Abstract
Young women tend to have lower blood pressure, and less risk of hypertension, compared to young men. As people age, both blood pressure and the risk of hypertension increase in both sexes; this occurs most strikingly in women after menopause. However, the mechanisms for these influences of sex and age remain incompletely understood. In this review we are specifically interested in the interaction between neural (sympathetic nerve activity; SNA) and haemodynamic factors (cardiac output, blood pressure and vascular resistance) and how these change with sex and age. While peripheral vascular SNA can vary 7- to 10–fold among normotensive young men and women, it is reproducible in a given individual. Surprisingly, higher levels of SNA are not associated with higher blood pressures in these groups. In young men, high SNA is associated with higher total peripheral vascular resistance (TPR), and appears to be balanced by lower cardiac output and less peripheral vascular responsiveness to adrenergic stimulation. Young women do not exhibit the SNA–TPR relationship. Recent evidence suggests that β–adrenergic vasodilatation offsets the vasoconstrictor effects of α–adrenergic vasoconstriction in young women, which may contribute to the generally lower blood pressures in this group. Sympathetic nerve activity increases with age, and in groups over 40, levels of SNA are more tightly linked to levels of blood pressure. The potentially protective β–adrenergic effect seen in young women appears to be lost after menopause and probably contributes to the increased blood pressure and increased risk of hypertension seen in older women.
Nisha Charkoudian's research interests focus on control mechanisms in the human circulation, as related to blood pressure regulation and thermoregulation. She was a member of the Physiology faculty at Mayo Clinic for several years, and recently moved to the US Army Research Institute of Environmental Medicine in Natick. Dr Charkoudian (left) acted as a co-mentor for Emma Hart (right) during her Postdoctoral Research Fellowship at the Mayo Clinic. Emma Hart's research interests centre on the role of the autonomic nervous system in blood pressure regulation and the influence sex hormones have on this. Emma has now recently moved to the University of Bristol after receiving a British Heart Foundation Intermediate Fellowship. Both are fortunate to be a part of a collaborative effort to understand sympathetic neural mechanisms in blood pressure control along with Gunnar Wallin and Michael Joyner. A main focus of this ongoing work has been the importance of inter-individual variability in blood pressure regulatory mechanisms.
 |
Introduction
Arterial blood pressure is a key regulated variable in the human cardiovascular system. Dysregulation of blood pressure leading to chronic hypertension or hypotensive disorders (such as orthostatic intolerance/hypotension) is the source of significant morbidity and mortality in the UK and developed countries around the world (Robertson, 1999; Fields 2004; Fu et al. 2004; Kearney et al. 2005). Importantly, the incidence of disorders at both ends of the blood pressure spectrum is significantly different between the sexes (Robertson, 1999; Kearney et al. 2005). Young women (prior to the menopause) have much lower incidence of chronic hypertension compared to men of similar age (Wiinberg et al. 1995; Kearney et al. 2005), whereas, compared to men, younger women are much more likely to develop hypotensive disorders such as orthostatic intolerance (Robertson, 1999; Ganzeboom et al. 2003; Fu et al. 2004; Olde Nordkamp et al. 2009). Several lines of evidence support the idea that female reproductive hormones have a major protective influence against the development of cardiovascular disease in young women. For example, ageing is associated with increased risk of hypertension in both men and women. However, at around the age of menopause, the incidence of hypertension increases markedly, where rates of hypertension meet or even exceed that in men of a similar age and demographic status (Burt et al. 1995; Wiinberg et al. 1995; Martins et al. 2001; Kearney et al. 2005). This is important to consider when the postmenopausal population is expected to increase 2- to 5–fold by 2025 (US Census Bureau on World Population; Meyer et al. 2008).
In the present discussion, our goal is to review evidence for differences between the sexes in terms of blood pressure regulation, with a focus on recent evidence regarding the importance of inter-individual variability in blood pressure regulatory mechanisms. In particular, we are interested in the interaction between neural factors (notably peripheral vascular sympathetic nerve activity, SNA) and haemodynamic factors (including cardiac output, peripheral blood flow and peripheral vascular resistance). Our overarching hypothesis is that sex and age interact to influence the transmission of muscle sympathetic nerve activity (MSNA) into vasomotor tone and thus alter the extent to which sympathetic nerve activity affects resting arterial pressure. We will focus primarily on data from human studies, with information from other models included where appropriate to complement the human work.
How is sympathetic nerve activity measured?
Various techniques are available to measure sympathetic nerve activity in humans (for review see Grassi & Esler, 1999; Esler et al. 2003; Wallin & Charkoudian, 2007), which include direct postganglionic recordings of efferent MSNA, measurement of whole body and regional noradrenaline spillover and measurements of plasma noradrenaline. Direct recordings of MSNA are conducted via microneurography, most commonly from the peroneal (fibular) nerve. In this case, the recorded neurogram contains nerve signals from vasoconstrictor nerve fibres, such that an increase in activity is associated with increased noradrenaline release (along with co-transmitters), vasoconstriction and increased vascular resistance. Noradrenaline spillover techniques are somewhat more invasive and use radiolabelled noradrenaline infusions to obtain information about regions not accessible by microneurography (Esler et al. 2003). Both techniques require a high level of technical skill and provide information that is mechanistically superior to simpler measurements of plasma noradrenaline. In general the three techniques correlate well with each other under resting conditions in male subjects (Wallin et al. 1992, 1996). However, it is unclear whether these data can be extrapolated to women or to conditions/interventions that cause sympatho-excitation (e.g. mental stress) or sympatho-inhibition (e.g. hyperoxia). To address the goals of our present review, we will focus primarily on data from studies using microneurographic recordings of MSNA.
What do we know about MSNA and blood pressure?
In humans, MSNA has several distinct characteristics. First, at rest MSNA varies around 7- to 10–fold among healthy normotensive men and women (Sundlöf & Wallin, 1977, 1978; Charkoudian et al. 2005; Narkiewicz et al. 2005). Second, acute changes in MSNA cause direct alterations in vasoconstrictor tone (Seals, 1989). Third, MSNA is tightly coupled to blood pressure via the arterial baroreflex. However, over this wide range of variability, MSNA is not linked to resting blood pressure values in young men and women (Fig. 1): young people with higher MSNA might have similar or lower brachial blood pressure compared to young people with lower MSNA (Sundlöf & Wallin, 1978; Charkoudian et al. 2005; Narkiewicz et al. 2005; Hart et al. 2011). This latter finding seems paradoxical: we know that SNA is linked to vasoconstriction and blood pressure. Therefore, why would young people who chronically have higher SNA not have higher blood pressure?
Figure 1. The relationship between muscle sympathetic nerve activity (MSNA) and mean arterial pressure (MAP) in young men and women (<40 years; top panels) and older men and women (>40 years; bottom panels).
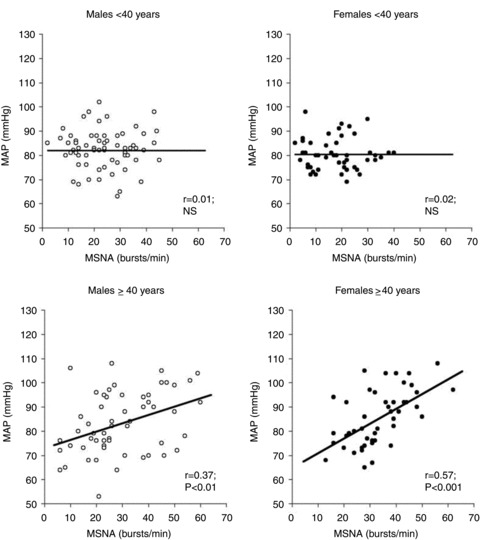
The relationship between MSNA and MAP becomes positive in older men and women. Taken from Narkiewicz et al. (2005).
The lack of relationship between MSNA and arterial pressure among young men and women has perplexed investigators for decades. Initially, this inter-individual variability in MSNA disappointed many investigators as it was thought to limit the use of microneurography as a clinical diagnostic tool (Vallbo et al. 2004). However, more recent work suggests that the variability of MSNA among men and women might provide important insights into cardiovascular and blood pressure regulation (Wallin, 2007).
Why is MSNA unrelated to blood pressure in young men?
Studies by Charkoudian et al. (2005, 2006b) indicated that MSNA was positively related to peripheral vasoconstrictor tone (total peripheral resistance; TPR) in young men. Therefore, young men with high MSNA had high levels of TPR. This has also been demonstrated by Hogarth et al. (2007) where calf vascular resistance was positively related to MSNA in young men. Why then is MSNA not related to the level of arterial pressure at rest in young men?Charkoudian and colleagues (2005) explained this by demonstrating that there was, in fact, an inverse relationship between MSNA and cardiac output in the group of young men. Consequently, young men with high MSNA and TPR had lower levels of cardiac output. Thus, cardiac output appears to balance high levels of MSNA (or vice versa) in young healthy men; that is, in young men the net effect of MSNA on the vasculature at rest is negated by a balance between cardiac output and TPR. Figure 2 demonstrates that these findings persist when a larger sample size is considered. Other observations also indicate that there is a balance between individual differences in vasoconstrictor responsiveness to noradrenaline and baseline MSNA (Charkoudian et al. 2006b). For example, young individuals with high MSNA had lower forearm vasoconstrictor responsiveness to α–adrenergic agonists, suggesting that the α–adrenergic receptors become relatively desensitised in individuals with high MSNA.
Figure 2. The relationship of MSNA to total peripheral resistance (TPR; top panels) and cardiac output (CO; bottom panels) in young men (left) and young women (right).
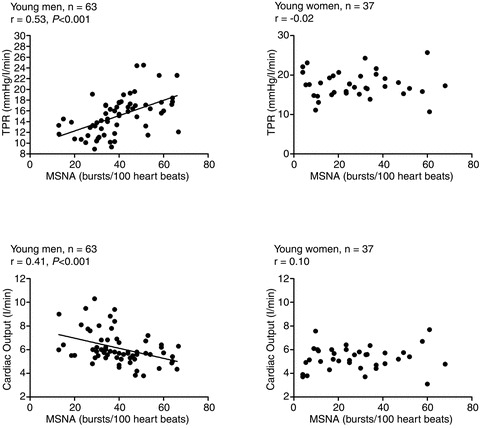
MSNA (bursts per 100 heart beats) is positively related to TPR but inversely related to cardiac output in young men. Conversely, there is no relationship among MSNA, TPR and cardiac output in young women. Data taken from Charkoudian et al. (2005, 2006a,b) and Hart et al. (2009a,b, 2011).
The findings from Charkoudian et al. indicate that cardiac output may to be important in buffering the effect of high MSNA and therefore high TPR on resting blood pressure in young men. Hart et al. (2009a) demonstrated that the inverse relationship between MSNA and cardiac output in young men was mediated by stroke volume rather than heart rate. Therefore, young men with high MSNA had a smaller stroke volume. However, whether the relationship between MSNA and cardiac output in young men is balanced or, in fact, driven by cardiac output is unclear. Moreover, it is possible that a loss of ability to balance high MSNA with lower cardiac output would lead to the development of high blood pressure and potentially hypertension in men.
Why is MSNA unrelated to blood pressure in young women?
There is a growing body of evidence suggesting that sex and/or sex hormones influence blood pressure regulation (Minson et al. 2000; Saleh et al. 2000; Shoemaker et al. 2001; Saleh & Connell, 2003; Christou et al. 2005; Fu et al. 2009; Hart et al. 2009a). Along these lines, women appear to have less autonomic (Christou et al. 2005) and α–adrenergic receptor (Schmitt et al. 2010) support of resting blood pressure, less ability to buffer increases in blood pressure via the baroreflex (Christou et al. 2005) and lower α–adrenergic receptor sensitivity to noradrenaline (Freedman et al. 1987; Kneale et al. 2000). Consequently, because the findings outlined above regarding balances among MSNA, cardiac output and TPR at rest were based on data recorded from young men, it was not clear whether such findings could be extended to women. Since there is no relationship between MSNA and arterial pressure in young women, it was expected that the relationships among MSNA, cardiac output and TPR would be similar to those observed in men (but perhaps blunted). Surprisingly, Hart et al. (2009a) reported that there was, in fact, no relationship of MSNA to TPR or cardiac output in young women. Thus, young women with high resting MSNA did not necessarily have high TPR or a low cardiac output. This suggests that MSNA is not a good indicator of vasoconstrictor tone in young women, and that some other factor(s) must balance out the pressor effects of high MSNA in this group. The disconnect between MSNA and vasoconstriction in young women is corroborated by Hogarth et al. (2007) who demonstrated that the relationship between MSNA and calf vascular resistance during sympathoexcitation (cold pressor test and ischaemic hand grip) was positive in young men, but did not exist in young women. These reports therefore suggest that the transduction of MSNA into vasomotor tone at rest (or during a sympathetic-excitatory stimulus) is blunted in young women.
The lack of relationship between MSNA and TPR in young women may point to a potential role for β–adrenergic receptors in explaining the differences in blood pressure regulation between the sexes. More specifically, a study by Kneale et al. (2000) suggested that localβ–adrenergic vasodilatation may offset α–adrenergic vasoconstriction in women to a greater extent than it does in men. Kneale et al. found that forearm vasoconstrictor responses to brachial artery noradrenaline infusions were greater in men compared to women but when propranolol (a non-selective β–adrenergic antagonist) was administered, forearm vasoconstriction was significantly greater in the women. Conversely, propranolol had no influence on forearm vasoconstriction to noradrenaline in the men. In addition to this, Kneale et al. demonstrated that the sensitivity of vascular β–adrenergic receptors to albuterol (specific β2–adrenergic agonist) in the human forearm was greater in young women compared to men.
These findings indicate that β–adrenergic receptor-mediated dilatation is enhanced in young women and thus might offset the vasoconstrictor effects of high MSNA. In this context, Hart et al. (2011) demonstrated that β–adrenergic vasodilatation does have an important systemic haemodynamic role in terms of overall sympathetic control of resting arterial blood pressure in women. As noted above, there is no relationship between MSNA and TPR at rest in young women. However, when the β–adrenergic receptors were blocked with high systemic doses of propranolol, the relationship between MSNA and TPR as well as the relationship between MSNA and MAP became significant and positive (Hart et al. 2011; Fig. 3). In other words, young women with high MSNA had a high blood pressure during β-blockade. Taken together these findings suggest that β-receptor-mediated vasodilatation is an important factor uncoupling MSNA from vasoconstriction in young women, and furthermore that it is an important component of the balance of factors maintaining normotension in young women. This mechanism may explain why young women are at less risk of developing hypertension (and other cardiovascular diseases) compared to young men.
Figure 3. The relationship between MSNA and TPR in young men, young women and postmenopausal (PM) women before (left) and during (right) systemic β-blockade with propranolol.
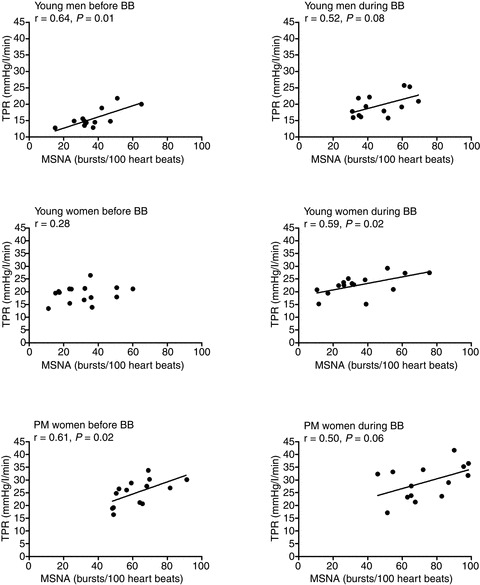
Systemic β-blockade caused the relationship between MSNA and TPR to become positive in young women, so it was similar to that observed in young men and postmenopausal women. BB, before β-blockade. Taken from Hart et al. (2011).
The mechanisms underlying increased vascular β–adrenergic receptor sensitivity in young women are unclear, but are most probably related to circulating female sex hormones. Along these lines oestrogen supplementation in ovariectomised rats increased β–adrenergic receptor sensitivity to isoproterenol in the mesenteric arteries (Ferrer et al. 1996). Moreover, the increased mesenteric artery vascular conductance in oestrogen-treated rats was abolished when propranolol was administered (Ferrer et al. 1996). Whether progesterone has a similar effect on the β–adrenergic receptors is unclear. Interestingly, progesterone supplementation selectively increases the density of the β2–adrenergic receptors in the rat myometrium (Vivat et al. 1992). However, it is unknown if progesterone up-regulates the vascular β2–adrenergic receptors in animals and humans. Exactly how the female sex hormones might increase the sensitivity of the β–adrenergic receptors to their respective agonists is ambiguous. Oestrogen is well known to increase vascular nitric oxide synthase activity and availability (Sudhir et al. 1996; Miller & Duckles, 2008). Furthermore, the β–adrenergic receptors cause vasodilatation partially through a nitric oxide mechanism (Cardillo et al. 1997; Ferro et al. 1999; Jordan et al. 2001; Eisenach et al. 2002). It is possible, therefore, that the female sex hormones enhance β–adrenergic-mediated dilatation by increasing nitric oxide availability.
What happens as we age?
As people age, resting blood pressure tends to increase, as does the risk of hypertension and related disorders (Kearney et al. 2005; Rothwell et al. 2005; Roger et al. 2011). In fact, more than 60% of men and women ≥65 years of age in England (Health Survey for England; Craig & Mindell, 2006) and over 65–70% of this population in the US have hypertension (National Centre for Health Statistics, USA, 2009). Furthermore, MSNA increases with age and is thought to contribute to the increased risk of developing hypertension in older individuals (Jones et al. 2001).
Starting at middle age in both men and women, the relationship between MSNA and arterial pressure becomes positive (Narkiewicz et al. 2005; Hart et al. 2009b, 2011), unlike the lack of relationship observed in younger people (Fig. 1). This suggests that MSNA becomes more important in determining resting arterial pressure in older men and women. The relationship between MSNA and blood pressure in older people appears to be due, in part, to the lack of balance between neural and haemodynamic factors. For example, the inverse relationship seen between MSNA and CO in younger men is absent in older men (Hart et al. 2009b). Interestingly, the positive relationship between MSNA and TPR that exists in young men also disappears in older men (Hart et al. 2009b), which suggests that factors other than MSNA, including endothelial function and/or circulating endothelin 1, become more important in maintaining peripheral vascular tone in older men. However, this area clearly needs further investigation. Additionally, older men do not demonstrate the inverse relationship between MSNA and vascular adrenergic responsiveness that is observed in younger groups (Hart et al. 2009b).
In older women, a change in the role of β–adrenergic receptors in maintaining vascular tone appears to be an important factor that influences resting blood pressure. Along these lines, the ability of the β–adrenergic receptors to offset noradrenaline-mediated vasoconstriction that is seen in younger women (Kneale et al. 2000; Hart et al. 2011), disappears in postmenopausal women without β-blockade (Hart et al. 2011). Postmenopausal women demonstrated a positive MSNA–TPR relationship in the absence of β-blockade (Fig. 3), such that older women with high MSNA had high levels of resting TPR and blood pressure. During β-blockade the relationship between MSNA and TPR remained positive in this group of women (Hart et al. 2011, Fig. 3) suggesting that the ability of the β–adrenergic receptors to buffer the transduction of MSNA into vasoconstrictor tone at rest is lost in postmenopausal women. Whether this change in the role of the β–adrenergic receptors is related to a decrease in circulating female sex hormones, or to ageing itself, is unclear. In postmenopausal women receiving transdermal oestrogen hormone replacement therapy there is also reduction in arterial pressure and MSNA (Vongpatanasin et al. 2001). Part of the beneficial effect of transdermal oestrogen on blood pressure may also be related to the potential effects of oestrogen on the vascular β-receptors. However, this remains to be addressed. Interestingly, some evidence indicates that vascular β–adrenergic receptor sensitivity is also attenuated in hypertensive individuals (Stein et al. 1995). Therefore, changes in the sensitivity of the β-receptors to noradrenaline may explain why the incidence of hypertension increases in postmenopausal women and becomes similar to (or sometimes greater than) that in men of the same age (Burt et al. 1995; Kearney et al. 2005).
Interestingly, in postmenopausal women at rest (with no β-blockers), the positive relationship between MSNA and TPR was not buffered by cardiac output as it is in young men. These data suggest that in postmenopausal women, cardiac output cannot balance the effect of high levels of MSNA on the vasculature. The mechanisms underlying this are unclear, but might be related to reductions in left ventricular compliance and thus stroke volume which occurs with age and sedentary lifestyle (Shibata et al. 2008; Fujimoto et al. 2010).
It should be noted that there are other physiological and behavioural changes, which occur as part of the normal ageing process in humans, and which may have an impact on the neural/haemodynamic interactions discussed here. These include a decline in physical activity, combined with increased fat mass (and diminished fat-free mass). Increased central adiposity can itself lead to an increase in sympathetic nerve activity (Jones et al. 1996, 1997a). Therefore the large increase in MSNA that occurs with age may be partially attributed to increased fat and total body mass (Jones et al. 1997a). Ageing is also associated with an increase in stiffness of the central elastic-type arteries (Tanaka et al. 1998, Monahan et al. 2001), endothelial dysfunction (Newcomer et al. 2005; Donato et al. 2009), desensitization of α–adrenergic receptors (Dinenno et al. 2002; Hart et al. 2011) (Smith et al. 2007), increased reactive oxygen species (Bailey et al. 2010) and decreased blood volume relative to fat-free mass (Davy & Seals, 1994; Jones et al. 1997b). Although these are outside the scope of the present discussion, any of these changes may contribute to, and/or interact with, SNA and its control of blood pressure as people age.
What about central aortic blood pressure?
The relationships between MSNA and blood pressure discussed in this manuscript are based on systemic arterial pressures measured in the brachial artery. It is well known that arterial pressure is different throughout the arterial tree, thus, systemic brachial arterial pressure does not necessarily reflect blood pressure in the aorta (or central arterial pressure). This is important to consider since the pressure in the ascending aorta partially determines the force that the left ventricle has to generate to pump blood into the arterial system (i.e. afterload). Consequently, high aortic systolic pressures are better predictors of cardiovascular risk and outcome than brachial arterial pressure (Vlachopoulos et al. 2010). Interestingly, the lack of relationship between MSNA and peripheral arterial pressure observed in young men and women persist when estimated aortic pressures are used (Casey et al. 2011). In addition, in postmenopausal women, the relationship between MSNA and aortic systolic pressure becomes positive, which follows the same pattern observed when brachial arterial pressures are considered (Hart et al. 2011; abstract only). Thus, older women with high MSNA and high peripheral arterial pressure, also have higher estimates of aortic systolic blood pressure.
Reflected blood pressure waves from peripheral sites that return to the aorta can affect aortic blood pressure depending on when they arrive (i.e. during systole or diastole). For example, a reflected pressure wave that returns during systole can increase aortic systolic arterial pressure. Aortic augmentation index is a measure of the percentage of systolic aortic pressure that is due to reflected blood pressure waves. Interestingly, in young men, MSNA is positively related to augmentation index, whereas in young women these variables are inversely related (Casey et al. 2011). Therefore, in young men with high MSNA, augmentation index is high and in young women, higher MSNA is associated with lower augmentation index. Importantly, higher augmentation index is linked to increased cardiovascular risk (Manisty et al. 2010). This might further explain why high MSNA is linked to increased cardiovascular risk profiles and moreover, why young women are protected against the development of cardiovascular disease.
Future directions
Throughout this review we have emphasized the recently identified importance of the vascular β–adrenergic receptors in modulating resting blood pressure and how these receptors might be either up-regulated or become more sensitive to noradrenaline in the presence of female sex hormones. Important future directions for this work include further understanding of the mechanisms involved in the interactions among sex steroids, noradrenaline and adrenergic receptors, as well as understanding of differences among vascular beds and how these differences are affected by age and sex. From a clinical perspective, it will ultimately be important to translate this understanding into treatments that are more specific to the sex and age of the patient.
Summary and perspectives
Studies focusing on inter-individual differences in blood pressure regulation have been fundamental in developing what we now know about resting blood pressure control. These studies indicate that blood pressure regulation varies among individuals and perhaps, more importantly, resting blood pressure control is dependent on sex and age. In this context, it has been demonstrated that the β–adrenergic receptors are essential in the control of resting blood pressure in young women (see Fig. 4 for summary schematic). That is, the β–adrenergic receptors offset the transduction of MSNA into vasoconstrictor tone and may prevent increases in TPR under conditions when resting MSNA is augmented in young women. However, this phenomenon does not occur in young men, where a balance among MSNA, TPR and cardiac output appears to be more important in controlling resting blood pressure. Interestingly, in postmenopausal women, the ability of the β-receptors to offset the transmission of MSNA into peripheral vasoconstrictor tone is lost. This, combined with elevations in sympathetic nerve activity, may explain why blood pressure and the incidence of hypertension increases around the age of menopause (Burt et al. 1995; Wiinberg et al. 1995; Kearney et al. 2005). Furthermore, it is possible that because the β-receptors cannot balance the pressor effects of acute elevations in resting MSNA (e.g. due to mental stress), postmenopausal women are more at risk of developing cerebrovascular or other cardiovascular-related events (Rothwell et al. 2005; Roger et al. 2011). In contrast to older women, the positive relationship between MSNA and MAP in ageing men appears to be due to a lack of balance among neural and haemodynamic factors. Along these lines, neither cardiac output nor vasoconstrictor responsiveness relate to MSNA in a way that helps to minimise its effect on blood pressure in older men.
Figure 4. Summary schematic representing our overall hypothesis regarding the role of sympathetic nerve activity in maintaining overall total peripheral vascular resistance (TPR) and blood pressure at rest.
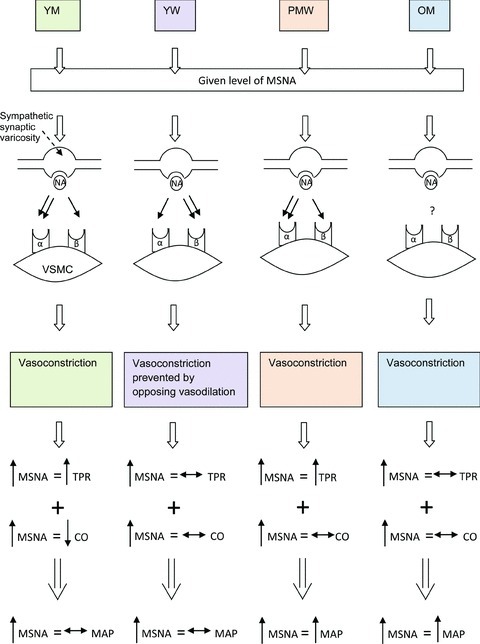
For a given level of resting MSNA, noradrenaline (NA) release from the sympathetic synapse can bind to α- and/or β–adrenergic receptors. The receptor that noradrenaline binds to subsequently influences how resting MSNA is transmitted into vasoconstrictor tone. The double arrow at the level of the synapse represents which receptor is primarily activated by noradrenaline in each specific group (NB: we do not know whether noradrenaline preferentially binds to the β–adrenergic receptors in young women (YW) or whether there is an increased density of those receptors). The effect of the given level of MSNA on total peripheral resistance combined with how cardiac output (CO) relates to MSNA, defines what effect resting MSNA has on resting arterial pressure. For example, young men (YM) with high MSNA (up arrow) have a high TPR and a low cardiac output (down arrow). The end result is that at rest MSNA has a minimal effect on resting blood pressure (horizontal double ended arrow) in young men. PMW, postmenopausal women; OM, older men; VSMC, vascular smooth muscle cell.
Clearly both sex and age have important influences on the integrative balance of neural and haemodynamic factors that determine the level of blood pressure in a given person. The investigation of inter-individual differences in these variables has provided important insight into the regulation of blood pressure in young and older men and women, and may contribute to the specificity of future therapeutic interventions in cardiovascular care.
Glossary
Abbreviations
- MSNA
muscle sympathetic nerve activity
- SNA
sympathetic nerve activity
- TPR
total peripheral vascular resistance
References
- Bailey DM, McEneny J, Mathieu-Costello O, Henry RR, James PE, McCord JM, Pietri S, Young IS, Richardson RS. Sedentary aging increases resting and exercise-induced intramuscular free radical formation. J Appl Physiol. 2010;109:449–456. doi: 10.1152/japplphysiol.00354.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- Cardillo C, Kilcoyne CM, Quyyumi AA, Cannon RO, 3rd, Panza JA. Decreased vasodilator response to isoproterenol during nitric oxide inhibition in humans. Hypertension. 1997;30:918–921. doi: 10.1161/01.hyp.30.4.918. [DOI] [PubMed] [Google Scholar]
- Casey DP, Curry TB, Joyner MJ, Charkoudian N, Hart EC. Relationship between muscle sympathetic nerve activity and aortic wave reflection characteristics in young men and women. Hypertension. 2011;57:421–427. doi: 10.1161/HYPERTENSIONAHA.110.164517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Barnes SA, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Relationship between muscle sympathetic nerve activity and systemic hemodynamics during nitric oxide synthase inhibition in humans. Am J Physiol Heart Circ Physiol. 2006a;291:H1378–H1383. doi: 10.1152/ajpheart.00234.2006. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol. 2006b;572:821–827. doi: 10.1113/jphysiol.2005.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111:494–498. doi: 10.1161/01.CIR.0000153864.24034.A6. [DOI] [PubMed] [Google Scholar]
- Craig R, Mindell J. The Information Centre, Leeds, 2008. 2006. Health Survey for England. Volume 1: Cardiovascular disease and risk factors in adults. [Google Scholar]
- Davy KP, Seals DR. Total blood volume in healthy young and older men. J Appl Physiol. 1994;76:2059–2062. doi: 10.1152/jappl.1994.76.5.2059. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional α–adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physio Heart Circ Physiol. 2009;297:H425–H432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach JH, Clark ES, Charkoudian N, Dinenno FA, Atkinson JLD, Fealey RD, Dietz NM, Joyner MJ. Effects of chronic sympathectomy on vascular function in the human forearm. J Appl Physiol. 2002;92:2019–2025. doi: 10.1152/japplphysiol.01025.2001. [DOI] [PubMed] [Google Scholar]
- Esler M, Lambert G, Brunner-La Rocca HP, Vaddadi G, Kaye D. Sympathetic nerve activity and neurotransmitter release in humans: translation from pathophysiology into clinical practice. Acta Physiol Scand. 2003;177:275–284. doi: 10.1046/j.1365-201X.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Meyer M, Osol G. Estrogen replacement increases β–adrenoceptor-mediated relaxation of rat mesenteric arteries. J Vasc Res. 1996;33:124–131. doi: 10.1159/000159140. [DOI] [PubMed] [Google Scholar]
- Ferro A, Queen LR, Priest RM, Xu B, Ritter JM, Poston L, Ward JP. Activation of nitric oxide synthase by β2–adrenoceptors in human umbilical vein endothelium in vitro. Br J Pharmacol. 1999;126:1872–1880. doi: 10.1038/sj.bjp.0702512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- Freedman R, Sabharwal S, Desai N. Sex differences in peripheral vascular adrenergic receptors. Circ Res. 1987;61:581–585. doi: 10.1161/01.res.61.4.581. [DOI] [PubMed] [Google Scholar]
- Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, Levine BD. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol. 2004;286:H449–H457. doi: 10.1152/ajpheart.00735.2002. [DOI] [PubMed] [Google Scholar]
- Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol. 2009;587:2019–2031. doi: 10.1113/jphysiol.2008.168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122:1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzeboom KS, Colman N, Reitsma JB, Shen WK, Wieling W. Prevalence and triggers of syncope in medical students. Am J Cardiol. 2003;91:1006–1008. doi: 10.1016/s0002-9149(03)00127-9. [DOI] [PubMed] [Google Scholar]
- Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17:719–734. doi: 10.1097/00004872-199917060-00001. [DOI] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009a;53:571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Johnson CP, Curry TB, Eisenach JH, Charkoudian N. Age-related differences in the sympathetic-hemodynamic balance in men. Hypertension. 2009b;54:127–133. doi: 10.1161/HYPERTENSIONAHA.109.131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart ECJ, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β–adrenergic receptors. J Physiol. 2011;589:5285–5297. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth AJ, Mackintosh AF, Mary DASG. Gender-related differences in the sympathetic vasoconstrictor drive of normal subjects. Clin Sci. 2007;112:353–361. doi: 10.1042/CS20060288. [DOI] [PubMed] [Google Scholar]
- Jones PP, Davy KP, Alexander S, Seals DR. Age-related increase in muscle sympathetic nerve activity is associated with abdominal adiposity. Am J Physiol Endocrinol Metab. 1997a;272:E976–E980. doi: 10.1152/ajpendo.1997.272.6.E976. [DOI] [PubMed] [Google Scholar]
- Jones PP, Davy KP, DeSouza CA, van Pelt RE, Seals DR. Absence of age-related decline in total blood volume in physically active females. Am J Physiol Heart Circ Physiol. 1997b;272:H2534–H2540. doi: 10.1152/ajpheart.1997.272.6.H2534. [DOI] [PubMed] [Google Scholar]
- Jones PP, Shapiro LF, Keisling GA, Jordan J, Shannon JR, Quaife RA, Seals DR. Altered autonomic support of arterial blood pressure with age in healthy men. Circulation. 2001;104:2424–2429. doi: 10.1161/hc4501.099308. [DOI] [PubMed] [Google Scholar]
- Jones PP, Snitker S, Skinner JS, Ravussin E. Gender differences in muscle sympathetic nerve activity: effect of body fat distribution. Am J Physiol Endocrinol Metab. 1996;270:E363–E366. doi: 10.1152/ajpendo.1996.270.2.E363. [DOI] [PubMed] [Google Scholar]
- Jordan J, Tank J, Stoffels M, Franke G, Christensen NJ, Luft FC, Boschmann M. Interaction between β–adrenergic receptor stimulation and nitric oxide release on tissue perfusion and metabolism. J Clin Endocrinol Metab. 2001;86:2803–2810. doi: 10.1210/jcem.86.6.7567. [DOI] [PubMed] [Google Scholar]
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- Manisty C, Mayet J, Tapp RJ, Parker KH, Sever P, Poulter NH, Thom SAM, Hughes AD ASCOT Investigators. Wave reflection predicts cardiovascular events in hypertensive individuals independent of blood pressure and other cardiovascular risk factors: an ASCOT (Anglo-Scandinavian Cardiac Outcome Trial) substudy. J Am Coll Cardiol. 2010;56:24–30. doi: 10.1016/j.jacc.2010.03.030. [DOI] [PubMed] [Google Scholar]
- Martins D, Nelson K, Pan D, Tareen N, Norris K. The effect of gender on age-related blood pressure changes and the prevalence of isolated systolic hypertension among older adults: data from NHANES III. J Gend Specif Med. 2001;4:10–13. [PubMed] [Google Scholar]
- Meyer MR, Haas E, Barton M. Need for research on estrogen receptor function: importance for postmenopausal hormone therapy and atherosclerosis. Gend Med. 2008;5:S19–S33. doi: 10.1016/j.genm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Tanaka H, Dinenno FA, Seals DR. Central arterial compliance is associated with age- and habitual exercise-related differences in cardiovagal baroreflex sensitivity. Circulation. 2001;104:1627–1632. doi: 10.1161/hc3901.096670. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- National Centre for Health Statistics, USA. 2009.
- Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am J Physiol Heart Circ Physiol. 2005;289:H308–H315. doi: 10.1152/ajpheart.01151.2004. [DOI] [PubMed] [Google Scholar]
- Olde Nordkamp LRA, van Dijk N, Ganzeboom KS, Reitsma JB, Luitse JSK, Dekker LRC, Shen W-K, Wieling W. Syncope prevalence in the ED compared to general practice and population: a strong selection process. Am J Emerg Med. 2009;27:271–279. doi: 10.1016/j.ajem.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317:75–77. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- Saleh TM, Connell BJ. Central nuclei mediating estrogen-induced changes in autonomic tone and baroreceptor reflex in male rats. Brain Res. 2003;961:190–200. doi: 10.1016/s0006-8993(02)03928-8. [DOI] [PubMed] [Google Scholar]
- Saleh TM, Connell BJ, Saleh MC. Acute injection of 17β-estradiol enhances cardiovascular reflexes and autonomic tone in ovariectomized female rats. Auton Neurosci. 2000;84:78–88. doi: 10.1016/s1566-0702(00)00196-x. [DOI] [PubMed] [Google Scholar]
- Schmitt JAM, Joyner MJ, Charkoudian N, Wallin BG, Hart EC. Sex differences in α–adrenergic support of blood pressure. Clin Auton Res. 2010;20:271–275. doi: 10.1007/s10286-010-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR. Sympathetic neural discharge and vascular resistance during exercise in humans. J Appl Physiol. 1989;66:2472–2478. doi: 10.1152/jappl.1989.66.5.2472. [DOI] [PubMed] [Google Scholar]
- Shibata S, Hastings JL, Prasad A, Fu Q, Okazaki K, Palmer MD, Zhang R, Levine BD. ‘Dynamic’ Starling mechanism: effects of ageing and physical fitness on ventricular–arterial coupling. J Physiol. 2008;586:1951–1962. doi: 10.1113/jphysiol.2007.143651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol. 2001;281:H2028–H2035. doi: 10.1152/ajpheart.2001.281.5.H2028. [DOI] [PubMed] [Google Scholar]
- Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional α–adrenergic vasoconstrictor responsiveness in healthy men. J Physiol. 2007;582:63–71. doi: 10.1113/jphysiol.2007.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CM, Nelson R, Deegan R, He H, Wood M, Wood AJ. Forearm β adrenergic receptor-mediated vasodilation is impaired, without alteration of forearm norepinephrine spillover, in borderline hypertension. J Clin Invest. 1995;96:579–585. doi: 10.1172/JCI118070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhir K, Jennings GL, Funder JW, Komesaroff PA. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension. 1996;28:330–334. doi: 10.1161/01.hyp.28.3.330. [DOI] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Hunt BE, Jones PP, DeSouza CA, Seals DR. Hemodynamic sequelae of age-related increases in arterial stiffness in healthy women. Am J Cardiol. 1998;82:1152–1155. A10. doi: 10.1016/s0002-9149(98)00578-5. [DOI] [PubMed] [Google Scholar]
- Vallbo ÅB, Hagbarth K-E, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol. 2004;96:1262–1269. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]
- Vivat V, Cohen-Tannoudji J, Revelli JP, Muzzin P, Giacobino JP, Maltier JP, Legrand C. Progesterone transcriptionally regulates the β2–adrenergic receptor gene in pregnant rat myometrium. J Biol Chem. 1992;267:7975–7978. [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–1871. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- Vongpatanasin W, Tuncel M, Mansour Y, Arbique D, Victor RG. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation. 2001;103:2903–2908. doi: 10.1161/01.cir.103.24.2903. [DOI] [PubMed] [Google Scholar]
- Wallin BG. Interindividual differences in muscle sympathetic nerve activity: a key to new insight into cardiovascular regulation? Acta Physiol (Oxf) 2007;190:265–275. doi: 10.1111/j.1748-1716.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Charkoudian N. Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle Nerve. 2007;36:595–614. doi: 10.1002/mus.20831. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol. 1996;491:881–887. doi: 10.1113/jphysiol.1996.sp021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiinberg N, Høegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens. 1995;8:978–986. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]


