Abstract
Individuals with high aerobic fitness have lower systolic left ventricular strain, rotation and twist (‘left ventricular (LV) mechanics’) at rest, suggesting a beneficial reduction in LV myofibre stress and more efficient systolic function. However, the mechanisms responsible for this functional adaptation are not known and the influence of aerobic fitness on LV mechanics during dynamic exercise has never been studied. We assessed LV mechanics, LV wall thickness and dimensions, central augmentation index (AIx), aortic pulse wave velocity (aPWV), blood pressure and heart rate in 28 males (age: 21 ± 2 years SD) with a consistent physical activity level (no change >6 months). Individuals were examined at rest and during exercise (40% peak exercise capacity) and separated post hoc into a moderate and high aerobic fitness group (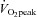 : 49 ± 5 and 63 ± 7 ml kg−1 min−1, respectively, P < 0.0001). At rest and during exercise, there were no significant differences in gross LV structure, AIx, blood pressure or heart rate (P > 0.05). However, for the same AIx, the high
: 49 ± 5 and 63 ± 7 ml kg−1 min−1, respectively, P < 0.0001). At rest and during exercise, there were no significant differences in gross LV structure, AIx, blood pressure or heart rate (P > 0.05). However, for the same AIx, the high 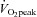 group had significantly lower LV apical rotation (P = 0.002) and LV twist (P = 0.003) while basal rotation and strain indices did not differ between groups (P > 0.05). We conclude that young males with high aerobic fitness have lower LV apical rotation at rest and during submaximal exercise that can occur without changes in gross LV structure, arterial haemodynamics or heart rate. The findings suggest a previously unknown type of physiological adaptation of the left ventricle that may have important implications for exercise training in older individuals and patient populations in which exercise training has previously failed to show clear benefits for LV function.
group had significantly lower LV apical rotation (P = 0.002) and LV twist (P = 0.003) while basal rotation and strain indices did not differ between groups (P > 0.05). We conclude that young males with high aerobic fitness have lower LV apical rotation at rest and during submaximal exercise that can occur without changes in gross LV structure, arterial haemodynamics or heart rate. The findings suggest a previously unknown type of physiological adaptation of the left ventricle that may have important implications for exercise training in older individuals and patient populations in which exercise training has previously failed to show clear benefits for LV function.
Key points
During cardiac contraction, left ventricular (LV) mechanics play an important role in equalising transmural fibre stress and ensuring efficient ejection of blood.
The factors responsible for altered LV mechanics in humans with high aerobic exercise capacity are unknown but are believed to be related to changes in LV structure or heart rate.
We performed a comprehensive assessment of LV mechanics and cardiovascular function at rest and during dynamic exercise in individuals with moderate and high aerobic exercise capacity.
Our novel data indicate that there is no direct association between altered LV mechanics in humans with high aerobic fitness and classic indicators of cardiovascular adaptation.
The findings provide evidence of a previously unknown type of physiological LV adaptation that may have important implications for exercise training in various healthy and diseased populations.
Introduction
Regular exercise training results in physiological adaptation of the left ventricle and a related improvement in maximal aerobic fitness (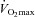 ), the latter being strongly associated with a reduced risk of cardiovascular morbidity and mortality (1986; Lee et al. 2000; Kodama et al. 2009). Typically, training-induced left ventricular (LV) adaptation is characterized by structural remodelling and concomitant improvements in systolic and diastolic function (Pluim et al. 2000; Scharhag et al. 2002; Hill & Olson, 2008; Baggish & Wood, 2011; Spence et al. 2011); however, the interdependence of structural and functional adaptation is not clear. For example, recent studies have shown that novel indices of LV function such as strain, rotation and twist (‘LV mechanics’) are significantly reduced in individuals with high aerobic fitness compared with sedentary controls despite a similar relative wall thickness (Nottin et al. 2008). The purpose of LV mechanics is to (1) minimise myofibre stress during ventricular contraction and (2) maximize the efficiency of LV ejection (Streeter et al. 1969; Beyar & Sideman, 1984, 1986; Vendelin et al. 2002). Accordingly, the previously observed reduction in resting LV strain and twist resulting from endurance exercise training (Zocalo et al. 2007; Nottin et al. 2008) suggests that LV myofibres of highly trained individuals experience less stress and produce a more efficient ejection. While the existing data clearly highlight the importance of assessing LV strain and twist as markers of functional myocardial adaptation, the influence of aerobic fitness on LV mechanics during exercise and the factors responsible for the different LV mechanics in highly fit individuals have not been studied.
), the latter being strongly associated with a reduced risk of cardiovascular morbidity and mortality (1986; Lee et al. 2000; Kodama et al. 2009). Typically, training-induced left ventricular (LV) adaptation is characterized by structural remodelling and concomitant improvements in systolic and diastolic function (Pluim et al. 2000; Scharhag et al. 2002; Hill & Olson, 2008; Baggish & Wood, 2011; Spence et al. 2011); however, the interdependence of structural and functional adaptation is not clear. For example, recent studies have shown that novel indices of LV function such as strain, rotation and twist (‘LV mechanics’) are significantly reduced in individuals with high aerobic fitness compared with sedentary controls despite a similar relative wall thickness (Nottin et al. 2008). The purpose of LV mechanics is to (1) minimise myofibre stress during ventricular contraction and (2) maximize the efficiency of LV ejection (Streeter et al. 1969; Beyar & Sideman, 1984, 1986; Vendelin et al. 2002). Accordingly, the previously observed reduction in resting LV strain and twist resulting from endurance exercise training (Zocalo et al. 2007; Nottin et al. 2008) suggests that LV myofibres of highly trained individuals experience less stress and produce a more efficient ejection. While the existing data clearly highlight the importance of assessing LV strain and twist as markers of functional myocardial adaptation, the influence of aerobic fitness on LV mechanics during exercise and the factors responsible for the different LV mechanics in highly fit individuals have not been studied.
Several factors have been postulated as being responsible for the lower resting LV strain and twist in athletes including structural LV remodelling, blood pressure and heart rate (Zocalo et al. 2007; Nottin et al. 2008; Weiner et al. 2010a). Equally, central arterial wave reflection and stiffness as determined by augmentation index (AIx) and aortic pulse wave velocity (aPWV, Sharman et al. 2005), respectively, are likely to interact with LV mechanics, yet this has never been examined. The simultaneous assessment of LV mechanics and AIx in individuals with different aerobic fitness would provide novel insight into the role of coupling between the LV and arterial function in the process of physiological LV adaptation. This may be of particular importance for older individuals and patient populations in which exercise training has previously failed to show clear benefits for cardiac function (Gates et al. 2003; Fujimoto et al. 2010).
To determine the mechanisms responsible for altered LV mechanics in individuals with high aerobic fitness, we performed a comprehensive assessment of cardiovascular function including LV strain, rotation and twist, AIx, aPWV, LV wall thickness, LV dimensions, blood pressure and heart rate at rest and during exercise in healthy individuals across a wide range of fitness levels. We hypothesised that in a young healthy population, individuals with high aerobic fitness would have significantly lower LV strain and twist at rest and during submaximal exercise and that this would be associated with lower AIx and aPWV.
Methods
Ethical approval and study population
Following ethical approval from the Cardiff Metropolitan University research ethics committee, 32 healthy, non-smoking males provided verbal and written informed consent to take part in the study. This study conforms to the standards set by the latest revision of the Declaration of Helsinki. Volunteers were not enrolled if they were professional athletes, if they were smoking or if they were currently taking any medication. Study participants with a consistent level of physical activity (no change >6 months) were recruited from a student population. We aimed to examine healthy young individuals across a wide range of aerobic fitness, for two reasons, firstly to ensure a wide spread of data to examine the relationships between LV mechanics and cardiovascular variables, and secondly to determine whether high aerobic capacity is related to LV mechanics in a general young population. Due to insufficient quality of echocardiographic images and AIx data in four volunteers, the final study group consisted of 28 participants. These 28 participants were split post hoc by the median into a moderate and a high aerobic fitness group (Fig. 1).
Figure 1. Gaussian distribution of 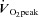 .
.
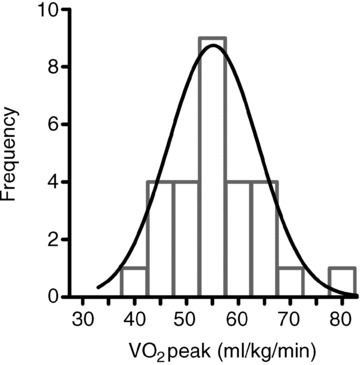
In the study group (n = 28), 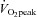 ranged from 38.7 to 79.7 ml kg−1 min−1 and was normally distributed (Shapiro–Wilk test: P = 0.81). The frequency of distribution is shown in groups of 5 ml kg−1 min−1.
ranged from 38.7 to 79.7 ml kg−1 min−1 and was normally distributed (Shapiro–Wilk test: P = 0.81). The frequency of distribution is shown in groups of 5 ml kg−1 min−1.
Experimental procedures
Participants attended the laboratory twice; for initial testing of their peak oxygen consumption (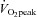 ) and on the experimental day for the assessment of cardiac and arterial function.
) and on the experimental day for the assessment of cardiac and arterial function. 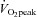 was determined using a standardised incremental exercise protocol performed on an upright bicycle ergometer (Lode Excalibur, Lode, Groningen, the Netherlands) until task failure. Breath-by-breath
was determined using a standardised incremental exercise protocol performed on an upright bicycle ergometer (Lode Excalibur, Lode, Groningen, the Netherlands) until task failure. Breath-by-breath 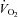 was acquired throughout the incremental exercise test (Oxycon Pro, Jaeger at Viasys Healthcare, Warwick, UK).
was acquired throughout the incremental exercise test (Oxycon Pro, Jaeger at Viasys Healthcare, Warwick, UK). 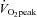 was determined as the highest 30 s average achieved during the test. On the experimental day, participants were placed in the left lateral position on a supine cycle ergometer tilted at 45 deg (Lode, Angio 2003). Following 10 min of rest in this position, LV mechanics, AIx, aPWV, LV dimensions, blood pressure and heart rate were recorded. Except for aPWV, data collection was then repeated during the last 3 min of a 5 min bout of supine cycling exercise (intensity: 40% peak power output achieved during the
was determined as the highest 30 s average achieved during the test. On the experimental day, participants were placed in the left lateral position on a supine cycle ergometer tilted at 45 deg (Lode, Angio 2003). Following 10 min of rest in this position, LV mechanics, AIx, aPWV, LV dimensions, blood pressure and heart rate were recorded. Except for aPWV, data collection was then repeated during the last 3 min of a 5 min bout of supine cycling exercise (intensity: 40% peak power output achieved during the 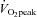 test; cadence: fixed at 60 rpm).
test; cadence: fixed at 60 rpm).
Data collection and analysis
Conventional echocardiography
A specialist cardiac sonographer who was blinded to participants’ acquired and analysed echocardiographic images for the assessment of LV structure and function according to current guidelines (Lang et al. 2006; Gorcsan & Tanaka, 2011). Three consecutive cardiac cycles were recorded on a commercially available ultrasound system (Vividq, GE Healthcare, Little Chalfont, UK) and saved for offline analysis (EchoPAC, GE healthcare, v. 110.1.1). LV parasternal long-axis views were analysed for end-diastolic wall thicknesses and dimensions. To account for the potential influence of body size on cardiac structure and function, absolute LV wall thicknesses and diameters as well as stroke volume were additionally allometrically scaled to body surface area0.46 (BSA0.46) according to Dewey et al. (2008). At rest and during exercise, stroke volume was calculated using the Teichholz method. To verify accuracy of these stroke volume results, in a sub-group of 16 individuals (n = 7 moderate
acquired and analysed echocardiographic images for the assessment of LV structure and function according to current guidelines (Lang et al. 2006; Gorcsan & Tanaka, 2011). Three consecutive cardiac cycles were recorded on a commercially available ultrasound system (Vividq, GE Healthcare, Little Chalfont, UK) and saved for offline analysis (EchoPAC, GE healthcare, v. 110.1.1). LV parasternal long-axis views were analysed for end-diastolic wall thicknesses and dimensions. To account for the potential influence of body size on cardiac structure and function, absolute LV wall thicknesses and diameters as well as stroke volume were additionally allometrically scaled to body surface area0.46 (BSA0.46) according to Dewey et al. (2008). At rest and during exercise, stroke volume was calculated using the Teichholz method. To verify accuracy of these stroke volume results, in a sub-group of 16 individuals (n = 7 moderate 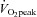 ; n = 9 high
; n = 9 high 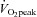 ), stroke volume was also assessed as the product of LV outflow tract area and the time–velocity integral obtained from a Doppler signal placed in the LV outflow tract on an apical five-chamber view. There were no differences in the results obtained by the two methods; the data obtained using the velocity time integral are reported in the tables and figures.
), stroke volume was also assessed as the product of LV outflow tract area and the time–velocity integral obtained from a Doppler signal placed in the LV outflow tract on an apical five-chamber view. There were no differences in the results obtained by the two methods; the data obtained using the velocity time integral are reported in the tables and figures.
Speckle tracking echocardiography
For the assessment of LV mechanics, parasternal short-axis images at the base and apex were acquired at 80 frames per second. Frame rate and imaging depth were kept constant during within-subject acquisition. Three consecutive cardiac cycles were recorded for off-line analysis (EchoPAC) of basal and apical rotation, rotation velocities, strain and strain rates. To adjust for inter- and intra-individual variability of heart rate, raw data were normalised to the percentage of systolic and diastolic duration using cubic spline interpolation of systolic (from the peak of the R-wave on the ECG to aortic valve closure) and diastolic (aortic valve closure to peak R-wave) data points (GraphPad Prism 5.00 for Windows; GraphPad Software Inc., La Jolla, CA, USA). Frame-by-frame twist and twist velocity values were obtained by subtracting the apical rotation/rotation velocity data from the basal rotation/rotation velocity data (Notomi et al. 2005; Sengupta et al. 2006). To ensure standardisation of apical data, apical short-axis images were checked for a consistent ratio of end-diastolic wall thickness to diameter (WT/D, see online Supplemental Material). The reliability of echocardiographic speckle tracking data (acquisition and analysis) for the present sonographer has been reported previously (Stöhr et al. 2011a).
Blood pressure and heart rate
Systolic and diastolic brachial blood pressure was obtained using standard auscultation. Heart rate was recorded continuously from a three-lead ECG inherent to the ultrasound (Vividq, GE Healthcare).
Pulse wave analysis, augmentation index (AIx) and aortic pulse wave velocity (aPWV)
At rest and during exercise, arterial pulse wave analysis was performed as previously described (Wilkinson et al. 1998a). Briefly, from the pressure wave form obtained at the radial artery (Colin CBM-7000, CMI, Komaki-City, Japan) a central (ascending aortic) pressure waveform was generated (SphygmoCor, Atcor Medical, Sydney, Australia) using a validated transfer function (Pauca et al. 2001). AIx was calculated as the difference between the second and first systolic peaks on the central aortic pressure waveform and expressed as a percentage of pulse pressure. The time to the reflected wave, Tr, was also obtained from the same pressure waveform. AIx data are reported as absolute values and normalised to a heart rate of 75 bpm. The ascending aortic waveform was also used to estimate central end systolic pressure (ESP) at the point of aortic valve closure (dicrotic notch). Current methods used to obtain central aortic AIx and ESP have previously been validated, both at rest and during exercise (Karamanoglu et al. 1993; Pauca et al. 2001; Sharman et al. 2006). Additionally, we assessed aortic pulse wave velocity (aPWV) at rest (SphygmoCor, Atcor Medical) using manual applanation tonometry by sequentially recording ECG-gated carotid and femoral artery waveforms as previously described in detail (Wilkinson et al. 1998b). The path length used for the determination of aPWV was measured as the surface distance between the supra-sternal notch and femoral pulse site minus the distance between the supra-sternal notch and carotid pulse site, using a tape measure. aPWV data were normalised to the mean arterial pressure of the study group. All data obtained for pulse wave analyses were calibrated to the manually obtained brachial systolic and diastolic blood pressures at rest and during exercise, respectively.
Statistical analysis
Differences in baseline characteristics between the moderate and the high aerobic fitness group were assessed using Student's t test for independent samples. Significant main effect of exercise or fitness level and their interaction were identified with two-way ANOVA. Relationships were determined using non-linear regression analysis based on the equation Y=Y0× exp(kX). α was set a priori to 0.05. All data are reported as means ± SD. Statistical analyses were performed with GraphPad Prism.
Results
Baseline characteristics
There were no significant differences in age, height, systolic and diastolic blood pressure, end-systolic pressure or heart rate between the moderate and high 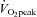 groups at rest (P > 0.05, Table 1). Body mass and body surface area (BSA) were significantly higher in the moderate aerobic fitness group (P < 0.05). In addition, there was no significant difference in stroke volume (Teichholz's method: 120 ± 26 and 118 ± 16 ml, moderate and high
groups at rest (P > 0.05, Table 1). Body mass and body surface area (BSA) were significantly higher in the moderate aerobic fitness group (P < 0.05). In addition, there was no significant difference in stroke volume (Teichholz's method: 120 ± 26 and 118 ± 16 ml, moderate and high 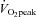 groups, respectively, P > 0.05), LV wall thicknesses, dimensions or AIxHR75 (all P > 0.05). Mean aPWV was significantly lower in highly fit individuals (P < 0.01).
groups, respectively, P > 0.05), LV wall thicknesses, dimensions or AIxHR75 (all P > 0.05). Mean aPWV was significantly lower in highly fit individuals (P < 0.01).
Table 1.
Baseline characteristics of the moderate and high aerobic fitness group
| Moderate aerobic fitness | High aerobic fitness | P value | |
|---|---|---|---|
| Age (years) | 21 ± 2 | 21 ± 3 | 0.38 |
| Height (cm) | 185 ± 6 | 181 ± 7 | 0.14 |
| Body mass (kg) | 84.3 ± 12.6 | 75.1 ± 7.8 | 0.03 |
| BSA (m2) | 2.08 ± 0.17 | 1.94 ± 0.15 | 0.03 |
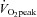 (ml kg-1 min−1) (ml kg-1 min−1) |
49 ± 5 | 63 ± 7 | <0.0001 |
| MAP (mmHg) | 96 ± 11 | 100 ± 6 | 0.28 |
| aPWV* (m s−1) | 5.7 ± 0.9 | 4.9 ± 0.5 | 0.01 |
| AIxHR75 (%) | 0 ± 10 | −7 ± 13 | 0.13 |
| IVSd (cm) | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.89 |
| IVSd index (cm/BSA0.46) | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.70 |
| LVPWd (cm) | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.57 |
| LVPWd index (cm/BSA0.46) | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.34 |
| LVIDd (cm) | 5.5 ± 0.4 | 5.4 ± 0.4 | 0.62 |
| LVIDd index (cm/BSA0.46) | 3.9 ± 0.2 | 4.0 ± 0.2 | 0.43 |
| LVIDs (cm) | 3.6 ± 0.4 | 3.5 ± 0.3 | 0.70 |
| LVIDs index (cm/BSA0.46) | 2.2 ± 0.3 | 2.3 ± 0.2 | 0.41 |
BP: blood pressure; BSA: body surface area; IVSd: inter-ventricular septum thickness at end-diastole; LVIDd/LVIDs: left ventricular internal diameter at end-diastole/end-systole; LVPWd: left ventricular posterior wall thickness at end-diastole. MAP: mean arterial pressure. aPWV*: aortic pulse wave velocity adjusted for mean arterial pressure. AIxHR75: augmentation index normalised to a heart rate of 75 bpm.
LV mechanics, AIx, blood pressure, heart rate and stroke volume
AIx, blood pressure, heart rate and stroke volume did not differ significantly between the groups at rest or during exercise (P > 0.05, Fig. 2). Similarly, strain and rotation at the LV base were not different between the moderate and high 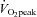 group (P > 0.05). In contrast, radial strain at the LV apex tended to be greater in the high aerobic fitness group (P = 0.09) and LV apical rotation was significantly lower at rest and during exercise in the high
group (P > 0.05). In contrast, radial strain at the LV apex tended to be greater in the high aerobic fitness group (P = 0.09) and LV apical rotation was significantly lower at rest and during exercise in the high 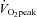 group (P < 0.04), which resulted in a trend towards a reduced LV twist (P = 0.09, Figs 3 and 4). No differences in systolic or diastolic strain rates or rotation velocities were observed between groups (P > 0.05, Table 2).
group (P < 0.04), which resulted in a trend towards a reduced LV twist (P = 0.09, Figs 3 and 4). No differences in systolic or diastolic strain rates or rotation velocities were observed between groups (P > 0.05, Table 2).
Figure 2. Cardiovascular responses at rest and during exercise in a moderate and high aerobic fitness group.
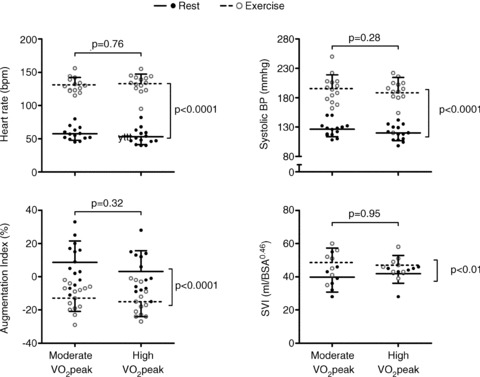
There were no significant differences in heart rate, systolic blood pressure, augmentation index (AIx) or stroke volume index (SVI) between the moderate and high aerobic fitness groups, indicating that cardiovascular demand and ventricular output were similar for both groups at rest and during exercise.
Figure 3. Peak left ventricular (LV) rotation and twist at rest and during cycling exercise.
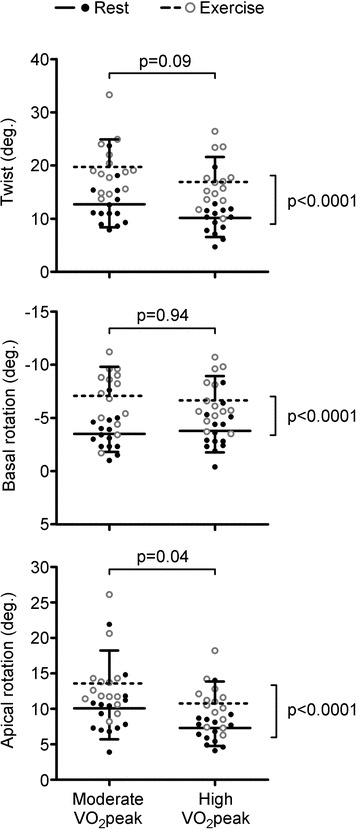
While exercise significantly increased LV mechanics to the same extent in both groups (P < 0.0001), LV apical rotation was significantly lower in the high aerobic fitness group at rest and during submaximal exercise (P = 0.04), resulting in a trend towards a decline in LV twist (P = 0.09). Note that the y-axis for LV basal rotation data has been inversed to reflect the increase in basal rotation during exercise.
Figure 4. Peak left ventricular (LV) strain at rest and during cycling exercise.
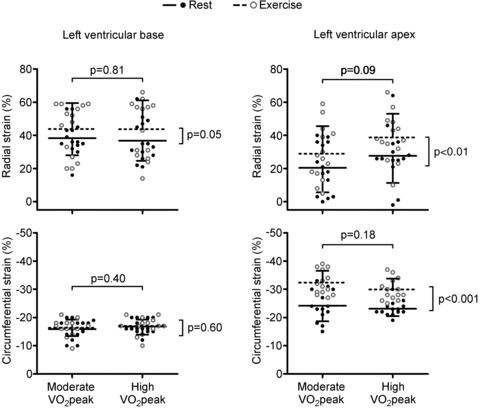
Similar to LV rotation, there were clear differences in the response between LV strain at the base and the apex.
Table 2.
Systemic cardiovascular responses and peak left ventricular mechanics at rest and during exercise in a moderate and high aerobic fitness group
| Moderate aerobic fitness | High aerobic fitness | ||||
|---|---|---|---|---|---|
| Rest | Exercise | Rest | Exercise | P value interaction | |
| Systemic cardiovascular responses | |||||
| Heart rate (bpm) | 58 ± 10 | 131 ± 11** | 53 ± 12 | 133 ± 14** | 0.19 |
| Systolic BP (mmHg) | 126 ± 13 | 196 ± 23** | 120 ± 13 | 189 ± 26** | 0.95 |
| Diastolic BP (mmHg) | 72 ± 8 | 77 ± 9 | 70 ± 11 | 79 ± 13 | 0.22 |
| SV (ml) | 82 ± 19 | 100 ± 22* | 81 ± 13 | 90 ± 10* | 0.33 |
| SV index (ml/BSA0.46) | 40 ± 9 | 49 ± 9* | 42 ± 6 | 47 ± 6* | 0.42 |
| ESP (mmHg) | 92 ± 9 | 123 ± 60* | 90 ± 13 | 135 ± 77* | 0.65 |
| AIx (%) | 9 ± 13 | −13 ± 8** | 3 ± 13 | −15 ± 9** | 0.37 |
| CAP (mmHg) | 2 ± 4 | −8 ± 6** | 1 ± 4 | −9 ± 6** | 0.94 |
| Tr (ms) | 169 ± 35 | 133 ± 5** | 166 ± 33 | 130 ± 8** | 0.95 |
| Peak systolic mechanics | |||||
| Twist velocity (deg s−1) | 82 ± 23 | 154 ± 44** | 75 ± 20 | 146 ± 40** | 0.94 |
| Basal rotation velocity (deg s−1) | −57 ± 22 | −95 ± 25** | −49 ± 14 | −98 ± 32** | 0.26 |
| Apical rotation velocity (deg s−1) | 84 ± 36 | 143 ± 47** | 54 ± 15 | 130 ± 34** | 0.21 |
| Basal circumferential SR (s−1) | −0.82 ± 0.19 | −1.10 ± 0.34** | −0.78 ± 0.12 | −1.20 ± 0.31** | 0.46 |
| Basal radial SR (s−1) | 1.27 ± 0.32 | 1.70 ± 0.41** | 1.28 ± 0.36 | 1.90 ± 0.85** | 0.51 |
| Apical circumferential SR (s−1) | −1.30 ± 0.43 | −2.81 ± 0.75** | −1.19 ± 0.19 | −2.69 ± 0.59** | 0.98 |
| Apical radial SR (s−1) | 0.89 ± 0.41 | 1.64 ± 0.92** | 0.97 ± 0.43 | 1.96 ± 0.78** | 0.44 |
| Peak diastolic mechanics | |||||
| Untwisting velocity (deg s−1) | −105 ± 21 | −251 ± 48** | −104 ± 25 | −218 ± 80** | 0.20 |
| Basal rotation velocity (deg s−1) | 46 ± 18 | 83 ± 24** | 55 ± 21 | 92 ± 25** | 0.91 |
| Apical rotation velocity (deg s−1) | −76 ± 20 | −203 ± 55** | −69 ± 20 | −167 ± 66** | 0.16 |
| Basal circumferential SR (s−1) | 1.36 ± 0.45 | 2.15 ± 0.51** | 1.47 ± 0.34 | 2.21 ± 0.63** | 0.80 |
| Basal radial SR (s−1) | −1.85 ± 0.60 | −2.62 ± 0.53** | −1.73 ± 0.56 | −2.71 ± 1.27** | 0.60 |
| Apical circumferential SR (s−1) | 2.25 ± 0.94 | 4.62 ± 1.05** | 2.25 ± 0.52 | 3.96 ± 0.94** | 0.11 |
| Apical radial SR (s−1) | −1.69 ± 0.92 | −2.86 ± 0.60** | −2.04 ± 0.98 | −2.97 ± 0.78** | 0.59 |
BP: blood pressure; SV: stroke volume; SVI: stroke volume index; ESP: end-systolic pressure; AIx: augmentation index; CAP: central augmented pressure; Tr: time to reflected wave; SR: strain rate. Note: there were no significant differences between groups at rest or during exercise.
P < 0.01 from rest within the same group;
P < 0.001 from rest within the same group.
Regression analysis revealed that there was no significant influence of aerobic fitness on the relationship between apical rotation and aPWV, systolic blood pressure, heart rate, body mass or BSA (Table 3). However, LV rotation and twist correlated with AIx and when the regression lines between AIx and LV mechanics were compared between both groups, highly fit individuals had a significantly lower apical rotation and twist for the same AIx (P = 0.002 and P = 0.003, respectively, Fig. 5). Interestingly, these differences were not present when LV strain was correlated with AIx (Fig. 6).
Table 3.
Comparison of the slope (k) and height (Y0) of regression lines between the moderate and high aerobic fitness group
| Moderate aerobic fitness Apical rotation | High aerobic fitness Apical rotation | k | Y0 | |
|---|---|---|---|---|
| aPWV (m s-1) | r2 < 0.001 | r2 < 0.01 | P = 0.91 | P = 0.95 |
| Systolic BP (mmHg) | r2= 0.14 | r2= 0.17 | P = 0.96 | P = 0.55 |
| Heart rate (bpm) | r2= 0.11 | r2= 0.25 | P = 0.75 | P = 0.24 |
| Body mass (kg) | r2= 0.02 | r2= 0.07 | P = 0.80 | P = 0.995 |
| BSA (m2) | r2= 0.14 | r2= 0.17 | P = 0.96 | P = 0.55 |
Note that data for body mass, body surface area (BSA) and aortic pulse wave velocity (aPWV) are rest only while data for systolic blood pressure (BP) and heart rate are rest and exercise combined.
Figure 5. Relationships between peak systolic left ventricular (LV) twist indices and central augmentation index (AIx).
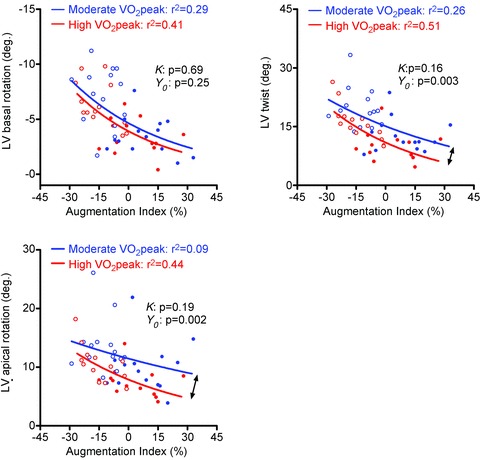
Central AIx and LV rotation and twist were inversely related in both the moderate and high  group. While the slope of the regression lines did not differ between groups (k: P > 0.05), LV twist was significantly lower at a given AIx in the high aerobic fitness group (Y0: P < 0.01). The lower LV twist was solely caused by a significantly reduced apical rotation (Y0: P < 0.01), suggesting that LV apical function can adapt independently of the LV base. Note that the Y-axis for basal rotation values was inversed to reflect the increase in rotation with reduced AIx. Filled and open circles represent rest and exercise data, respectively.
group. While the slope of the regression lines did not differ between groups (k: P > 0.05), LV twist was significantly lower at a given AIx in the high aerobic fitness group (Y0: P < 0.01). The lower LV twist was solely caused by a significantly reduced apical rotation (Y0: P < 0.01), suggesting that LV apical function can adapt independently of the LV base. Note that the Y-axis for basal rotation values was inversed to reflect the increase in rotation with reduced AIx. Filled and open circles represent rest and exercise data, respectively.
Figure 6. Relationships between peak systolic left ventricular (LV) radial and circumferential strain at the LV base and apex and augmentation index (AIx).
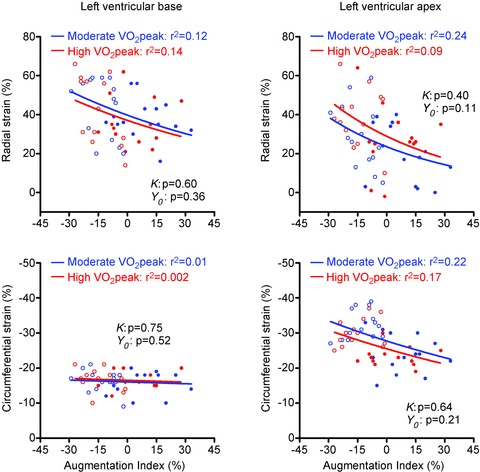
Radial and circumferential strain showed only weak or no relationships with AIx and there were no differences between groups (k and Y0: P > 0.05, respectively). Filled and open circles represent rest and exercise data, respectively.
Discussion
The primary aim of the present study was to determine the factors responsible for altered LV strain, rotation and twist (‘LV mechanics’) in individuals with high aerobic fitness. There were three novel findings. In a young, healthy population (1) LV apical rotation is significantly lower at rest and during submaximal exercise in individuals with high aerobic fitness, (2) for the same AIx, highly fit individuals have a significantly lower LV apical rotation and twist while the relationship between AIx and strain (radial and circumferential) is not influenced by aerobic fitness, and (3) the significant difference in LV apical rotation between moderately and highly fit individuals cannot be explained by AIx, aPWV, LV wall thickness, systolic blood pressure or heart rate in young healthy individuals. Thus, the present data show for the first time that individuals with high aerobic fitness have significant adaptations in LV mechanics that are region (base vs. apex) and function specific (strain vs. twist), despite maintenance of gross LV structure, arterial haemodynamics and heart rate. Together, these results suggest a benefit of increased aerobic fitness upon LV function that is not necessarily dependent on the marked changes in cardiovascular function typically associated with ‘athlete's heart’ (Baggish & Wood, 2011). This novel finding in a non-athletic population may have important implications for exercise training in older individuals and patient populations in which exercise training has previously failed to show clear benefits for LV function using classical indices of cardiac structure and function.
Baseline characteristics
When assessing the influence of exercise training on cardiovascular function, previous studies have often compared sedentary individuals with competitive athletes. These studies have provided important insight into the full range of cardiovascular adaptation, from the influence of bed rest at one end of the spectrum to cardiovascular adaptation in ultra-endurance athletes at the other (McGuire et al. 2001; George et al. 2011). However, in the present investigation, we purposefully chose to study a healthy young population with a normally distributed level of aerobic fitness for two reasons, firstly to ensure a wide spread of data to examine the relationships between LV mechanics and other cardiovascular variables, and secondly, because only a few people achieve elite sporting status, examining the adaptation of LV mechanics across a wide range of fitness levels will help to determine whether exercise training is of benefit in a general young population. Given our choice of population, it is not surprising that cardiac dimensions were not significantly different between the two groups. Although it is the common consensus that exercise training can result in cardiac remodelling (Baggish et al. 2008; Weiner et al. 2010a; Spence et al. 2011), some studies have shown that even when comparing extreme ends of a normal population, such as sedentary individuals and elite athletes, the magnitude of cardiac remodelling can be quite small, despite clear differences in 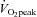 (Scharhag et al. 2002). Therefore, we chose to use
(Scharhag et al. 2002). Therefore, we chose to use 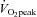 to classify individuals as moderately or highly fit. For the aforementioned reasons, it is also not surprising that heart rate, blood pressure and stroke volume did not significantly differ between the moderate and high
to classify individuals as moderately or highly fit. For the aforementioned reasons, it is also not surprising that heart rate, blood pressure and stroke volume did not significantly differ between the moderate and high 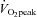 groups in the present study.
groups in the present study.
The significantly lower apical rotation at rest in the highly fit group in this study agrees with the previous findings of Nottin et al. (2008). In contrast, Weiner et al. (2010a) showed an increase in apical rotation following exercise training. Although the exact reasons for the disparity in findings remains to be elucidated, the previous studies differ in the time that LV mechanics would have had to adapt. In our opinion, the elegant longitudinal study by Weiner et al. (2010a) provides insight into the initial adaptation of LV mechanics to exercise training following a relatively short training period (90 days), which may not reflect the final stage of structural and functional remodelling. Support for this interpretation is provided by the trend towards a difference in relative wall thickness pre- and post-training reported by Weiner et al. (2010a) whilst relative wall thickness was the same between groups in the present study (see Supplemental Material).
With regard to the consistently elevated resting blood pressure in all of our participants, this was likely to have been caused by the position in which individuals were assessed. To standardise data collection at rest and during exercise, assessment of cardiovascular function was performed in the left lateral position on the exercise bed. This position probably caused an elevated postural muscle tension resulting in a mild increase in blood pressure from normal resting values. However, all participants were assessed in the same position and, hence, blood pressure was likely elevated to the same extent in all individuals. Consequently, this should not have affected the comparison of blood pressure between the moderate and high aerobic fitness group.
Despite the significant baseline differences in aPWV between moderately and highly fit individuals, there was no association between aPWV and LV apical rotation. Furthermore, the time to reflected arterial wave, Tr, was not significantly different between the groups at rest and during exercise, suggesting that aerobic fitness did not influence central arterial stiffness (Sharman et al. 2005). In accordance with this, McEniery et al. (2005) have previously shown that AIx may be a more sensitive indicator of arterial function/stiffness than aPWV in young individuals. In the present study, the similar AIx and AIxHR75 between the two groups further indicate that arterial stiffness was not significantly influenced by aerobic fitness. Since stroke volume and end-systolic pressure were also similar between the moderate and high 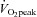 group, it seems reasonable to conclude that central arterial function was not responsible for the significant differences in LV apical mechanics.
group, it seems reasonable to conclude that central arterial function was not responsible for the significant differences in LV apical mechanics.
Dissociation of LV mechanics with other cardiovascular indices
Despite the assessment of several indicators of cardiac and vascular function that have previously been used to evidence cardiac adaptation to exercise training, none explained the chronic difference in LV apical mechanics with aerobic fitness. Specifically, there were no significant differences in AIx, Tr, LV wall thickness (including the apical WT/D ratio), blood pressure, stroke volume or heart rate; yet LV apical rotation was significantly lower in the high compared with the moderate aerobic fitness group. Furthermore, during exercise LV apical rotation remained significantly lower while all other parameters were the same between the moderate and high aerobic fitness group. The identical response in LV mechanics and cardiovascular indices at rest and during exercise significantly strengthens the present finding that LV apical rotation is indeed reduced in highly fit individuals without concomitant adaptation in gross LV structure, arterial haemodynamics or heart rate.
In accordance with our hypothesis, LV mechanics correlated well with AIx. However, upon closer inspection of the data it becomes apparent that these relationships cannot be reflective of a direct ‘cause-and-effect’ interaction. One observation was that the increase in LV mechanics during exercise was greater than the reduction in AIx as evidenced by the exponential shape of relationships. This implies that during exercise AIx plateaus prior to LV mechanics and, thus, strict interdependence seems unlikely. Furthermore, the relationship between apical rotation and AIx was very weak in the moderate compared with the high 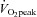 group. Accordingly, the cross-talk between LV mechanics and arterial wave reflection may be enhanced with high aerobic fitness. Using longitudinal study designs, future studies may want to explore the development of coupling between LV apical rotation and AIx over the course of an exercise training programme. Overall, the coupling of LV mechanics and arterial haemodynamics is an attractive concept that may still hold true in other populations, but the results from this study do not provide clear evidence for such a direct interaction in healthy young men.
group. Accordingly, the cross-talk between LV mechanics and arterial wave reflection may be enhanced with high aerobic fitness. Using longitudinal study designs, future studies may want to explore the development of coupling between LV apical rotation and AIx over the course of an exercise training programme. Overall, the coupling of LV mechanics and arterial haemodynamics is an attractive concept that may still hold true in other populations, but the results from this study do not provide clear evidence for such a direct interaction in healthy young men.
Similar to AIx, blood pressure, heart rate and relative wall thickness did not explain the chronically lowered apical rotation in highly fit individuals. Whether regular exercise training results in permanent changes in systolic blood pressure in young individuals is currently not clear. The present findings provide evidence that chronic changes in systolic blood pressure do not explain the lower LV apical rotation as BP was the same in the moderate and high 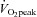 group at rest and also during exercise. Similarly, heart rate was the same in both groups at rest and during exercise and, therefore, is also unlikely to explain LV mechanical adaptation. Finally, the present findings also agree with Nottin et al. (2008) who showed that the different LV apical rotation in individuals with high aerobic fitness is not related to remodelling of the LV macrostructure as relative wall thickness was maintained.
group at rest and also during exercise. Similarly, heart rate was the same in both groups at rest and during exercise and, therefore, is also unlikely to explain LV mechanical adaptation. Finally, the present findings also agree with Nottin et al. (2008) who showed that the different LV apical rotation in individuals with high aerobic fitness is not related to remodelling of the LV macrostructure as relative wall thickness was maintained.
Potential mechanisms for differences in LV mechanics
Evidence exists that the LV apex is more responsive to exercise training than the LV base (Zocalo et al. 2007; Nottin et al. 2008; Weiner et al. 2010a). This observation related to chronic LV adaptation, parallels the more dynamic behaviour of the LV apex during acute physiological challenges (Doucende et al. 2010; Stöhr et al. 2011c). The present results advance the previous understanding by showing that LV apical rotation is not only lower at rest but also during exercise in individuals with high aerobic fitness. Still, the underlying mechanisms for adaptation in LV apical rotation remain unclear. In recent studies it was shown that acute changes in LV preload and afterload impact differently on LV basal and apical mechanics (Burns et al. 2010a,b; Weiner et al. 2010b; Stöhr et al. 2011b). However, the acute response to enhanced LV preload, which results in a significantly increased apical rotation (Weiner et al. 2010b), is the direct opposite of the chronic reduction observed in the present study. Furthermore, in the present study we show that the lower apical rotation in participants with high aerobic fitness is paralleled by a greater radial strain. At present, few data are available on the interplay between the different components of LV mechanics and it is not clear why radial strain may be greater in individuals with higher aerobic fitness. Uncoupling of LV mechanics has been shown previously in response to ageing and acute physiological stimuli (Lumens et al. 2006; Stöhr et al. 2011a) while Nottin et al. (2008) reported a concomitant decrease in both rotation and radial strain in elite cyclists. It is possible that the significant reduction in radial strain in cyclists is the result of remodelling caused by a marked and sustained volume expansion of the LV. In contrast, the higher radial strain in this study may represent a compensatory mechanism to maintain LV stroke volume despite a lower apical rotation, when LV volumes are similar between individuals with high and moderate aerobic fitness.
While the influence of repeated systemic stimuli such as an increase in preload during exercise on LV mechanical adaptation will need to be assessed in future studies, it is also possible that more intrinsic myocardial mechanisms could be involved in the observed LV adaptation. One factor that may be related to the lower LV apical rotation with high aerobic fitness is a change in the LV microstructure and a subsequent re-arrangement of oblique LV myofibres. Anatomical as well as mathematical modelling studies have demonstrated that the oblique fibre architecture is responsible for LV rotation and twist (Taber et al. 1996; Sengupta et al. 2006). As outlined above, changes in the absolute and relative wall thickness per se do not appear to be the cause for LV mechanical adaptation. Indeed, myocardial adaptation is possible without affecting relative LV wall thickness. For example, exercise training significantly influences matrix metalloproteinase 1, collagen volume fraction and microRNAs (Xu et al. 2008; Baggish et al. 2011). Such molecular alterations within the myocardium are likely to transform LV mechanics, perhaps through a change in fibre function or in the structural alignment between the subendocardial and subepicardial fibres. Combining the assessment of comprehensive LV mechanical function with indicators of molecular remodelling would further advance the current understanding of physiological adaptation of LV mechanics.
Analogous to skeletal muscle, which shows rapid adaptation in neural function to exercise training (Vila-Cha et al. 2010), adaptation in myocardial mechanics may also be influenced by alterations in neural control. This could involve structural adaptation of the components of the autonomic nervous system as well as changes in function for example by altered delivery or uptake of sympathetic and parasympathetic neurotransmitters. Interestingly, studies on the distribution and density of sympathetic nerve endings and β-adrenergic receptors have shown a heterogeneous pattern across the LV base and apex (Mori et al. 1993; Kawano et al. 2003), fitting the hypothesis of a neural origin to region-specific LV mechanical adaptation. Since in vivo data on the distribution and density of adrenoreceptors are extremely difficult to obtain, future studies may be able to explore differences in the electrical activation patterns between the LV base and apex that may correspond to the adaptation in LV mechanics observed in individuals with high aerobic fitness.
Significance of findings and future directions
Typically, physiological LV remodelling and functional adaptation to exercise training is identified by assessing LV dimensions, wall thicknesses, compliance and alterations in systolic and diastolic LV function such as transmitral filling or longitudinal tissue velocities. These markers of systolic and diastolic function reflect chronic alterations in LV structure and functional outcome but they do not provide any insight into the underpinning mechanics performed by the LV to achieve a certain systolic ejection or diastolic filling. The low number of studies examining the physiological adaptation of LV mechanics is surprising as LV mechanics undoubtedly play an essential role in overall LV performance in pathology (Sengupta et al. 2006; Russel et al. 2009; Geyer et al. 2010; Gorcsan & Tanaka, 2011). Knowledge of the adaptive potential of LV mechanics to physiological stimuli may help to understand changes observed in cardiovascular disease and offer possibilities for treatment. The present study contributes to a greater understanding of LV mechanics by showing that healthy individuals with high aerobic fitness have different LV mechanics despite similar arterial haemodynamics and LV output, suggesting an improved myocardial efficiency. This interpretation is in agreement with Vendelin et al. (2002) who demonstrated that LV mechanics are important for the optimisation of ventricular myofibre stress and efficiency.
The regional and functional heterogeneity of LV mechanical adaptation (LV base vs. apex and strain vs. twist, respectively) demonstrates that myocardial adaptation is complex and that some functional changes may not be captured by the measurement of conventional systolic and diastolic LV indices. Previous studies that have elegantly examined the impact of exercise training on cardiac function in ageing humans without assessing LV mechanics have failed to identify clear benefits of endurance training on cardiac function (Gates et al. 2003; Fujimoto et al. 2010). It is possible that LV apical rotation may be a more sensitive indicator of myocardial adaptation to exercise training. It must be noted, however, that variability of normal baseline data is high and that recognised ‘normal’ values in different patient populations are still required. This precludes, at present, the use of LV apical rotation per se as a diagnostic clinical marker of cardiac performance. Furthermore, we acknowledge that the present findings were obtained in healthy young males and that other populations may demonstrate a different interaction between aerobic fitness and LV mechanics. Nonetheless, the novel findings in this study give rise to the hypothesis that exercise training may enhance LV mechanics without concomitant adaptations in classic indices of cardiovascular structure and function.
Conclusions
In young males, high aerobic fitness is associated with a significantly lower apical rotation at rest and during sub-maximal exercise. Importantly, the differences in LV mechanics between moderately and highly fit individuals are present despite maintained markers of LV adaptation that are classically associated with ‘athlete's heart’. This novel finding in non-athletic individuals has important implications for exercise training in older individuals and patient populations in which exercise training has previously failed to show clear benefits for LV function.
Acknowledgments
The authors would like to thank James Cox, Michael Harrison, Joshua Howard and Geraint Phillips for their valuable contribution to the project.
Glossary
Abbreviations
- AIx
augmentation index
- AIxHR75
augmentation index normalized to a heart rate of 75 bpm
- aPWV
aortic pulse wave velocity
- BP
blood pressure
- BSA
body surface area
- CAP
central augmented pressure
- ESP
end-systolic pressure
- IVSd
inter-ventricular septum at end-diastole
- LV
left ventricular
- LVIDd
left ventricular internal diameter at end-diastole
- LVIDs
left ventricular internal diameter at end-systole
- LVPWd
left ventricular posterior wall thickness at end-diastole
- MAP
mean arterial pressure
- SR
strain rate
- SV
stroke volume
- Tr
time to reflected wave
Author contributions
E.J.S., B.M. and R.S. contributed to the conception and design of the experiment, data collection, analysis, interpretation of the data and the drafting of the manuscript. J.T., K.S., T.B., R.H. and J.C. contributed to data collection and analysis and the critical revision of the manuscript for its intellectual content. All authors have approved the final version of the manuscript. The authors have no conflict of interest to report.
References
- Baggish AL, Hale A, Weiner RB, Lewis GD, Systrom D, Wang F, Wang TJ, Chan SY. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol. 2011;589:3983–3994. doi: 10.1113/jphysiol.2011.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, Picard MH, Hutter AM, Jr, Wood MJ. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol. 2008;104:1121–1128. doi: 10.1152/japplphysiol.01170.2007. [DOI] [PubMed] [Google Scholar]
- Baggish AL, Wood MJ. Athlete's heart and cardiovascular care of the athlete: scientific and clinical update. Circulation. 2011;123:2723–2735. doi: 10.1161/CIRCULATIONAHA.110.981571. [DOI] [PubMed] [Google Scholar]
- Beyar R, Sideman S. A computer study of the left ventricular performance based on fiber structure, sarcomere dynamics, and transmural electrical propagation velocity. Circ Res. 1984;55:358–375. doi: 10.1161/01.res.55.3.358. [DOI] [PubMed] [Google Scholar]
- Beyar R, Sideman S. Left ventricular mechanics related to the local distribution of oxygen demand throughout the wall. Circ Res. 1986;58:664–677. doi: 10.1161/01.res.58.5.664. [DOI] [PubMed] [Google Scholar]
- Burns AT, La Gerche A, D’Hooge J, MacIsaac AI, Prior DL. Left ventricular strain and strain rate: characterization of the effect of load in human subjects. Eur J Echocardiogr. 2010a;11:283–289. doi: 10.1093/ejechocard/jep214. [DOI] [PubMed] [Google Scholar]
- Burns AT, La Gerche A, Prior DL, Macisaac AI. Left ventricular torsion parameters are affected by acute changes in load. Echocardiography. 2010b;27:407–414. doi: 10.1111/j.1540-8175.2009.01037.x. [DOI] [PubMed] [Google Scholar]
- Dewey FE, Rosenthal D, Murphy DJ, Jr, Froelicher VF, Ashley EA. Does size matter? Clinical applications of scaling cardiac size and function for body size. Circulation. 2008;117:2279–2287. doi: 10.1161/CIRCULATIONAHA.107.736785. [DOI] [PubMed] [Google Scholar]
- Doucende G, Schuster I, Rupp T, Startun A, Dauzat M, Obert P, Nottin S. Kinetics of left ventricular strains and torsion during incremental exercise in healthy subjects: the key role of torsional mechanics for systolic-diastolic coupling. Circ Cardiovasc Imaging. 2010;3:586–594. doi: 10.1161/CIRCIMAGING.110.943522. [DOI] [PubMed] [Google Scholar]
- Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122:1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates PE, Tanaka H, Graves J, Seals DR. Left ventricular structure and diastolic function with human ageing. Relation to habitual exercise and arterial stiffness. Eur Heart J. 2003;24:2213–2220. doi: 10.1016/j.ehj.2003.09.026. [DOI] [PubMed] [Google Scholar]
- George KP, Warburton DE, Oxborough D, Scott JM, Esch BT, Williams K, Charlesworth S, Foulds H, Oxborough A, Hoffman MD, Shave R. Upper limits of physiological cardiac adaptation in ultramarathon runners. J Am Coll Cardiol. 2011;57:754–755. doi: 10.1016/j.jacc.2010.05.070. [DOI] [PubMed] [Google Scholar]
- Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–369. doi: 10.1016/j.echo.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Gorcsan J, Tanaka H. Echocardiographic assessment of myocardial strain. J Am Coll Cardiol. 2011;58:1401–1413. doi: 10.1016/j.jacc.2011.06.038. [DOI] [PubMed] [Google Scholar]
- Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- Karamanoglu M, O’Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14:160–167. doi: 10.1093/eurheartj/14.2.160. [DOI] [PubMed] [Google Scholar]
- Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 2003;18:32–39. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Lee IM, Sesso HD, Paffenbarger RS., Jr Physical activity and coronary heart disease risk in men: does the duration of exercise episodes predict risk? Circulation. 2000;102:981–986. doi: 10.1161/01.cir.102.9.981. [DOI] [PubMed] [Google Scholar]
- Lumens J, Delhaas T, Arts T, Cowan BR, Young AA. Impaired subendocardial contractile myofiber function in asymptomatic aged humans, as detected using MRI. Am J Physiol Heart Circ Physiol. 2006;291:H1573–1579. doi: 10.1152/ajpheart.00074.2006. [DOI] [PubMed] [Google Scholar]
- McEniery CM, Yasmin –, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT) J Am Coll Cardiol. 2005;46:1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, Mitchell JH. A 30-year follow-up of the Dallas Bedrest and Training Study: II. Effect of age on cardiovascular adaptation to exercise training. Circulation. 2001;104:1358–1366. [PubMed] [Google Scholar]
- Mori H, Ishikawa S, Kojima S, Hayashi J, Watanabe Y, Hoffman JI, Okino H. Increased responsiveness of left ventricular apical myocardium to adrenergic stimuli. Cardiovasc Res. 1993;27:192–198. doi: 10.1093/cvr/27.2.192. [DOI] [PubMed] [Google Scholar]
- Notomi Y, Lysyansky P, Setser RM, Shiota T, Popovic ZB, Martin-Miklovic MG, Weaver JA, Oryszak SJ, Greenberg NL, White RD, Thomas JD. Measurement of ventricular torsion by two-dimensional ultrasound speckle tracking imaging. J Am Coll Cardiol. 2005;45:2034–2041. doi: 10.1016/j.jacc.2005.02.082. [DOI] [PubMed] [Google Scholar]
- Nottin S, Doucende G, Schuster-Beck I, Dauzat M, Obert P. Alteration in left ventricular normal and shear strains evaluated by 2D-strain echocardiography in the athlete's heart. J Physiol. 2008;586:4721–4733. doi: 10.1113/jphysiol.2008.156323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffenbarger RS, Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314:605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete's heart. A meta-analysis of cardiac structure and function. Circulation. 2000;101:336–344. doi: 10.1161/01.cir.101.3.336. [DOI] [PubMed] [Google Scholar]
- Russel IK, Gotte MJ, Bronzwaer JG, Knaapen P, Paulus WJ, van Rossum AC. Left ventricular torsion: an expanding role in the analysis of myocardial dysfunction. JACC Cardiovasc Imaging. 2009;2:648–655. doi: 10.1016/j.jcmg.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Scharhag J, Schneider G, Urhausen A, Rochette V, Kramann B, Kindermann W. Athlete's heart: right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J Am Coll Cardiol. 2002;40:1856–1863. doi: 10.1016/s0735-1097(02)02478-6. [DOI] [PubMed] [Google Scholar]
- Sengupta PP, Korinek J, Belohlavek M, Narula J, Vannan MA, Jahangir A, Khandheria BK. Left ventricular structure and function: basic science for cardiac imaging. J Am Coll Cardiol. 2006;48:1988–2001. doi: 10.1016/j.jacc.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Sharman JE, Lim R, Qasem AM, Coombes JS, Burgess MI, Franco J, Garrahy P, Wilkinson IB, Marwick TH. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension. 2006;47:1203–1208. doi: 10.1161/01.HYP.0000223013.60612.72. [DOI] [PubMed] [Google Scholar]
- Sharman JE, McEniery CM, Campbell RI, Coombes JS, Wilkinson IB, Cockcroft JR. The effect of exercise on large artery haemodynamics in healthy young men. Eur J Clin Invest. 2005;35:738–744. doi: 10.1111/j.1365-2362.2005.01578.x. [DOI] [PubMed] [Google Scholar]
- Spence AL, Naylor LH, Carter HH, Buck CL, Dembo L, Murray CP, Watson P, Oxborough D, George KP, Green DJ. A prospective randomised longitudinal MRI study of left ventricular adaptation to endurance and resistance exercise training in humans. J Physiol. 2011;589:5443–5452. doi: 10.1113/jphysiol.2011.217125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr EJ, González-Alonso J, Pearson J, Low DA, Ali L, Barker H, Shave R. Effects of graded heat stress on global left ventricular function and twist mechanics at rest and during exercise in healthy humans. Exp Physiol. 2011a;96:114–124. doi: 10.1113/expphysiol.2010.055137. [DOI] [PubMed] [Google Scholar]
- Stöhr EJ, González-Alonso J, Pearson J, Low DA, Ali L, Barker H, Shave RE. Dehydration reduces left ventricular filling at rest and during exercise independent of twist mechanics. J Appl Physiol. 2011b;111:897–897. doi: 10.1152/japplphysiol.00528.2011. [DOI] [PubMed] [Google Scholar]
- Stöhr EJ, González-Alonso J, Shave R. Left ventricular mechanical limitations to stroke volume in healthy humans during incremental exercise. Am J Physiol Heart Circ Physiol. 2011c;301:H478–487. doi: 10.1152/ajpheart.00314.2011. [DOI] [PubMed] [Google Scholar]
- Streeter DD, Jr, Spotnitz HM, Patel DP, Ross J, Jr, Sonnenblick EH. Fiber orientation in the canine left ventricle during diastole and systole. Circ Res. 1969;24:339–347. doi: 10.1161/01.res.24.3.339. [DOI] [PubMed] [Google Scholar]
- Taber LA, Yang M, Podszus WW. Mechanics of ventricular torsion. J Biomech. 1996;29:745–752. doi: 10.1016/0021-9290(95)00129-8. [DOI] [PubMed] [Google Scholar]
- Vendelin M, Bovendeerd PH, Engelbrecht J, Arts T. Optimizing ventricular fibers: uniform strain or stress, but not ATP consumption, leads to high efficiency. Am J Physiol Heart Circ Physiol. 2002;283:H1072–1081. doi: 10.1152/ajpheart.00874.2001. [DOI] [PubMed] [Google Scholar]
- Vila-Cha C, Falla D, Farina D. Motor unit behavior during submaximal contractions following six weeks of either endurance or strength training. J Appl Physiol. 2010;109:1455–1466. doi: 10.1152/japplphysiol.01213.2009. [DOI] [PubMed] [Google Scholar]
- Weiner RB, Hutter AM, Jr, Wang F, Kim J, Weyman AE, Wood MJ, Picard MH, Baggish AL. The impact of endurance exercise training on left ventricular torsion. JACC Cardiovasc Imaging. 2010a;3:1001–1009. doi: 10.1016/j.jcmg.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Weiner RB, Weyman AE, Khan AM, Reingold JS, Chen-Tournoux AA, Scherrer-Crosbie M, Picard MH, Wang TJ, Baggish AL. Preload dependency of left ventricular torsion: the impact of normal saline infusion. Circ Cardiovasc Imaging. 2010b;3:672–678. doi: 10.1161/CIRCIMAGING.109.932921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson IB, Cockcroft JR, Webb DJ. Pulse wave analysis and arterial stiffness. J Cardiovasc Pharmacol. 1998a;32(Suppl 3):S33–37. [PubMed] [Google Scholar]
- Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998b;16:2079–2084. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- Xu X, Wan W, Powers AS, Li J, Ji LL, Lao S, Wilson B, Erikson JM, Zhang JQ. Effects of exercise training on cardiac function and myocardial remodeling in post myocardial infarction rats. J Mol Cell Cardiol. 2008;44:114–122. doi: 10.1016/j.yjmcc.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocalo Y, Bia D, Armentano RL, Arias L, Lopez C, Etchart C, Guevara E. Assessment of training-dependent changes in the left ventricle torsion dynamics of professional soccer players using speckle-tracking echocardiography. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:2709–2712. doi: 10.1109/IEMBS.2007.4352888. [DOI] [PubMed] [Google Scholar]


