between-group comparisons, limiting our search to the same anatomic ROIs and statistical thresholds mentioned previously. This strategy allowed for investigation of between-group differences in activation, independent of group differences in % ideal body weight (i.e., body mass index and frame size). A neuroanatomist (N.M.), who was blind to study hypotheses and specific nuclei of interest, labelled activated clusters within the amygdala and anterior insula by visual inspection.
Anatomic overlays were used on each participant’s statistical maps to acquire signal change values across ROIs. Values indicated the degree of change in magnetic resonance signal detected between the high-calorie food and object conditions. Average % signal change values (β weights averaged across all voxels within functional clusters identified in the group contrasts) were obtained using the REX toolbox for SPM8.42 We used these values to calculate effect sizes for the difference between groups in these same clusters (e.g., effect size = [control group mean (high-calorie foods – objects % signal change) – active group mean (high-calorie foods –objects % signal change)] ÷ standard deviation of % signal change value of the whole sample). We also used the values in brain–behaviour correlational analyses.
We analyzed behavioural data using SPSS software, version 19 (SPSS Inc.). Demographic and clinical characteristics were analyzed using independent t tests. Appetite, anxiety and stimuli ratings were analyzed using analyses of variance (ANOVAs) with post hoc correction for multiple comparisons using the Tukey test. Pearson correlations were used to quantify relations between individual % signal change values and appetite and stimuli ratings in each group. These separate within-group correlations were compared using Fisher z transformation to verify significant between-group differences in correlations. We considered results to be significant at p < 0.05.
Results
Demographic, clinical and behavioural data
We enrolled 12 women with anorexia nervosa–restricting type, 10 weight-restored women (restricting type n = 8, binge-eating/purging type n = 2) and 11 healthy-weight control women in our study. All 3 groups were matched for sex and handedness (Table 1). Although the weight-restored group was slightly (p = 0.039) older than the healthy control group, the mean group difference was only 1.8 years. All weight-restored women had regular menses.
Table 1.
Demographic and clinical characteristics of women with active disease, women with weight-restored anorexia nervosa and healthy controls
| Group; mean (SD)* | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | Active, n = 12 | Weight-restored, n = 10 | Control, n = 11 | Between-group comparison |
| Age, yr | 21.8 (2.7) | 23.4 (2.3) | 21.6(1.3) | Weight-restored > controls, p = 0.039 |
| % ideal body weight | 81.3 (4.0) | 98.6 (10.3) | 96.7 (6.4) | Weight-restored > active, p < 0.001 Controls > active, p < 0.001 |
| Body mass index | 18.0 (0.8) | 22.1 (2.2) | 22.4 (1.3) | Weight-restored > active, p < 0.001 Controls > active, p < 0.001 |
| Age at symptom onset, yr | 16.7 (3.1) | 15.6 (2.3) | ||
| Duration of illness, yr | 5.0 (2.7) | 4.0 (2.3) | ||
| Duration of recovery, yr | 3.5 (2.9) | |||
| Eating Disorder Examination Questionnaire,27 global score | 3.4 (1.3) | 1.6 (0.9) | 0.2 (0.2) | Active > weight-restored, p = 0.010 Active > controls, p < 0.001 Weight-restored > controls, p = 0.001 |
| Beck Depression Inventory-2,28 total score | 15.3 (11.1) | 7.7 (6.9) | 0.7 (1.4) | Active > controls, p < 0.001 Weight-restored > controls, p = 0.011 |
| Spielberger State-Trait Anxiety Inventory,29 trait anxiety score† | 52.3 (13.3) | 36.2 (13.3) | 27.3 (5.4) | Active > weight-restored, p = 0.001 Active > controls, p < 0.001 Weight-restored > controls, p < 0.001 |
| Right-handedness, no. (%) | 11 (91.7) | 10 (100) | 10 (90.9) | |
| History of purging, no. (%) | 2 (18.2) | 7 (70.0) | Weight-restored > active, p = 0.027 | |
| Current psychotropic medication, no. (%)‡ | 4 (33.3) | 2 (20.0) | ||
| Comorbid diagnosis, no. (%)§ | ||||
| Current | 5 (41.7) | 1 (10.0) | ||
| Past | 4 (33.3) | 1 (10.0) | ||
Unless otherwise indicated.
The Spielberger State-Trait Anxiety Inventory is a self-report of current and “usual” levels of anxiety. Forty statements are rated on a scale from 1 to 4 (1 = statement poorly reflects feelings of anxiety; 4 = statement accurately reflects feelings of anxiety). The standardized global score reflects how the individual feels in general (trait-level anxiety). Any score < 50 is in the low normative range.
Four women with active anorexia nervosa were taking psychotropic medications: 1 was taking venlafaxine, 1 was taking fluoxetine, 1 was taking a low dose of amphetamine/dextroamphetamine (5 mg 24 h before the scan) and 1 was taking escitalopram and aripiprazole. Two weight-restored women were taking psychotropic medications: 1 was taking bupropion and lorazepam and 1 was taking fluoxetine.
Comorbid Axis I diagnoses in the active group included 3 patients with current generalized anxiety disorder (GAD); 1 patient with current attention-deficit/hyperactivity disorder not otherwise specified (ADHD NOS); 1 patient with current GAD, history of bipolar I, history of ADHD NOS and history of posttraumatic stress disorder; 2 patients with a history of major depressive disorder (MDD); and 1 patient with a history of depressive disorder NOS. Comorbid Axis I diagnoses in the weight-restored group included 1 participant with current MDD and GAD and 1 participant with a history of MDD and social phobia.
Per design, women with active disease had significantly lower % ideal body weight than weight-restored and control women (Table 1). Women with active disease had significantly greater eating disorder symptoms and trait anxiety symptoms than weight-restored and control women. Weight-restored women and those with active disease had comparable depressive symptoms that were greater than those of controls.
Groups did not differ in overall caloric intake during consumption of breakfast, indicating that differences in brain activity during the postmeal session could not be attributed to differences in caloric intake (Table 2). On premeal appetite ratings (and not postmeal ratings), control women exhibited greater hunger and desire to eat their favourite food than women with active disease (and to a lesser degree, weight-restored women). Control women rated high-calorie food stimuli as more appetizing than women with active disease pre- and postmeal, and more appetizing premeal than weight-restored women. Women with active disease exhibited higher levels of state anxiety than controls and weight-restored women before and after scanning. Results reveal differences in self-report of hunger and food motivation between weight-restored women, those with active disease and healthy controls, despite similar caloric intake.
Table 2.
Bionutrition data and anxiety, appetite and food stimuli ratings
| Group mean (SD) | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | Active, n = 12 | Weight-restored, n = 10 | Controls, n = 11 | Between-group comparison* |
| Calories consumed at breakfast | 390.1 42.8) | 408.8 (18.1) | 405.4 (7.7) | |
| Spielberger State-Trait Anxiety Inventory,29 state anxiety scores† | ||||
| Premeal scan 1 | 46.7 (10.4) | 29.1 (8.6) | 26.9 (6.2) | Active > controls, p < 0.001 Active > weight-restored, p < 0.001 |
| Postmeal scan 1 | 48.8 (11.2) | 32.0 (9.5) | 25.0 (4.5) | Active > controls, p < 0.001 Active > weight-restored, p = 0.001 |
| Premeal scan 2 | 49.5 (12.5) | 30.5 (9.0) | 24.5 (4.7) | Active > controls, p < 0.001 Active > weight-restored, p = 0.001 |
| Postmeal scan 2 | 49.1 (9.9) | 31.6 (10.5) | 24.5 (3.4) | Active > controls, p < 0.001 Active > weight-restored, p = 0.001 |
| Appetite ratings‡ | ||||
| Premeal scan | ||||
| Prescan hunger | 5.0 (2.3) | 5.2 (1.7) | 6.2 (1.8) | |
| Prescan desire to eat favourite food | 2.5 (2.5) | 3.0 (2.3) | 5.7 (2.5) | Controls > active, p = 0.010 Controls > weight-restored, p = 0.044 |
| Postscan hunger | 5.3 (2.7) | 6.3 (1.2) | 7.5 (0.9) | Controls > active, p = 0.024 |
| Postscan desire to eat favourite food | 4.1 (3.1) | 4.9 (2.6) | 7.0 (1.9) | Controls > active, p = 0.031 |
| Postmeal scan | ||||
| Prescan hunger | 2.2 (2.1) | 1.9 (1.7) | 1.7 (2.2) | |
| Prescan desire to eat favourite food | 3.3 (2.9) | 1.7 (2.0) | 2.7 (2.8) | |
| Postscan hunger | 3.0 (2.8) | 2.7 (2.4) | 2.9 (2.6) | |
| Postscan desire to eat favourite food | 2.7 (2.5) | 2.4 (2.1) | 4.5 (3.3) | |
| Food stimuli ratings§ | ||||
| Premeal scan | ||||
| High-calorie food | 3.0 (1.2) | 4.8 (1.6) | 6.4 (1.3) | Controls > active, p < 0.001 Controls > weight-restored, p = 0.029 Weight-restored > active, p = 0.011 |
| Low-calorie food | 5.8 (1.7) | 5.6 (1.4) | 6.0 (1.7) | |
| Objects | 5.3 (0.9) | 5.5 (0.9) | 5.2 (2.2) | |
| Postmeal scan | ||||
| High-calorie food | 4.1 (1.7) | 5.0 (1.6) | 5.8 (1.0) | Controls > active, p = 0.036 |
| Low-calorie food | 6.4 (1.2) | 6.7 (1.0) | 6.9 (1.2) | |
| Objects | 5.6 (1.0) | 5.7 (0.9) | 5.7 (1.2) | |
Corrected for multiple comparisons (Tukey test).
The Spielberger State-Trait Anxiety Inventory is a self-report of current and “usual” levels of anxiety. Forty statements are rated on a scale from 1 to 4 (1 = statement poorly reflects feelings of anxiety; 4 = statement accurately reflects feelings of anxiety). The standardized global score reflects how the individual feels in general (trait-level anxiety). Any score < 50 is in the low normative range.
Participants rated their appetite immediately before and after each functional magnetic resonance imaging scanning session. These scales consisted of several questions using a visual analogue scale, each presented with a set of 100 mm lines anchored by “not at all” on the left and “extremely” on the right.
After each functional magnetic resonance imaging scanning session, participants rated a selection of the food stimuli on valence using a visual analogue scale, each presented with a set of 100 mm lines anchored by “highly unappetizing/unpleasant” on the left and “highly appetizing/pleasant” on the right.
Functional MRI data
Data are presented here for the 12 women with active anorexia nervosa, 10 weight-restored women and 11 healthy controls described in the previous section. One additional healthy control participant was excluded owing to technical error during data acquisition, and 2 additional participants (1 woman with chronic anorexia nervosa, 1 control) were excluded owing to excessive movement.
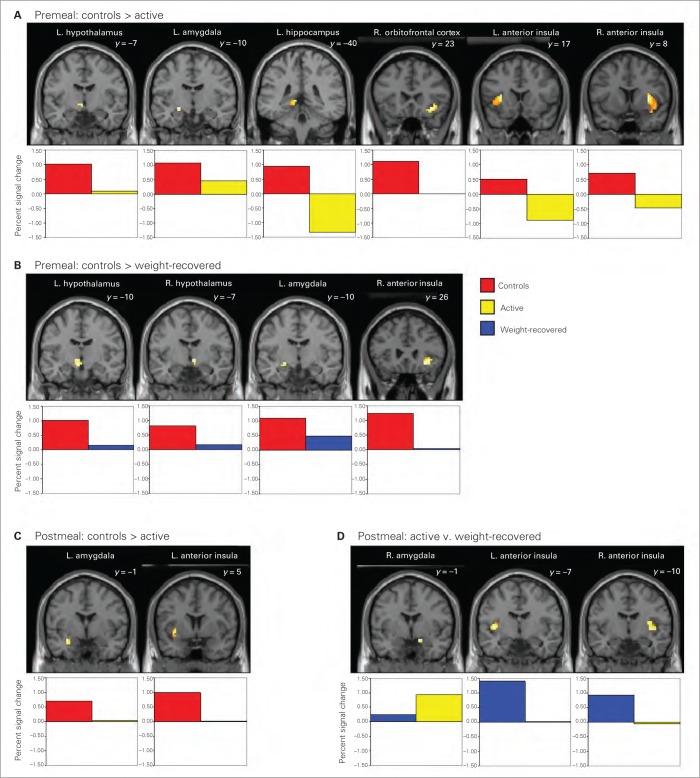
The main contrasts of interest focused on differences between groups in response to high-calorie foods pre- and post-meal. Compared with controls, women with active disease exhibited hypoactivation premeal in the hypothalamus, amygdala, hippocampus, orbitofrontal cortex and anterior insula and postmeal in the amygdala and insula (Table 3, Fig. 1). After controlling for % ideal body weight, premeal between-group differences remained significant in the anterior insula and amygdala; postmeal differences remained significant in the anterior insula and showed a trend toward significance in the amygdala. Women with active disease did not exhibit greater activation than controls in response to high-calorie foods either pre- or postmeal. Controls displayed greater activation than weight-restored women premeal in the hypothalamus, amygdala and anterior insula, with results showing a trend toward significance even after controlling for % ideal body weight (Table 3, Fig. 1). There were no differences in activation between controls and weight-restored women postmeal. Differences between women with active disease and weight-restored women were restricted to postmeal, with women with active disease exhibiting amygdala hyperactivation and anterior insula hypoactivation compared with weight-restored women. This result remained significant after controlling for % ideal body weight (Table 3, Fig. 1). Comparisons of individual % signal change values confirmed these results, with effect sizes ranging from three-quarters to more than 1 full standard deviation difference, and a more modest effect size difference (0.54) between women with active disease and weight-restored women in amygdala hyperactivation postmeal (Table 3). Within-group analyses of the high-calorie versus object contrast (premeal, postmeal and premeal v. postmeal) are summarized in the Appendix (Tables S1–S3), as are results of between-group analyses at the whole-brain level (Table S4).
Table 3.
Regions of activation in comparisons of high–calorie food to objects: between–group contrasts
| Condition; contrast | Brain region | MNI coordinate | z score | Voxels | Voxel-level, FWE–corrected | Effect size | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| x | y | z | p value* | p value controlling for % IBW | |||||
| Premeal | |||||||||
| Controls > active | Left hypothalamus | −3 | −7 | −5 | 2.42 | 5 | 0.93 | ||
| Left amygdala | −21 | −10 | −11 | 2.55 | 23 | 0.08 | 0.031 | 0.84 | |
| Left hippocampus | −9 | −40 | 1 | 3.41 | 11 | 0.022 | NS | 1.35 | |
| Right orbitofrontal cortex | 36 | 23 | −11 | 3.50 | 42 | 0.05 | 0.19 | 1.05 | |
| Right insula | 33 | 8 | 4 | 4.14 | 284 | 0.003 | 0.05 | 1.33 | |
| Left insula | −30 | 17 | 7 | 3.66 | 243 | 0.016 | 0.023 | 1.27 | |
| Active > controls | None | ||||||||
| Controls > weight-restored | Right hypothalamus | 9 | −7 | −5 | 2.56 | 14 | 0.06 | 0.07 | 0.89 |
| Left hypothalamus | −6 | −10 | −5 | 2.54 | 19 | 0.06 | 0.07 | 0.93 | |
| Left amygdala | −24 | −10 | −11 | 2.63 | 19 | 0.07 | 0.07 | 0.75 | |
| Right insula | 39 | 26 | −8 | 3.25 | 97 | 0.05 | 0.07 | 0.94 | |
| 30 | 26 | −11 | 3.16 | 97 | 0.06 | 0.09 | 1.08 | ||
| Weight-restored > controls | None | ||||||||
| Active > weight-restored | None | ||||||||
| Weight-restored > active | None | ||||||||
| Postmeal | |||||||||
| Controls > active | Left amygdala | −30 | −1 | −20 | 3.63 | 7 | 0.006 | 0.08 | 0.95 |
| −24 | −10 | −14 | 2.71 | 8 | 0.06 | 0.07 | 0.78 | ||
| Left insula | −39 | −7 | 4 | 3.61 | 29 | 0.021 | 0.016 | 1.07 | |
| −33 | 5 | −5 | 3.46 | 52 | 0.032 | 0.22 | 1.07 | ||
| Active > controls | None | ||||||||
| Controls > weight-restored | None | ||||||||
| Weight-restored > controls | None | ||||||||
| Active > weight-restored | Right amygdala | 15 | −1 | −17 | 2.74 | 6 | 0.06 | 0.42 | 0.54 |
| Weight-restored > active | Right insula | 36 | −10 | 13 | 3.15 | 48 | 0.08 | 0.38 | 1.07 |
| Left insula | −39 | −7 | 4 | 3.89 | 156 | 0.010 | 0.003 | 1.25 | |
FWE = family-wise error; IBW = ideal body weight; MNI = Montreal Neurological Institute; ROI = region of interest.
The family–wise error rate was used for small volume correction: voxel-level significance (FWE–corrected within the search volume of interest).
Fig. 1.
Significant hypoactivation of food motivation circuitry regions in women with active anorexia nervosa and weight-restored women in comparison with healthy controls. Activations of hypothesized regions of interest were derived using restriction to within anatomic borders (defined by a brain manually segmented in Montreal Neurological Institute space) with the small-volume correction tool in SPM8. Activations in Fig. 1 are selected from Table 3, centred on the peak voxel of activation at a significance level of p < 0.05, uncorrected.
Relations between % signal change values and visual analogue scales for stimuli and appetite ratings examined whether group differences in activation in response to high-calorie food stimuli were partially driven by individual differences in subjective appetite and stimuli ratings. Ratings of high-calorie food stimuli valence were positively correlated with premeal % signal change in the insula in control women but not in women with active disease (Table 4). Premeal insula % signal change was positively related to appetite ratings in controls, including hunger and desire to eat favourite foods, but not in women with active disease. In weight-restored women, premeal % signal change in the hypothalamus was positively associated with desire to eat favourite foods, a relation not present in controls. Premeal % signal change in the amygdala was positively correlated with desire to eat favourite foods in controls and weight-restored women, but not in women with active disease. These results suggest that during a state of high motivation, higher ratings on behavioural evaluations of subjective appetite and rewarding food-related stimuli correspond to greater activation in the insula in controls, the hypothalamus in weight-restored women and the amygdala in controls and weight-restored women, but not in women with active disease.
Table 4.
Correlations between premeal activation to high-calorie food versus objects and stimuli and appetite ratings: group differences
| Active | Weight-restored | Controls | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Condition/rating item | Brain region | r | p value | r | p value | r | p value | Between-group comparison |
| High-calorie food stimuli | Right insula | 0.24 | 0.45 | 0.66 | 0.027 | Active v. controls, z = 1.13, p = 0.13 | ||
| Left insula | 0.39 | 0.20 | 0.62 | 0.043 | Active v. controls, z = 0.65, p = 0.26 | |||
| Hunger | Right insula | −0.21 | 0.52 | 0.66 | 0.026 | Active v. controls, z = 2.1, p = 0.019 | ||
| Desire to eat favourite food | Right hypothalamus | 0.81 | 0.008 | −0.27 | −0.42 | Weight-restored v. controls, z = 2.7, p = 0.003 | ||
| Left amygdala | 0.10 | 0.76 | 0.62 | 0.05 | 0.66 | 0.026 | Weight-restored v. controls, z = 1.2, p = 0.08 Active v. weight-restored, z = 1.4, p = 0.11 |
|
| Right insula | −0.08 | 0.80 | 0.79 | 0.004 | Active v. controls, z = 2.4, p = 0.009 | |||
Discussion
Elucidation of the brain phenotype associated with active disease and recovery in individuals with anorexia nervosa will contribute to understanding the neurobiology and development of more effective treatment strategies. We report systematic hypoactivations in the hypothalamus, amygdala and anterior insula in women with active anorexia nervosa and in weight-restored women compared with healthy-weight controls during a state of high motivation (premeal), suggesting that these deficits might be enduring traits of anorexia nervosa. Further, during low motivation (postmeal), anterior insula hypoactivation persisted in women with active disease (but not in weight-restored women) compared with controls, suggesting that insula deficits might also reflect a clinical state of anorexia nervosa, with restored ability to regulate appetitive signals following food intake after recovery. Importantly, anterior insula and amygdala hypoactivations in both anorexia nervosa groups premeal were independent of body weight, providing additional evidence that among the women with active disease, these deficits were unassociated with low weight or starvation. Finally, activations in the hypothalamus, amygdala and anterior insula in controls and weight-restored women were significantly associated with behavioural indicators of hedonic (desire for favourite foods) and nonhedonic (hunger) aspects of appetite, relations that were not present in women with active disease. Taken together, these results provide evidence for an association between active anorexia nervosa and amygdala and insula dysfunction after eating, with additional findings suggesting that the phenotypic abnormal brain response in these regions and in the hypothalamus during a state of hunger and high appetitive motivation may persist after weight restoration.
To our knowledge, widespread food motivation circuitry hypoactivation in response to high-calorie foods has not previously been reported in studies of anorexia nervosa. We found that, in contrast to controls, women with active disease exhibited significantly less activation in the hypothalamus, amygdala, hippocampus, orbitofrontal cortex and anterior insula during hunger and in the amygdala and anterior insula during satiety, with effect sizes greater than three-quarters of a standard deviation difference in % signal change. In comparison to 2 studies that examined neural circuitry related to processing of visual food stimuli in women with anorexia nervosa using similar paradigms,14,15 our results are largely dissimilar. Although Santel and colleagues14 reported hypoactivation in women with active anorexia nervosa compared with healthy controls, the decreased activation was observed largely in the lingual gyrus premeal and in the inferior posterior lobule postmeal — posterior regions not typically involved in food-related processing of hunger and satiation. Furthermore, in contrast to Gizewski and colleagues,15 who reported hyperactivation in women with active anorexia nervosa in the posterior cingulate premeal and in the right mid-insula postmeal, we did not find any regions in which women with active anorexia nervosa demonstrated elevated activation in response to food images. These discrepancies could be related to a variety of methodological differences between the studies (e.g., magnetic resonance field strength, activation paradigm, data analytic approach). However, similar to Gizewski and colleagues,15 we found hypoactivation postmeal in the left insula in women with active disease. In addition, our results of premeal insula hypoactivation in weight-restored women are in agreement with those of a previous report of insula hypoactivation in response to gustatory stimuli in a similar group19 despite significant differences in methodology. Thus, with respect to the larger context of neuroimaging studies on anorexia nervosa focused on hunger and satiety mechanisms, our findings most strongly support the evidence for significant hypoactivation in the insula in women with active anorexia nervosa.
Our findings of hypothalamus, amygdala and anterior insula hypoactivation in weight-recovered women during a state of high appetitive motivation, even after controlling for % ideal body weight, offer compelling evidence that these hypoactivations may be traits of anorexia nervosa. We note that, importantly, the functions of these regions are not limited to processes related to appetite and food intake regulation. These regions are also highly involved in the response to stressful stimuli in healthy controls43 and have been implicated in anxiety disorders, including generalized anxiety disorder,44,45 social anxiety disorder46 and obsessive–compulsive disorder.47,48 Although activity in the amygdala44,46,48 and insula45–47 is generally heightened in individuals with anxiety disorders (rather than hypoactive, as observed here in women with anorexia nervosa), we cannot rule out the possibility that dysfunction in these regions in women with anorexia nervosa might reflect a generalized anxiety response to food images.
Having stated this caveat, we focus on interpretation of activation in these regions with respect to hunger and satiation given our design and hypotheses. The hypothalamus governs several homeostatic functions regulating arousal and food intake, with receptors for several potent appetite hormones and neuropeptides, including ghrelin, leptin, neuropeptide Y, agouti-related protein, insulin and glucose identified in hypothalamic nuclei involved in hunger and satiation signalling (i.e., arcuate nucleus).8,49,50 Hypoactivation in response to high-calorie foods in the hypothalamus premeal in women with active disease and weight-restored women, which to our knowledge has not been reported previously, might be related to well-established neuroendocrine abnormalities in individuals with anorexia nervosa.51–55 The amygdala is involved in learning cues associated with satiation, approach behaviours related to food and assessing reward value of food,56–59 and has dense connections with the anterior insula.60,61 The anterior “limbic” insula contains the primary taste cortex,62,63 plays a role in the assessment of affective tone and motivational behaviour64–66 and is involved in autonomic integration of visceral and proprioceptive signalling, assisting in the generation of a representation of one’s physical and internal state that is integral to self-awareness.65 It is also involved in aspects of cognitive control, such as intentional inhibition, awareness of performance errors66 and prediction of future emotional responses.67 Efferent outputs from the anterior insula to the amygdala are responsible for relay of sensory and visceral in-formation to the limbic system,60 providing information regarding physical and emotional comfort and discomfort, including during conditioned taste aversion.68 Thus, associations between activations in these regions and subjective ratings of homeostatic and rewarding aspects of food motivation were observed in controls, but not in women with active disease, providing evidence that these deficits may contribute to disrupted food-related behaviours.
Limitations
Our findings are limited by a relatively small sample size. However, we included a larger sample of participants than the single previous fMRI study examining food motivation circuitry in women with active disease, weight-restored women and healthy controls, and we report substantial effect sizes of between-group differences, suggesting that our study had adequate statistical power. Although use of an ANOVA model would have allowed for full exploration of within- and between-group interaction effects, we did not have sufficient power to use this statistical approach. We included participants who were taking psychotropic medication, which may have had an effect on some activations, but would not have produced differential within-subject patterns of results pre- and postmeal. Future studies should attempt to recruit medication-free participants. Two weight-restored women showed signs of moderate depression, although % signal change values for these participants were close to the mean for the group in all regions (data not shown). Finally, we were not able to counterbalance the order of our scanning sessions, thus we cannot rule out the possibility that postmeal group differences reflect physiologic phenomena (other than the effect of food intake), such as slower habituation to high-calorie foods. However, if post-meal findings were simply related to to physiologic phenomena, then one would not expect our region-specific results.
Conclusion
Self-report of hunger and perception of the reward value of food are significantly altered in women with active anorexia nervosa, with ongoing debate regarding whether these abnormalities are driven by physiologic or psychological processes. Although weight gain through restoration of healthy eating patterns is the primary goal (especially early) in treatment, eating behaviour disturbances remain even after recovery, with life-long risk of relapse. Our data provide evidence of potential state and trait patterns of hypoactivation in food motivation regions involved in appetitive behaviours, assessment of food’s reward value and integration of these with interoceptive signalling of one’s internal state of well-being and homeostatic and hedonic aspects of appetite. These findings stress the importance of examining state and trait characteristics in investigations of brain phenotypes in individuals with anorexia nervosa.
Acknowledgements
L.M. Holsen and E.A. Lawson were funded by the Harvard K12 HD051959 Building Interdisciplinary Research Careers in Women’s Health Program supported by National Institutes of Health Office of Research in Women’s Health. The research was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR025758) and the Davis Foundation (P.K. Fazeli). We thank Daniel Donoho, Lauren Ogden and Tara Minaker for help in earlier phases of the study and David Herzog, MD, Kamryn Eddy, PhD, and Erinne Meenaghan, NP, for clinical assessment of the participants.
Footnotes
Competing interests: As above for L.M. Holsen, E.A. Lawson and P.K. Fazeli. None declared for J. Blum. E. Ko, N. Makris, A. Klibanski and J.M. Goldstein declare having received grant support from the National Institutes of Health through their respective insitutions.
Contributors: L.M. Holsen, E.A. Lawson, A. Klibanski and J.M. Goldstein designed the study. L.M. Holsen, E.A. Lawson, E. Ko and P.K. Fazeli acquired the data. L.M. Holsen, E.A. Lawson, J. Blum, E. Ko, N. Makris and J.M. Goldstein analyzed the data. L.M. Holsen and J.M. Goldstein wrote the article. E.A. Lawson, J. Blum, E. Ko, N. Makris, P.K. Fazeli, A. Klibanski and J.M. Goldstein reviewed the article. All authors approved its publication.
References
- 1.Steinhausen HC. The outcome of anorexia nervosa in the 20th century. Am J Psychiatry. 2002;159:1284–93. doi: 10.1176/appi.ajp.159.8.1284. [DOI] [PubMed] [Google Scholar]
- 2.Guarda AS. Treatment of anorexia nervosa: insights and obstacles. Physiol Behav. 2008;94:113–20. doi: 10.1016/j.physbeh.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Steinhausen HC, Boyadjieva S, Grigoroiu-Serbanescu M, et al. A transcultural outcome study of adolescent eating disorders. Acta Psychiatr Scand. 2000;101:60–6. doi: 10.1034/j.1600-0447.2000.101001060.x. [DOI] [PubMed] [Google Scholar]
- 4.Hetherington MM, Rolls BJ. Eating behavior in eating disorders: response to preloads. Physiol Behav. 1991;50:101–8. doi: 10.1016/0031-9384(91)90505-i. [DOI] [PubMed] [Google Scholar]
- 5.Robinson RG, Tortosa M, Sullivan J, et al. Quantitative assessment of psychologic state of patients with anorexia nervosa or bulimia: response to caloric stimulus. Psychosom Med. 1983;45:283–92. doi: 10.1097/00006842-198308000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Huse DM, Lucas AR. Dietary patterns in anorexia nervosa. Am J Clin Nutr. 1984;40:251–4. doi: 10.1093/ajcn/40.2.251. [DOI] [PubMed] [Google Scholar]
- 7.Robinson PH. Perceptivity and paraceptivity during measurement of gastric emptying in anorexia and bulimia nervosa. Br J Psychiatry. 1989;154:400–5. doi: 10.1192/bjp.154.3.400. [DOI] [PubMed] [Google Scholar]
- 8.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 9.Holsen LM, Zarcone JR, Thompson TI, et al. Neural mechanisms underlying food motivation in children and adolescents. Neuroimage. 2005;27:669–76. doi: 10.1016/j.neuroimage.2005.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaBar KS, Gitelman DR, Parrish TB, et al. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 11.Stoeckel LE, Weller RE, Cook EW, III, et al. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–47. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 12.Ellison Z, Foong J, Howard R, et al. Functional anatomy of calorie fear in anorexia nervosa. Lancet. 1998;352:1192. doi: 10.1016/S0140-6736(05)60529-6. [DOI] [PubMed] [Google Scholar]
- 13.Uher R, Murphy T, Brammer MJ, et al. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry. 2004;161:1238–46. doi: 10.1176/appi.ajp.161.7.1238. [DOI] [PubMed] [Google Scholar]
- 14.Santel S, Baving L, Krauel K, et al. Hunger and satiety in anorexia nervosa: fMRI during cognitive processing of food pictures. Brain Res. 2006;1114:138–48. doi: 10.1016/j.brainres.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 15.Gizewski ER, Rosenberger C, de Greiff A, et al. Influence of satiety and subjective valence rating on cerebral activation patterns in response to visual stimulation with high-calorie stimuli among restrictive anorectic and control women. Neuropsychobiology. 2010;62:182–92. doi: 10.1159/000319360. [DOI] [PubMed] [Google Scholar]
- 16.Joos AA, Saum B, van Elst LT, et al. Amygdala hyperreactivity in restrictive anorexia nervosa. Psychiatry Res. 2011;191:189–95. doi: 10.1016/j.pscychresns.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Vocks S, Herpertz S, Rosenberger C, et al. Effects of gustatory stimulation on brain activity during hunger and satiety in females with restricting-type anorexia nervosa: an fMRI study. J Psychiatr Res. 2011;45:395–403. doi: 10.1016/j.jpsychires.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Uher R, Brammer MJ, Murphy T, et al. Recovery and chronicity in anorexia nervosa: brain activity associated with differential out-comes. Biol Psychiatry. 2003;54:934–42. doi: 10.1016/s0006-3223(03)00172-0. [DOI] [PubMed] [Google Scholar]
- 19.Wagner A, Aizenstein H, Mazurkewicz L, et al. Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology. 2008;33:513–23. doi: 10.1038/sj.npp.1301443. [DOI] [PubMed] [Google Scholar]
- 20.Cowdrey FA, Park RJ, Harmer CJ, McCabe C. Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biol Psychiatry. 2011;70:736–43. doi: 10.1016/j.biopsych.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 21.Goldstone AP, Prechtl de Hernandez CG, Beaver JD, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30:1625–35. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- 22.Dellava JE, Hamer RM, Kanodia A, et al. Diet and physical activity in women recovered from anorexia nervosa: a pilot study. Int J Eat Disord. 2011;44:376–82. doi: 10.1002/eat.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner A, Barbarich-Marsteller NC, Frank GK, et al. Personality traits after recovery from eating disorders: Do subtypes differ? Int J Eat Disord. 2006;39:276–84. doi: 10.1002/eat.20251. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994. [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; Nov, 2002. [Google Scholar]
- 26.1983 metropolitan height and weight tables. Stat Bull Metrop Life Found. 1983;64:3–9. [PubMed] [Google Scholar]
- 27.Fairburn CG, Cooper PJ. The Eating Disorder Examination. In: Fairburn CG, Wilson GT, editors. Binge eating: nature, assessment, and treatment. 12th ed. New York (NY): Guilford Press; 1993. pp. 317–60. [Google Scholar]
- 28.Beck AT, Steer RA, Brown GK. BDI II Manual. San Antonio (TX): Psychological Corporation; 2002. [Google Scholar]
- 29.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press; 1983. [Google Scholar]
- 30.Devlin JT, Russell RP, Davis MH, et al. Susceptibility-induced loss of signal: comparing PET and fMRI on a semantic task. Neuroimage. 2000;11:589–600. doi: 10.1006/nimg.2000.0595. [DOI] [PubMed] [Google Scholar]
- 31.Gorno-Tempini ML, Hutton C, Josephs O, et al. Echo time dependence of BOLD contrast and susceptibility artifacts. Neuroimage. 2002;15:136–42. doi: 10.1006/nimg.2001.0967. [DOI] [PubMed] [Google Scholar]
- 32.Ojemann JG, Akbudak E, Snyder AZ, et al. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–67. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- 33.Andersson JL, Hutton C, Ashburner J, et al. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–19. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- 34.Hutton C, Bork A, Josephs O, et al. Image distortion correction in fMRI: A quantitative evaluation. Neuroimage. 2002;16:217–40. doi: 10.1006/nimg.2001.1054. [DOI] [PubMed] [Google Scholar]
- 35.Breiter HC, Gollub RL, Weisskoff RM, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 36.Caviness JN, Makris N, Meyer JW, et al. MRI-based topographic parcellation of human neocortex: an anatomically specific method with estimate of reliability. J Cogn Neurosci. 1996;8:566–87. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- 37.Makris N, Gasic GP, Seidman LJ, et al. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44:729–40. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 38.Makris N, Meyer JW, Bates JF, et al. MRI-Based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. Neuroimage. 1999;9:18–45. doi: 10.1006/nimg.1998.0384. [DOI] [PubMed] [Google Scholar]
- 39.Rademacher J, Galaburda A, Kennedy D, et al. Human cerebral cortex: Localization, parcellation and morphometry with magnetic resonance imaging. J Cogn Neurosci. 1992;4:352–74. doi: 10.1162/jocn.1992.4.4.352. [DOI] [PubMed] [Google Scholar]
- 40.Maldjian JA, Laurienti PJ, Kraft RA, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 41.Friston KJ, Ashburner JT, Kiebel SJ, et al., editors. Statistical parametric mapping: the analysis of functional brain images. London: Academic Press; 2007. [Google Scholar]
- 42.Whitfield-Gabrieli S. Region of Interest Extraction (REX) Toolbox. Boston (MA): MIT Department of Brain and Cognitive Sciences; 2009. [Google Scholar]
- 43.Goldstein JM, Jerram M, Abbs B, et al. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci. 2010;30:431–8. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klumpp H, Angstadt M, Nathan PJ, et al. Amygdala reactivity to faces at varying intensities of threat in generalized social phobia: an event-related functional MRI study. Psychiatry Res. 2010;183:167–9. doi: 10.1016/j.pscychresns.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol Psychol. 2012;89:273–6. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah SG, Klumpp H, Angstadt M, et al. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. J Psychiatry Neurosci. 2009;34:296–302. [PMC free article] [PubMed] [Google Scholar]
- 47.Schienle A, Schafer A, Stark R, et al. Neural responses of OCD patients towards disorder-relevant, generally disgust-inducing and fear-inducing pictures. Int J Psychophysiol. 2005;57:69–77. doi: 10.1016/j.ijpsycho.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Simon D, Kaufmann C, Musch K, et al. Fronto-striato-limbic hyperactivation in obsessive-compulsive disorder during individually tailored symptom provocation. Psychophysiology. 2010;47:728–38. doi: 10.1111/j.1469-8986.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 49.Hosoda H, Kojima M, Kangawa K. Biological, physiological, and pharmacological aspects of ghrelin. J Pharmacol Sci. 2006;100:398–410. doi: 10.1254/jphs.crj06002x. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz MW, Woods SC, Porte D, Jr, et al. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 51.Misra M, Miller KK, Kuo K, et al. Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289:E373–81. doi: 10.1152/ajpendo.00041.2005. [DOI] [PubMed] [Google Scholar]
- 52.Misra M, Miller KK, Kuo K, et al. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289:E347–56. doi: 10.1152/ajpendo.00615.2004. [DOI] [PubMed] [Google Scholar]
- 53.Misra M, Miller KK, Herzog DB, et al. Growth hormone and ghrelin responses to an oral glucose load in adolescent girls with anorexia nervosa and controls. J Clin Endocrinol Metab. 2004;89:1605–12. doi: 10.1210/jc.2003-031861. [DOI] [PubMed] [Google Scholar]
- 54.Otto B, Cuntz U, Fruehauf E, et al. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001;145:669–73. [PubMed] [Google Scholar]
- 55.Nakahara T, Kojima S, Tanaka M, et al. Incomplete restoration of the secretion of ghrelin and PYY compared to insulin after food ingestion following weight gain in anorexia nervosa. J Psychiatr Res. 2007;41:814–20. doi: 10.1016/j.jpsychires.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 56.Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- 57.Petrovich GD, Setlow B, Holland PC, et al. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci. 2002;22:8748–53. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–9. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 59.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 60.Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 61.Sims KS, Williams RS. The human amygdaloid complex: a cytologic and histochemical atlas using Nissl, myelin, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate diaphorase staining. Neuroscience. 1990;36:449–72. doi: 10.1016/0306-4522(90)90440-f. [DOI] [PubMed] [Google Scholar]
- 62.Scott TRYS, Sienkiewicz ZJ, Rolls ET. Gustatory responses in the frontal opercular cortex of the alert cynomolgus monkey. J Neurophysiol. 1986;56:876–90. doi: 10.1152/jn.1986.56.3.876. [DOI] [PubMed] [Google Scholar]
- 63.Sudakov K, MacLean PD, Reeves A, et al. Unit study of exteroceptive inputs to claustrocortex in awake, sitting, squirrel monkey. Brain Res. 1971;28:19–34. doi: 10.1016/0006-8993(71)90521-x. [DOI] [PubMed] [Google Scholar]
- 64.Kringelbach ML, Berridge KC. Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn Sci. 2009;13:479–87. doi: 10.1016/j.tics.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 66.Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 67.Brass M, Haggard P. The hidden side of intentional action: the role of the anterior insular cortex. Brain Struct Funct. 2010;214:603–10. doi: 10.1007/s00429-010-0269-6. [DOI] [PubMed] [Google Scholar]
- 68.Lamprecht R, Dudai Y. The amygdala in conditioned taste aversion: It’s there, but where? In: Aggleton JP, editor. The amygdala: a functional analysis. 2 ed. Oxford: Oxford University Press; 2000. pp. 331–51. [Google Scholar]