Abstract
Central chemoreception is the mechanism by which the brain regulates breathing in response to changes in tissue CO2/H+. A brainstem region called the retrotrapezoid nucleus (RTN) contains a population of CO2/H+-sensitive neurons that appears to function as an important chemoreceptor. Evidence also indicates that CO2-evoked ATP release from RTN astrocytes modulates activity of CO2/H+-sensitive neurons; however, the extent to which purinergic signalling contributes to chemoreception by RTN neurons is not clear and the mechanism(s) underlying CO2/H+-evoked ATP release is not fully elucidated. The goals of this study are to determine the extent to which ATP contributes to RTN chemoreception both in vivo and in vitro, and whether purinergic drive to chemoreceptors relies on extracellular Ca2+ or gap junction hemichannels. We also examine the possible contribution of P2Y1 receptors expressed in the RTN to the purinergic drive to breathe. We show that purinergic signalling contributes, in part, to the CO2/H+ sensitivity of RTN neurons. In vivo, phrenic nerve recordings of respiratory activity in adult rats show that bilateral injections of pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS, a P2 receptor blocker) decreased the ventilatory response to CO2 by 30%. In vitro, loose-patch recordings from RTN neurons show that P2 receptor blockers decreased responsiveness to both 10% and 15% CO2 also by 30%. In the slice, the contribution of purinergic signalling to RTN chemoreception did not increase with temperature (22–35°C) and was retained in low extracellular Ca2+ medium. Conversely, the gap junction blockers carbenoxolone and cobalt decreased neuronal CO2/H+ sensitivity by an amount similar to P2 receptor antagonists. Inhibition of the P2Y1 receptor in the RTN had no effect on CO2 responsivness in vitro or in vivo; thus, the identity of P2 receptors underlying the purinergic component of RTN chemoreception remains unknown. These results support the possibility that CO2/H+-evoked ATP release is mediated by a mechanism involving gap junction hemichannels.
Key points
The retrotrapezoid nucleus (RTN) is an important site of chemoreception, i.e. the mechanism by which the brain regulates breathing in response to changes in tissue CO2/H+.
Mechanisms underlying RTN chemoreception appear to involve direct CO2/H+-mediated neuronal activation and indirect neuronal activation by CO2-evoked ATP release (i.e. purinergic signalling) from astrocytes.
Here, we show in vitro and in vivo that purinergic signalling in the RTN contributes to ∼30% of RTN chemoreception.
Purinergic drive in the RTN involves gap junction hemichannels but not P2Y1 receptors.
These results clearly indicate that purinergic signalling contributes to integrated output of the RTN during hypercapnia and thus is an important determinant of respiratory drive.
Introduction
Carbon dioxide provides the primary stimulus to breathe. Central respiratory chemoreceptors sense changes in tissue CO2, H+ and/or HCO3− to regulate the rate and depth of breathing (for review see Huckstepp & Dale, 2011). It is now well established that the retrotrapezoid nucleus (RTN) is an important site of chemoreception. Specifically, the RTN contains a subset of neurons that is highly CO2/H+ sensitive in vivo (Nattie et al. 1993; Mulkey et al. 2004; Takakura et al. 2008) and in vitro (Mulkey et al. 2004; Ritucci et al. 2005), is glutamatergic (Mulkey et al. 2004; Weston et al. 2004) and projects to respiratory centres to directly influence breathing (Mulkey et al. 2004; Abbott et al. 2009). The mechanism by which RTN neurons sense pH involves inhibition of an unidentified voltage-independent K+ conductance (Mulkey et al. 2004, 2007). Evidence also indicates that purinergic signalling contributes to the ventilatory response to CO2 (Thomas et al. 1999; Thomas & Spyer, 2000; Gourine et al. 2003; Gourine, 2005). At the level of the RTN, hypercapnia has been shown to evoke the discrete release of ATP near the ventral medullary surface (Gourine et al. 2005, 2010; Huckstepp et al. 2010b), and ATP has been shown to contribute to respiratory drive by increasing activity of CO2/H+-sensitive RTN neurons (Mulkey et al. 2006; Gourine et al. 2010; Wenker et al. 2010).
There is increasing evidence that astrocytes are the source of purinergic drive to RTN chemoreceptors (Gourine et al. 2010; Huckstepp et al. 2010b; Wenker et al. 2010); however, the extent to which ATP contributes to RTN chemoreception is questionable and the mechanisms underlying purinergic modulation of RTN chemoreceptors are not clear (Mulkey & Wenker, 2011). For example, a recent study reported that H+ sensitivity of RTN neurons could be blocked with a P2 receptor antagonist (Gourine et al. 2010), suggesting that RTN chemoreception is entirely dependent on purinergic signalling. The same study also suggested that the purinergic drive to RTN neurons is dependent on extracellular Ca2+; it showed that acidification triggered a Ca2+ wave that propagated between ventral surface astrocytes, possibly by activating Ca2+-permeable channels (e.g. P2X1 or P2X3), to potentiate additional ATP release and subsequently activate Phox2b-expressing RTN neurons in vitro and increased respiratory activity in vivo. Conversely, we have shown in vitro that RTN neurons are highly pH sensitive in the presence of P2 receptor blockers (Mulkey et al. 2004, 2006; Wenker et al. 2010), and that purinergic signalling contributes to only a portion of neuronal CO2/H+ sensitivity (Wenker et al. 2010). Still further evidence obtained using the brainstem–spinal cord preparation reported that purinergic signalling does not contribute to CO2 responsiveness of neurons in the parafacial respiratory group (pFRG)/RTN of newborn rats (Onimaru et al. 2012). In addition, the mechanism underlying CO2-evoked ATP release in the RTN appears to be mediated by direct gating of gap junction hemichannels in a Ca2+-independent manner (Huckstepp et al. 2010b).
The objectives of this study are to establish the extent to which purinergic signalling contributes to RTN chemoreception both in vivo and in vitro, and to gain insight into the mechanisms underlying CO2-evoked ATP release and downstream activation of RTN neurons. To make these determinations, we recorded phrenic nerve activity in vivo or the firing rate response of CO2/H+-sensitive RTN neurons in the brain slice preparation during exposure to hypercapnia alone and in the presence of P2 receptor blockers. In addition, we used a pharmacological approach to manipulate potential mechanisms of ATP release and activity of downstream P2 receptors. We find that purinergic signalling is an important component of RTN chemoreception; application of P2 receptor antagonists into the RTN reduced the ventilatory response to CO2 by ∼30%in vivo, and blunted the firing rate response of RTN neurons to both 10% and 15% CO2 also by 30%. This purinergic drive to breathe was independent of temperature, stimulus strength and changes in the extracellular Ca2+ gradient, but could be blocked with gap junction hemichannel antagonists. These results are most consistent with a recent study by Huckstepp and colleagues (Huckstepp et al. 2010b), and suggest that astrocytes but not neurons mediate CO2-evoked ATP release by a mechanism involving gap junction hemichannels.
Methods
In vivo preparation
Animal use was in accordance with guidelines approved by the University of São Paulo Animal Care and Use Committee and conforms to the principles of UK regulations, as described in Drummond (2009). All efforts were made to minimize the number of animals used and their suffering. All in vivo experiments were performed in male Wistar rats weighing 250–280 g; a total of 38 rats were used for these in vivo experiments. The surgical procedures and experimental protocols were similar to those previously described (Takakura & Moreira, 2011; Takakura et al. 2011). Briefly, general anaesthesia was induced with 5% halothane in 100% O2. A tracheostomy was made and the halothane concentration was reduced to 1.4–1.5% until the end of surgery. The femoral artery was cannulated (polyethylene tubing, 0.6 mm o.d., 0.3 mm i.d., Scientific Commodities, Lake Havasu City, AZ, USA) for measurement of arterial pressure (AP). The femoral vein was cannulated for administration of fluids and drugs. The occipital plate was removed, and a micropipette was placed in the medulla oblongata via a dorsal transcerebellar approach for microinjection of drugs. A skin incision was made over the lower jaw for placement of a bipolar stimulating electrode, next to the mandibular branch of the facial nerve, as previously described (Moreira et al. 2006; Takakura et al. 2011). The phrenic nerve was accessed by a dorsolateral approach after retraction of the right shoulder blade. To prevent any influence of artificial ventilation on phrenic nerve activity (PNA), the vagus nerve was cut bilaterally. Additionally, a complete baro- and peripheral chemoreceptor deafferentation was performed by sectioning the vagosympathetic trunks, the superior laryngeal nerves and the glossopharyngeal nerves (proximal to the junction with the carotid sinus nerves). Upon completion of the surgical procedures, halothane was replaced with urethane (1.2 g kg−1) administered slowly i.v. All rats were ventilated with 100% O2 throughout the experiment. Rectal temperature was maintained at 37°C. End-tidal CO2 was monitored throughout each experiment with a capnometer (CWE, Inc., Ardmore, PA, USA) that was calibrated twice per experiment with a calibrated CO2–N2 mix. This instrument provided a reading of <0.1% CO2 during inspiration in animals breathing 100% O2, and an asymptotic, nearly horizontal reading during expiration. The adequacy of anaesthesia was monitored during a 20 min stabilization period by testing for the absence of withdrawal responses, pressor responses and changes in PNA to a firm toe pinch. After these criteria were satisfied, the muscle relaxant pancuronium was administered at an initial dose of 1 mg kg−1i.v. and the adequacy of the anaesthesia was thereafter gauged solely by the lack of increase in AP and PNA rate or amplitude to a firm toe pinch. Approximately hourly supplements of one-third of the initial dose of urethane were needed to satisfy these criteria throughout the recording period (2 h).
In vivo recordings of physiological variables
As previously described (Mulkey et al. 2004; Moreira et al. 2006; Takakura et al. 2011), mean arterial pressure (MAP), phrenic nerve activity (PNA) and end-expiratory CO2 (etCO2) were digitized with a micro1401 (Cambridge Electronic Design), stored on a computer and processed off-line with version 6 of Spike 2 software (Cambridge Electronic Design). Integrated phrenic nerve activity (iPNA) was obtained after rectification and smoothing (τ= 0.015 s) of the original signal, which was acquired with a 30–300 Hz bandpass filter. PNA amplitude (PNA amp) and PNA frequency (PNA freq) were expressed for each rat on a scale from 0 (value during apnoea) to 100 (value while breathing 10% CO2).
Hypercapnia was produced by addition of pure CO2 to the 100% O2 supplied by artificial ventilation to increase the maximum end-expiratory CO2 to 9.5–10%. End-expiratory CO2 was maintained for 5 min, and CO2 was removed. Each rat was submitted to three sessions of hypercapnia: one session 10 min after bilateral saline injections into the RTN, and two additional sessions, 10 and 60 min after bilateral injections of saline, PPDAS or MRS2179 into the RTN.
Histology
At the end of each in vivo experiment, rats were deeply anaesthetized with halothane and perfused through the heart with PBS (pH 7.4) followed by paraformaldehyde (4% in 0.1 m phosphate buffer, pH 7.4). The brains were removed and stored in fixative for 24 h at 4°C. The medulla was cut in 40-µm-thick coronal sections with a vibrating microtome (Vibratome 1000S Plus, USA). Sections were stored at −20°C in a cryoprotectant solution. The injection sites were confirmed with an Axioskop 2 microscope (Zeiss, Oberkochen, Germany). Sections from different brains were aligned with respect to a reference section, which was the most caudal section containing an identifiable cluster of facial motor neurons. To this reference section was assigned a value of 11.6 mm caudal to bregma (bregma −11.6 mm, Paxinos & Watson, 1998). Levels rostral or caudal to this reference section were determined by adding or subtracting the number of intervening sections × 40 μm.
Brain slice preparation
All procedures were performed in accordance with National Institutes of Health and University of Connecticut Animal Care and Use Guidelines and conformed to the principles of UK regulations, as described in Drummond (2009). A total of 64 rat pups were used for these in vitro experiments. Slices containing the RTN were prepared as previously described (Mulkey et al. 2004). Briefly, neonatal rats (7–12 days postnatal) were decapitated under ketamine–xylazine anaesthesia and transverse brainstem slices (300 μm) were cut using a microslicer (DSK 1500E; Dosaka, Kyoto, Japan) in ice-cold substituted Ringer solution containing (in mm): 260 sucrose, 3 KCl, 5 MgCl2, 1 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose and 1 kynurenic acid. Slices were incubated for ∼30 min at 37°C and subsequently at room temperature in normal Ringer solution (in mm): 130 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3 and 10 glucose. Both substituted and normal Ringer solutions were bubbled with 95% O2–5% CO2 (extracellular pH (pHo) 7.35).
Slice-patch electrophysiology
Individual slices were transferred to a recording chamber mounted on a fixed-stage microscope (Zeiss Axioskop FS) and perfused continuously (∼2 ml min−1) with a bath solution of normal Ringer solution (same as incubation Ringer solution above) bubbled with 95% O2–5% CO2 (pHo 7.35). The pH of the bicarbonate-based bath solution was decreased to 6.90 by bubbling with 15% CO2 or 7.10 by bubbling with 10% CO2. All recordings were made with an Axopatch 200B patch-clamp amplifier, digitized with a Digidata 1322A A/D converter, and recorded using pCLAMP 10.0 software (Molecular Devices). Recordings were obtained at room temperature (∼22°C) with patch electrodes pulled from borosilicate glass capillaries (Warner Instruments) on a two-stage puller (P89; Sutter Instrument) to a DC resistance of 4–6 MΩ when filled with an internal solution containing the following (in mm): 120 KCH3SO3, 4 NaCl, 1 MgCl2, 0.5 CaCl2, 10 Hepes, 10 EGTA, 3 Mg-ATP and 0.3 GTP-Tris (pH 7.2) plus 0.2% biocytin; electrode tips were coated with Sylgard 184 (Dow Corning). All recordings of neuronal firing rate were performed in the loose-patch configuration to ensure minimal alteration of the intracellular milieu. Firing rate histograms were generated by integrating action potential discharge in 10 s bins and plotted using Spike 5.0 software. Excitatory postsynaptic currents (EPSCs) were measured from chemosensitive neurons (identified in loose-patch) in whole-cell voltage clamp (Ihold=−60 mV) and analysed off-line with AxoGraph X using the following parameters: amplitude =−25 pA, rise constant = 0.7 ms and decay constant = 1.6 ms, with a threshold of detection set to −7 SD.
Drugs
All drugs were purchased from Sigma. For in vivo experiments, the non-specific P2 receptor antagonist pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), the P2Y1-receptor antagonist MRS2179 and the P2Y1-receptor agonist MRS2365 were diluted to 100 μm in sterile saline (pH 7.4) and injected into the RTN using single-barrel glass pipettes (tip diameter of 20 μm) connected to a pressure injector (Picospritzer III, Parker Hannifin Corp, Cleveland, OH, USA). For each injection we delivered a volume of 50 nl over a period of 5 s. These glass pipettes also allowed recordings of field potential properties that were used to help direct the electrode tip to the desired site. Injections in the RTN region were guided by recordings of the facial field potential (Brown & Guyenet, 1985), and were placed 250 μm below the lower edge of the field, 1.7 mm lateral to the midline and 200 μm rostral to the caudal end of the field. Recordings were made on one side only; the second injection was made 1–2 min later at the same level on the contralateral side. We included a 5% dilution of fluorescent latex microbeads (Lumafluor, New City, NY, USA) with all drug applications to mark the injection sites and verify the spread of the injections (Takakura & Moreira, 2011; Takakura et al. 2011). For the in vitro experiments, we bath applied PPADS (100 μm) or suramin (100 μm) to block P2 receptors, MRS2179 (3 μm) to block P2Y1 receptors, and carbenoxolone (CBX, 100 μm) or cobalt (500 μm) to block gap junction hemichannels. A low Ca2+, high Mg2+ synaptic block solution was used to block potential ATP release from neurons and to limit Ca2+ influx into astrocytes from the extracellular space. The composition of the synaptic block medium used in this study is similar to normal Ringer solution except that MgCl2 was increased to 11.4 mm, CaCl2 was decreased to 0.2 mm, and to maintain osmolality, NaCl was decreased to 124 mm. The efficacy of this synaptic blocking medium is well established (Richards & Sercombe, 1970; Mason, 1980; Hatton, 1982) and confirmed here by recording its effects on spontaneous EPSCs. In addition, the specific P2Y1 receptor agonist (MRS2365; 100 μm in Hepes-buffered medium, pH 7.3) was delivered focally using low-resistance pipettes connected to a Picospritzer III (Parker Instrumentation, Cleveland, OH, USA) and manoeuvered into close proximity of the target neurons. Application times were 600 ms, and vehicle control experiments were performed to ensure agonist responses were not attributable to pressure artifacts.
Statistics
Data are reported as mean ± standard error of the mean. Statistical analysis was performed using Sigma Stat version 3.0 software (Jandel Corporation, Point Richmond, CA, USA). t test, paired t test or one-way ANOVA followed by the Newman–Keul multiple comparisons test were used as appropriate (P < 0.05).
Results
This study consists of three sets of experiments. First, to determine the extent to which purinergic signalling contributes to central chemoreception, we tested the effects of P2 receptor antagonists (PPADS or suramin) on the hypercapnic ventilatory response in vivo, and on the firing rate response of RTN neurons to 10% and 15% CO2 at room or body temperature in vitro. Second, to determine if purinergic drive to RTN neurons is dependent on synaptic input or requires extracellular Ca2+, as previously reported, we compared effects of P2 receptor antagonists on neuronal CO2/H+ responsiveness under control conditions and in low Ca2+–high Mg2+ synaptic block medium. Third, to determine if purinergic drive to CO2/H+-sensitive RTN neurons is mediated by hemichannels, we tested neuronal CO2/H+ sensitivity in the presence of the gap junction blockers CBX or cobalt. Note that chemosensitive RTN neurons have been shown to express the transcription factor Phox2b (Stornetta et al. 2006; Lazarenko et al. 2009); however, Phox2b has also been shown to be expressed by other cells in relatively close proximity to the RTN, including catecholaminergic neurons (C1 and A5), facial motor neurons and the superior salivatory nucleus (Kang et al. 2007). Therefore, we chose to functionally identify CO2/H+-sensitive RTN neurons based on their characteristic firing rate response to CO2/H+. Neurons were considered chemosensitive if they were spontaneously active in 5% CO2 (pHo 7.35) and responded to 15% CO2 (pHo∼6.90) with at least 1.5 Hz increase in firing rate. This level of CO2/H+ responsiveness is similar to what we, and others, have reported for RTN chemosensitive neurons (Ritucci et al. 2005; Mulkey et al. 2006; Wenker et al. 2010). RTN neurons that did not exhibit this minimum firing rate response to 15% CO2 were considered non-chemosensitive and excluded from this study.
Purinergic drive to RTN chemoreceptors in vivo
As expected, when urethane-anaesthetized, sino-aortic denervated and vagotomized rats (n = 6 per group) are exposure to hypercapnia (10% CO2) PNA amplitude and PNA frequency increased by 100 ± 2% and 99 ± 2% (Fig. 1A, D and E). Injections of PPADS (3 mm in 50 nl) were placed bilaterally in the RTN in these rats (Fig. 1B and C). The injection centre was 250 μm below the facial motor nucleus and 200 μm rostral to the caudal end of this nucleus, targeting the region that contains the highest density of CO2-sensitive RTN neurons (Mulkey et al. 2004; Takakura & Moreira, 2011; Takakura et al. 2011). Application of PPADS into the RTN did not change resting PNA activity (99 ± 7% of control value; P > 0.05), but did reduce the hypercapnia-induced increase in PNA amplitude (62 ± 9% of the control) and PNA frequency (77 ± 3% of the control) (Fig. 1D and E).
Figure 1. P2 receptor antagonist injections into the RTN attenuate effects of hypercapnia on arterial pressure and PNA in anaesthetized vago-sino-aortic denervated rats.
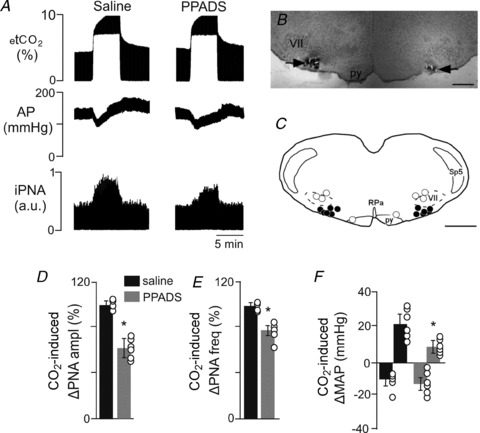
A, recordings from one rat showing the effect of injection of PPADS into the RTN on changes in arterial pressure (AP) and phrenic nerve activity (PNA) elicited by an increase of end-expiratory CO2 from 5 to 10%. Responses were recorded 10 min after bilateral injection of saline into the RTN, and 10 min after bilateral injection of PPADS (3 mm, 50 nl each side) in the RTN. B, photomicrograph of a coronal section showing bilateral injections in the RTN. Scale bar is 500 μm. C, computer-assisted plot of the centre of injection sites revealed by presence of fluorescent microbeads included in the injectate (coronal plane at Bregma −11.6; Paxinos & Watson, 1998). Scale bar is 1 mm. D–F, summary data (n = 6) show changes in PNA amplitude (ΔPNA ampl) (D), PNA frequency (ΔPNA freq) (E) and mean arterial pressure (ΔMAP) (F) elicited by stepping the end-expiratory CO2 from 5 to 10% during saline or PPADS injections into the RTN. Differences expressed as a percentage of the response to the CO2 challenge elicited during saline injection. *Different from saline (P < 0.05). Abbreviations: py, pyramid; RPa, raphe pallidus; Sp5, spinal trigeminal tract; VII, facial motor nucleus.
As shown in Fig. 1A, exposure to hypercapnia also caused hypotension (−11 ± 7 mmHg) followed by a gradual return of mean arterial pressure (MAP) to control level, 30 to 40 s later. Immediately after hypercapnia ended, the MAP increased by 21 ± 6 mmHg, and returned to control values within 5 min (Fig. 1A and F). Injection of PPADS into the RTN did not change resting MAP (124 ± 6 mmHg compared with saline 126 ± 5 mmHg), but did reduce the hypercapnia-induced pressor response from 21 ± 6 to 8 ± 4 mmHg (Fig. 1A and F).
Injections located outside the RTN region often (3 out of 4) reached the facial motor nucleus or dorsal to it and one injection (1 out of 4) was located in the parapyramidal region (data not shown). Bilateral injections of PPADS in the facial motor nucleus or in the parapyramidal region did not change the hypercapnic-induced respiratory or pressor responses in these animals (data not shown).
Purinergic drive to RTN chemoreceptors in vitro
Previous evidence indicates that temperature greatly increased pH sensitivity of RTN neurons in vitro (Guyenet et al. 2005). Therefore, we wanted to determine if the contribution of purinergic drive to CO2/H+-sensitive RTN neurons also increased with temperature. We found that increasing temperature from ∼22°C to 35°C increased baseline activity by 2.01 ± 0.18 Hz (Fig. 2A) and increased the firing rate response to 15% CO2 from 1.9 ± 0.09 Hz to 3.3 ± 0.23 Hz (n = 4) (Fig. 2B). The temperature coefficient (Q10) for the CO2/H+ sensitivity of RTN neurons was estimated to be ∼1.5 ± 0.1 (n = 9); this value is very similar to the pH-sensitive Q10 of RTN neurons over a similar pH range (∼1.55, extrapolated from Fig. 8D; Guyenet et al. 2005]. At 35°C, exposure to the P2 receptor antagonists PPADS (100 μm) or suramin (100 μm) decreased firing rate by 0.73 ± 0.18 Hz, consistent with the hypothesis that CO2/H+-sensitive RTN neurons receive tonic purinergic input (Wenker et al. 2010). Prior to testing CO2/H+ sensitivity at 35°C, a hyperpolarizing current (∼5 pA) was delivered to approximate the control level of neuronal activity. In the continued presence of PPADS or suramin at 35°C (with baseline activity adjusted by DC current injection to near-control levels), exposure to 15% CO2 increased neuronal activity by 2.38 ± 0.23 Hz (n = 8); this response was significantly less than the CO2/H+ response at 35°C in the absence of the P2 receptor antagonist (initial response or after wash out) (Fig. 2A–B). This purinergic-sensitive component of RTN chemoreception has an estimated Q10 of ∼1.3. It is worth noting that this value is similar to that described for connexin-mediated ATP release or current flux (Q10= 1.2–1.4; Bukauskas et al. 1995; Valiunas et al. 1999; Leybaert et al. 2003). In addition, heating from 22°C to 35°C did not significantly increase effects of P2 receptor antagonists on CO2/H+ sensitivity of RTN neurons; PPADS and suramin decreased chemoreceptor activity by 27.3 ± 4.8% (n = 12) and 27.9 ± 4.5% (n = 8) at 22°C and 35°C, respectively (Fig. 2C). These results indicate that purinergic drive to CO2/H+-sensitive RTN neurons does not vary over this temperature range; therefore, all subsequent in vitro experiments were performed at room temperature.
Figure 2. Purinergic contribution to CO2/H+ sensitivity of RTN neurons is not temperature dependent.
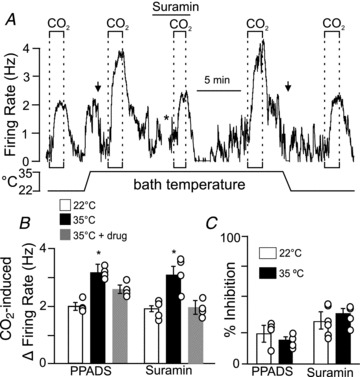
A, traces of firing rate and bath temperature show CO2/H+ responsiveness at room temperature (∼22°C) and 35°C with and without suramin (Sur, 100 μm), a P2 receptor antagonist. At room temperature, increasing bath CO2 from 5 to 15% increased firing rate ∼2 Hz. Heating to 35°C increased firing rate ∼2 Hz. Prior to testing CO2/H+ sensitivity at 35°C we delivered a hyperpolarizing current (−5 nA; left arrow) into the cell to match firing rate to control activity. Under these conditions exposure to 15% CO2 increased firing rate ∼3.5 Hz. A 10 min incubation in suramin (*) decreased the firing rate response to 15% CO2 by 40%. CO2/H+ sensitivity returned to control levels in wash. B, average data show the CO2/H+-induced firing rate response at room temperature (a portion of these results were published previously Wenker et al. 2010) and at 35°C with or without the P2 receptor antagonist PPADS (100 μm, n = 4) or suramin (100 μm, n = 4). Based on these results we calculated the Q10 of CO2/H+ sensitivity and purinergic drive to RTN neurons to be ∼1.5 and ∼1.3, respectively. C, purinergic drive to chemosensitive RTN neurons shown as % inhibition of CO2/H+ sensitivity by PPADS (n = 11) or suramin (n = 11) at 22°C and 35°C. Note that increasing temperature did not increase the purinergic drive to chemosensitive RTN neurons.
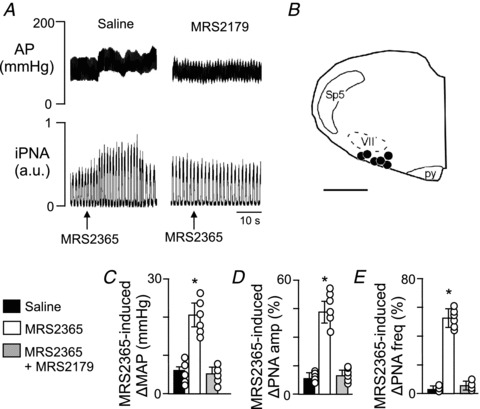
Figure 8. P2Y1 receptors do not contribute to CO2-induced changes in breathing or blood pressure.
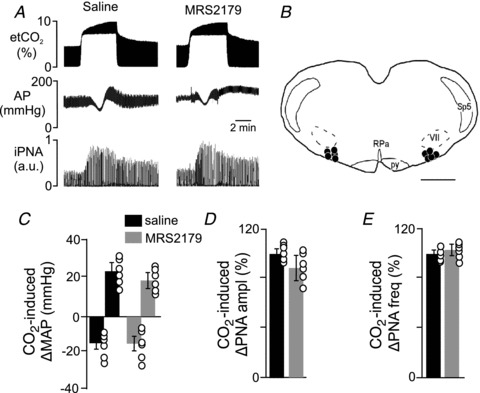
A, traces of end-expiratory CO2 (etCO2), arterial pressure (AP) and integrated PNA (iPNA) show CO2 responses 10 min after RTN injections of saline or MRS2179 (100 μm, 50 nl each side). B, computer-assisted plot of the centre of injection sites (Bregma −11.6; Paxinos & Watson, 1998). Scale bar in B, 1 mm. C–E, summary data (n = 5) showing changes in mean arterial pressure (ΔMAP) (C), PNA amplitude (ΔPNA ampl) (D) and PNA frequency (ΔPNA freq) (E) elicited by stepping the end-expiratory CO2 from 5 to 10% after RTN injections of saline or MRS2179. Differences are expressed as a percentage of the response to CO2 after saline injection. Abbreviations: py, pyramid; RPa, raphe pallidus; Sp5, spinal trigeminal tract; VII, facial motor nucleus.
To address the possibility that purinergic drive to RTN neurons is stimulus dependent, we compared effects of suramin on the firing rate response of RTN neurons to 10% and 15% CO2. As shown in Fig. 3A and B, exposure to 10% (pHo 7.10) and 15% CO2 (pHo 6.90) increased chemoreceptor activity by 1.83 ± 0.17 Hz and 2.48 ± 0.20 Hz (n = 3), respectively. In addition, suramin had similar effects on the firing rate response of RTN neurons to 10% and 15% CO2. Previous (Wenker et al. 2010) and current results (Fig. 3C and D) show that ∼10 min incubation in suramin (100 μm) decreased the response of RTN neurons to 10% and 15% CO2 by 33.5 ± 3.3% and 31.9 ± 7.2%, respectively (Fig. 3C and D), suggesting that purinergic drive to these cells is relatively constant over this CO2 range.
Figure 3. Purinergic drive does not increase with increased CO2 intensity.
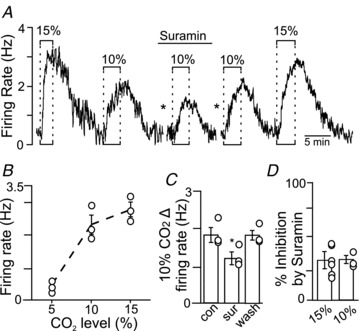
A, firing rate trace shows that exposure to 10 and 15% CO2 increased neuronal activity by 2.9 and 2.1 Hz, respectively. In the presence of suramin (100 μm), exposure to 10% CO2 increased neuronal activity only 1.6 Hz. Responsiveness to 10 and 15% CO2 was fully recovered when suramin was washed out. The asterisks denote 10 min time breaks. B, average data (n = 3) show that exposure to 10 and 15% CO2 increased neuronal activity in a relatively linear manner. C, summary data (n = 3) show that suramin decreased the firing rate response to 10% CO2. D, summary data (n = 3) show that suramin decreased the responsiveness to 10 and 15% CO2 by similar amounts, indicating that purinergic drive to chemosensitive RTN neurons does not increase in response to this range of stimulus intensities.
Changes in the extracellular Ca2+ gradient do not affect purinergic drive to chemoreceptors
Evidence suggests that H+-evoked ATP release triggers Ca2+ influx into ventral surface astrocytes, possibly via P2X receptors, and this leads to bulk ATP release and subsequent activation of CO2/H+-sensitive RTN neurons (Gourine et al. 2010). To explore this possibility further, we tested the effects of a high MgCl2 and low CaCl2 medium on the CO2/H+ sensitivity of RTN neurons. We used low (200 μm) rather than zero Ca2+ in order to minimize the effects of low Ca2+ on gap junctions. In addition, this medium has been shown to effectively block Ca2+-dependent exocytosis with little disruption of intrinsic membrane characteristics (Richards & Sercombe, 1970). As before, CO2/H+-sensitive RTN neurons were identified based on their robust firing rate response to 15% CO2 in normal Ringer solution. Exposure to low Ca2+–high Mg2+ medium alone did not consistently increase baseline activity, suggesting that this low level of extracellular Ca2+ did not facilitate gap junction-mediated ATP release. However, as noted below low Ca2+–high Mg2+ medium can inhibit excitatory input to RTN chemoreceptors (Fig. 4C), and so potentially offset excitatory effects of connexin-mediated ATP release. A second exposure to 15% CO2, this time after ∼10 min incubation in low Ca2+–high Mg2+ solution, also elicited a strong firing rate response (Fig. 4A and B), indicating that a 10-fold decrease in the extracellular Ca2+ gradient did not affect CO2/H+ sensitivity (Fig. 4B). To confirm that Ca2+-dependent exocytosis was effectively attenuated by these incubation conditions, we recorded excitatory post-synaptic currents (EPSCs) from RTN chemoreceptor neurons during exposure to the low Ca2+–high Mg2+ solution. As expected, ∼5 min perfusion with low Ca2+–high Mg2+ solution caused a robust and reversible decrease in EPSC frequency with only a modest effect on amplitude (Fig. 4C–E). These results suggest that the CO2/H+ sensitivity of RTN neurons, including the purinergic component, is not dependent on neuronal vesicle release.
Figure 4. Purinergic drive to RTN neurons is not dependent on extracellular Ca2+.
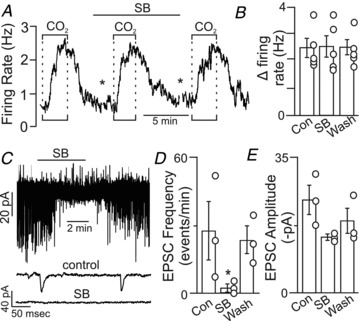
A, firing rate trace shows that the CO2/H+ sensitivity of an RTN neuron was unaffected by low Ca2+–high Mg2+ synaptic block (SB) medium. The asterisks denote 8 min time breaks. B, average data (n = 5) show that SB medium did not significantly affect CO2/H+ sensitivity (P = 0.982). C, traces of holding current (at a potential of −60 mV) show that exposure to SB medium blocked spontaneous excitatory post-synaptic currents (EPSCs) in chemosensitive RTN neurons. D and E, as expected, SB medium inhibited EPSC frequency (D, n = 3) but not EPSC amplitude (E, n = 3). Average decay time constant was 4.8 ± 0.2 ms (not shown). These results suggest that extracellular Ca2+ is not required for the purinergic drive to breathe.
Gap junction hemichannels contribute to CO2/H+-evoked ATP release in the RTN
There is evidence that connexin 26 hemichannels are directly gated by CO2 (Huckstepp et al. 2010a) and contribute to CO2-evoked ATP release in the RTN (Huckstepp et al. 2010b). To test whether connexin-mediated transmitter release contributes to the CO2/H+ sensitivity of RTN neurons, we used CBX and cobalt to block hemichannels. We found that ∼10 min incubation in CBX (100 μm) decreased neuronal CO2/H+ sensitivity by 0.74 ± 0.18 Hz (n = 6) or 27.7 ± 5.5% (Fig. 5A and B). Likewise, incubation in cobalt (500 μm) decreased neuronal CO2/H+ sensitivity by 0.64 ± 0.08 Hz (n = 5) or 26.36 ± 2.73% (Fig. 5C and D). Note that both gap junction blockers decreased neuronal CO2/H+ sensitivity by an amount similar to the previously reported effects of P2 receptor antagonists (PPADS and suramin); at room temperature P2 receptor antagonists decreased CO2/H+ sensitivity of RTN neurons by 0.7 ± 0.18 Hz or 27.3 ± 4.8% (Wenker et al. 2010). Furthermore, in combination suramin and cobalt decreased CO2/H+ sensitivity of RTN neurons by 28 ± 3.0% (Fig. 6A and B). The per cent inhibition by suramin and cobalt acting together was not different from that of either blocker alone (Fig. 6C), further suggesting that the majority of CO2-evoked ATP release is mediated by gap junction hemichannels. Together, these results strongly suggest that gap junction hemichannels mediate CO2-evoked ATP release in the RTN.
Figure 5. Gap junction blockers decrease purinergic drive to RTN neurons.
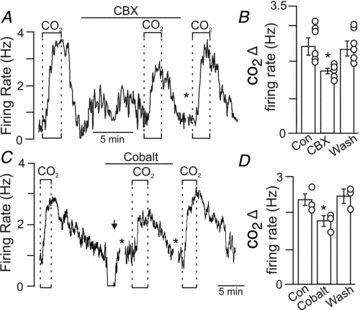
A, trace of firing rate shows that under control conditions, exposure to 15% CO2 increased the neuronal activity ∼3 Hz. A second exposure to 15% CO2, this time after 10 min incubation with carbenoxolone (CBX, 100 μm), increased firing rate only ∼2 Hz. The asterisk indicates a gap in recording during the CBX wash. B, average data (n = 6) show that CBX decreased CO2/H+ sensitivity by ∼30%. C, trace of firing rate shows that exposure to 15% CO2 increased neuronal activity 2.1 Hz. After returning to control conditions, exposure to cobalt (500 μm) inhibited neuronal activity; therefore a depolarizing current (5 nA; arrow) was injected into the cell to approximate baseline activity under control conditions. In the continued presence of cobalt (with a holding current of 5.0 nA), a second exposure to 15% CO2 this time only increased firing rate ∼1.5 Hz. CO2 responsivness was fully recovered after washing out cobalt for ∼10 min. D, average data (n = 5) show that cobalt decreased CO2/H+ sensitivity by ∼30%.
Figure 6. In combination, P2 receptor antagonist and gap junction blockers decrease CO2/H+ sensitivity by an amount similar to each blocker alone.

A, trace of firing rate shows that under control conditions exposure to 15% CO2 increased neuronal activity ∼2 Hz. A second exposure to 15% CO2, this time in the presence of suramin to block P2 receptors and cobalt to block gap junction hemichannels, increased firing rate only ∼1 Hz or 72% of control. The asterisk indicates an 8 min time break. CO2 sensitivity fully recovered after washing out suramin and cobalt. B, summary data (n = 3) show that in combination suramin and cobalt decreased CO2/H+ sensitivity of RTN neurons by 28 ± 3.0%. C, the per cent inhibition by suramin and cobalt acting together is not different from that of either blocker alone.
P2Y1 receptors in the RTN do not contribute to central chemoreception in vitro or in vivo
Evidence suggests that P2Y receptors mediate the excitatory effects of ATP on CO2/H+-sensitive RTN neurons (Mulkey et al. 2007). Of these, P2Y1 receptors have been shown to function as the primary substrate for ATP-mediated activation of inspiratory neurons in the preBötzinger complex (Lorier et al. 2007, 2008), a region critically involved in inspiratory rhythm generation (Smith et al. 1991). Therefore, we considered the possibility that P2Y1 receptors also contribute to up-stream purinergic modulation of CO2/H+-sensitive RTN neurons. To make this determination, we tested effects of MRS2179 (a potent and specific P2Y1 receptor blocker) on CO2 sensitivity of RTN neurons in vitro and in vivo. In the brain slice preparation, incubation (∼10 min) in MRS2179 (3 μm) had negligible effects on baseline activity and CO2/H+ sensitivity of RTN neurons (Fig. 7A and B). In addition, focal application of a specific P2Y1 receptor agonist (MRS2365, 100 μm) had no effect on activity of CO2/H+-sensitive RTN neurons (Fig. 7A). These results suggest P2Y1 receptors are not expressed by CO2/H+-sensitive RTN neurons and do not contribute to their CO2 sensitivity.
Figure 7. Purinergic drive to RTN neurons does not depend on P2Y1 receptors.
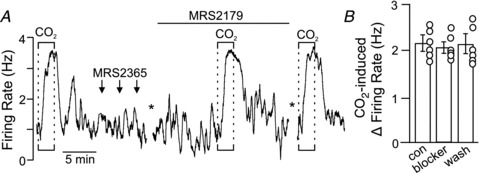
A, trace of firing rate shows that under control conditions exposure to 15% CO2 increased neuronal activity ∼3 Hz. A second exposure to 15% CO2, this time in the presence of the P2Y1 receptor-specific antagonist MRS2179 (3 μm), also increased firing rate by ∼3 Hz. The asterisk indicates a 10 min time break during which the tissue was incubated in MRS2179. Arrows indicate when the P2Y1-specific agonist MRS2365 (100 μm in 7.3 Hepes buffer) was focally applied. B, summary data (n = 6) show that MRS2179 had no effect on the CO2/H+ sensitivity of RTN neurons.
To determine if P2Y1 receptors contribute to CO2-induced changes in breathing or blood pressure in vivo, we measured CO2 responsivness after bilateral RTN injections of saline or MRS2179. As described above, under control conditions (i.e. after saline injections), exposure to hypercapnia (10% CO2) increases PNA and causes hypotension (−16 ± 6 mmHg) followed by an increase in MAP (24 ± 5 mmHg) (Figs 1 and 8). Bilateral RTN injections of MRS2179 (100 μm in 50 nl) did not change resting MAP (119 ± 5 mmHg compared with saline 121 ± 3 mmHg) or baseline PNA activity (102 ± 5% of control). In addition, injections of MRS2179 did not affect the CO2-induced pressor response (24 ± 5 mmHg vs. saline 19 ± 4 mmHg) or the CO2-induced increase in PNA amplitude (86 ± 15% of control) and PNA frequency (108 ± 5% of the control) (Fig. 8A and C–E). However, unilateral RTN injections of MRS2365 (100 μm in 50 nl) increased MAP by 23 ± 1 mmHg (compared with 4 ± 1 mmHg in control experiments) and increased PNA amplitude and frequency by 59 ± 6% and 53 ± 5%, respectively (Fig. 9C–E). Further, bilateral injections of MRS2179 entirely blocked the effects of MRS2365 (Fig. 9A–E), thus confirming effectiveness of the antagonist. These results are consistent with our in vitro evidence that P2Y1 receptors are not expressed by CO2/H+-sensitive RTN neurons and do not contribute to central CO2 sensitivity.
Figure 9. RTN injections of a specific P2Y1 agonist increased arterial pressure and PNA in anaesthetized vago-sino-aortic-denervated rats.
A, traces of arterial pressure (AP) and integrated phrenic nerve activity (iPNA) show that unilateral RTN injections of the specific P2Y1 receptor agonist MRS2365 (100 μm in 50 nl) increased AP and PNA. B, computer-assisted plot of injection sites (coronal plane at Bregma −11.6 (Paxinos & Watson, 1998). Scale bar in B, 1 mm. C–E, summary data (n = 6) show MRS2365-induced ΔMAP (B), ΔPNA amp (C) and ΔPNA freq (D) after RTN injections of saline or MRS2179. Differences expressed as a percentage of the response to the CO2 challenge elicited during saline injection. Abbreviations: py, pyramid; Sp5, spinal trigeminal tract; VII, facial motor nucleus.
Discussion
There is compelling evidence that CO2-evoked ATP release from ventral surface astrocytes contributes to respiratory drive by activating chemosensitive RTN neurons (Gourine, 2005; Gourine et al. 2010; Wenker et al. 2010). Here, we demonstrate in vivo that blocking P2 receptors in the RTN lessened the changes in PNA amplitude, PNA frequency and pressor responses elicited by central chemoreflex activation. Additionally, we show in vitro that bath application of P2 receptor antagonists (PPADS and suramin) blunted the firing rate response of RTN neurons to 10% and 15% CO2 by 30%. We show that the contribution of purinergic signalling to chemosensitive RTN neuronal activity is independent of temperature and stimulus strength. These results clearly indicate that purinergic signalling contributes in part to the integrated output of the RTN during hypercapnia. We also found that the purinergic drive to chemosensitive RTN neurons was wholly retained in low Ca2+ synaptic block medium but was significantly attenuated by gap junction blockers (CBX and cobalt), suggesting that astrocytes release ATP via gap junction hemichannels. Our results also show that P2Y1 receptors do not contribute to CO2/H+ sensitivity of the RTN; however, P2Y1 receptors are expressed in the region and their activation can influence respiratory and sympathetic outflow of the RTN. The identity and function of these P2Y1-expressing neurons remain unknown. We conclude that RTN chemoreception is determined primarily by CO2/H+-sensitive neurons but is augmented by gap junction-mediated, CO2-evoked ATP release most probably from astrocytes.
Experimental limitations
Our in vivo experiments were performed is anaesthetized rats and it is well known that anaesthetics can modify centrally mediated cardio-respiratory reflexes. Therefore, it will be important for future studies to confirm the role of purinergic signalling in central chemoreception using conscious animals. In addition, we did not measure ATP directly but rather used the activity of RTN chemoreceptors as a functional measure of ATP release. This approach is reasonable considering that CO2-evoked ATP release in the region of the RTN is well established (Gourine, 2005; Gourine et al. 2010; Huckstepp et al. 2010b) and the focus of the current study is on the purinergic drive to RTN neurons, which has received only limited attention. This study was also limited by the lack of pharmacological tools that allow for the specific manipulation of astrocytes without confounding effects on neurons. Nevertheless, the results presented here provide important direction for future experiments that take advantage of astrocyte-specific transgenic animal models, e.g. IP3-receptor type 2 (IP3R2) knockout mice (Petravicz et al. 2008) or the dominant negative synaptobrevin 2 model (Pascual et al. 2005), to further dissect neuron–astrocyte interactions in the context of chemoreception.
Contribution of purinergic signalling to RTN chemoreception
Bilateral injection of PPADS into the RTN of adult rats decreased the ventilatory response to CO2 by ∼30%. These results are entirely consistent with previous evidence showing that direct application of PPADS onto the exposed ventral surface also decreased the hypercapnic ventilatory response in vivo by up to ∼30% (Gourine et al. 2005). In addition, we show by using brain slices from neonatal rat pups that bath application of PPADS or suramin decreased the response of functionally identified CO2/H+-sensitive RTN neurons by ∼30% regardless of stimulus strength or bath temperature. Considering that P2 receptor blockers had similar effects on chemosensitivity in neonatal (postnatal days 7–12) and adult rats suggests that the purinergic drive to RTN neurons does not increase over this age range. However, astrocyte are known to undergo dramatic changes during the first postnatal week, and in other brain regions it is not until ∼day 4 that characteristically mature astrocytes first appear (i.e. electrically passive) (Zhou et al. 2006). In the RTN, CO2/H+-sensitive astrocytes exhibit an electrically passive signature and presumably these cells are the source of ATP released in this region during hypercapnia (Wenker et al. 2010). Therefore, if mature astrocytes are absent from the RTN in newborn animals, than we expect CO2/H+ sensitivity to be determined exclusively by intrinsic neuronal properties. This is exactly what Onimaru et al. (2012) demonstrated using the in vitro brainstem–spinal cord preparation from newborn rats; bath application of P2 receptor blockers (MRS2179 or PPADS) had no effect on CO2 responsivness of pFRG/RTN neurons. Our results clearly show in neonatal and adult rats that CO2-evoked ATP release contributes to a portion of the hypercapnic ventilatory response by increasing baseline activity of chemosensitive RTN neurons at any given level of CO2. However, it remains questionable whether purinergic signalling directly contributes to the H+-sensing mechanism of RTN neurons. In addition, the purinergic-independent component of RTN chemoreception (∼70%) was retained when synaptic activity was blocked which strengthens the possibility that RTN neurons are CO2/H+ sensitive (Mulkey et al. 2004, 2006).
It is well established that hypercapnia produces biphasic changes in vascular tone, i.e hypotension followed by hypertension. The initial hypotension probably results from a direct effect of CO2/H+ on vasculature smooth muscle cells. Compensatory activation of sympathetic activity allows arterial pressure to recover to near control levels during hypercapnia, and accounts for the increase in arterial pressure observed at the end of the hypercapnia episode (Moreira et al. 2006; Takakura & Moreira, 2011). Application of PPADS into the RTN region decreased the sympathetic-mediated pressure response to hypercapnia, suggesting a novel role of purinergic signalling in regulation of vascular tone during hypercapnia. For example, it is possible that CO2-evoked ATP release provides excitatory drive to the nearby RVLM/C1 presympathetic neurons. In the presence of PPADS this excitatory drive would be reduced and so potentially decrease sympathetic output. However, this possibility remains speculative and in need of further investigation.
The identity of P2 receptors expressed by CO2/H+-sensitive RTN neurons remains unknown. It was shown previously that P2Y1 receptors mediate the excitatory effects of ATP on neurons in the preBötzinger complex (Lorier et al. 2007, 2008). However, we found that P2Y1 receptors are not expressed by chemosensitive RTN neurons and do not contribute to CO2/H+ sensitivity of these cells in vitro or to CO2-induced changes in breathing or blood pressure in vivo. Therefore, P2Y1 receptors do not contribute to RTN chemoreception. However, activation of P2Y1 receptors in the RTN increased breathing and MAP, suggesting that P2Y1 receptors are expressed in the region and, when activated, can influence respiratory and sympathetic outflow. Considering that ADP not ATP is the preferential ligand for P2Y1 receptors, we speculate that ATP released during hypercapnia is not readily broken down to ADP and adenosine, thus limiting the contribution of these unknown P2Y1-expressing neurons to regulation of breathing or blood pressure. A focus of future work will be to determine the identity and function of these P2Y1-expressing RTN neurons. Nevertheless, the absence of an identifiable G-protein-coupled purinergic receptor on chemosensitive RTN neurons suggests that one or more P2X receptors may contribute to purinergic modulation of chemosensitive RTN neurons, possibly P2X1 and/or P2X3 (Gourine et al. 2010).
Requisite role of gap junction hemichannels in purinergic drive to RTN chemoreceptors
Our evidence that gap junction blockers (CBX and cobalt), but not synaptic block medium, decreased chemosensitive RTN neuronal activity by an amount proportional to inhibition by P2 receptor antagonists, suggests that the purinergic drive to RTN neurons is dependent on hemichannels. We consider it unlikely that communication between astrocytes via gap junctions is a major determinant of purinergic drive to neurons in this region for two reasons. First, RTN astrocytes are unusual in that they exhibit limited dye-coupling compared with astrocytes in other brain regions (Wenker et al. 2010), suggesting that these cells are not extensively gap junction coupled. In addition, previous evidence showed that cobalt preferentially blocks hemichannel current with little effect on transjunctional current through gap junctions in embryonic stem cells (Huettner et al. 2006). Our results are consistent with a recent in vivo study that showed that application of connexin hemichannel antagonists near the RTN inhibited CO2-evoked ATP release and decreased the whole animal ventilatory response to CO2 by ∼25% (Huckstepp et al. 2010b).
There is convincing evidence that CO2-evoked ATP release in the RTN is dependent on connexin 26 (Cx26) (Huckstepp et al. 2010a). The mechanism by which CO2 influences the gating of Cx26 hemichannels is not fully understood. Evidence suggests that molecular CO2 binds directly to Cx26 to increase the open probability of the hemichannel (Huckstepp et al. 2010a). However, it has also been shown that CO2/H+ sensitivity of RTN astrocytes is determined, in part, by inhibition of Kir4.1–Kir5.1 channels (Wenker et al. 2010), suggesting that membrane depolarization could trigger ATP release. This mechanism could be parsimonious with the current data considering that certain hemichannels exhibit voltage-dependent gating (Sáez et al. 2005). However, rat Cx26 does not demonstrate voltage dependence (Gonzalez et al. 2006); thus, if Cx26 is the main conduit for ATP release from RTN astrocytes then acid-induced changes in membrane potential might not contribute. Consistent with this possibility, a recent in vivo study reported that Kir5.1 knockout mice exhibit normal ventilatory response to CO2in vivo (Trapp et al. 2011), suggesting that astrocytic membrane depolarization is not required for CO2-evoked ATP release. However, it should be recognized that Kir5.1 channels are expressed in other organs, including the kidneys, and as described by Trapp et al. these conventional knockout mice have profound metabolic acidosis which could conceivably interfere with this acid-sensing mechanism. Thus, the link between CO2/H+-induced changes in astrocytic membrane potential and ATP release remains to be definitively established.
Role of astrocytes in RTN purinergic signalling
There is general agreement that astrocytes (or more precisely glial-like cells that express astrocytic markers) are the most likely source of ATP release (Gourine et al. 2010, Huckstepp et al. 2010b; Wenker et al. 2010). For example, Gourine and colleagues demonstrated, using astrocyte-specific expression of channel rhodopsin 2, a light-sensitive cation channel, that generating calcium waves in astrocytes results in ATP release that can activate RTN neurons in vitro and increase breathing in vivo (Gourine et al. 2010). Likewise, we have shown that fluorocitrate-mediated activation of astrocytes resulted in increased activity of chemosensitive RTN neurons by a purinergic-dependent mechanism (Wenker et al. 2010). In addition, Huckstepp and colleagues showed that hypercapnia opened connexin hemichannels on glial fibrillary acidic protein (GFAP)-expressing cells (Huckstepp et al. 2010a), thus suggesting that direct CO2 gating of connexin hemichannels results in ATP release from astrocytes. We show here that CO2 sensitivity of RTN neurons is fully retained in low Ca2+–high Mg2+ solution, thus further suggesting that that purinergic drive to RTN neurons is not dependent on excitatory synaptic transmission.
In summary, the RTN contains a population of CO2/H+-sensitive neurons that sends excitatory glutamatergic projections to respiratory centres to stimulate breathing (Mulkey et al. 2004) and modulate sympathetic drive to the heart and vasculature (Moreira et al. 2006; Takakura & Moreira, 2011). In addition to activation by CO2/H+, RTN neurons are also modulated by various neurotransmitters, including ATP (Mulkey et al. 2006; Gourine et al. 2010; Huckstepp et al. 2010b). Based on previous and present results, astrocytes (but not neurons) most probably mediate CO2-evoked ATP release by a mechanism involving gap junction hemichannels.
Acknowledgments
This work was supported by the National Institutes of Health Grant HL104101 (D.K.M.), American Heart Association grant 11PRE7580037 (I.C.W.) and The University of Connecticut Outstanding Graduate Student Fellowship (I.C.W.). This research was also supported by public funding from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grants 10/19336-0 (T.S.M.), 10/09776-3 (A.C.T.) and 11/13462-7 (C.R.S.).
Glossary
Abbreviations
- etCO2
end-expiratory CO2
- MAP
mean arterial pressure
- pFRG
parafacial respiratory group
- NA
phrenic nerve activity
- RTN
retrotrapezoid nucleus
Author contributions
I.C.W.: experimental design; collection and analysis of in vitro data; revising the manuscript. C.R.S.: collection and analysis of in vivo data; revising the manuscript. A.C.T.: experimental design; collection and analysis of in vivo data; revising the manuscript. T.S.M.: experimental design; collection, analysis and interpretation of in vivo data; revising the manuscript. D.K.M.: experimental design; data analysis; revising the manuscript; drafting the manuscript. All authors approved the final version.
References
- Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DL, Guyenet PG. Electrophysiological study of cardiovascular neurons in the rostral ventrolateral medulla in rats. Circ Res. 1985;56:359–369. doi: 10.1161/01.res.56.3.359. [DOI] [PubMed] [Google Scholar]
- Bukauskas FF, Elfgang C, Willecke K, Weingart R. Biophysical properties of gap junction channels formed by mouse connexin40 in induced pairs of transfected human HeLa cells. Biophys J. 1995;68:2289–2298. doi: 10.1016/S0006-3495(95)80411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez D, Gomez-Hernandez JM, Barrio LC. Species specificity of mammalian connexin-26 to form open voltage-gated hemichannels. FASEB J. 2006;20:2329–2338. doi: 10.1096/fj.06-5828com. [DOI] [PubMed] [Google Scholar]
- Gourine AV. On the peripheral and central chemoreception and control of breathing: an emerging role of ATP. J Physiol. 2005;568:715–724. doi: 10.1113/jphysiol.2005.095968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Atkinson L, Deuchars J, Spyer KM. Purinergic signaling in the medullary mechanisms of respiratory control in the rat: respiratory neurons express the P2X2 receptor subunit. J Physiol. 2003;552:197–211. doi: 10.1113/jphysiol.2003.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI. Phasic bursting activity of rat paraventricular neurones in the absence of synaptic transmission. J Physiol. 1982;327:273–284. doi: 10.1113/jphysiol.1982.sp014231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, Dale N. Redefining the components of central CO2 chemosensitivity – towards a better understanding of mechanism. J Physiol. 2011;589:5561–5579. doi: 10.1113/jphysiol.2011.214759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, Eason R, Sachdev A, Dale N. CO2-dependent opening of connexin 26 and related beta connexins. J Physiol. 2010a;588:3921–3931. doi: 10.1113/jphysiol.2010.192096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol. 2010b;588:3901–3920. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner JE, Lu A, Qu Y, Wu Y, Kim M, McDonald JW. Gap junctions and connexon hemichannels in human embryonic stem cells. Stem Cells. 2006;24:1654–1667. doi: 10.1634/stemcells.2005-0003. [DOI] [PubMed] [Google Scholar]
- Kang BJ, Chang DA, Mackay DD, West GH, Moreira TS, Takakura AC, Gwilt JM, Guyenet PG, Stornetta RL. Central nervous system distribution of the transcription factor Phox2b in the adult rat. J Comp Neurol. 2007;503:627–641. doi: 10.1002/cne.21409. [DOI] [PubMed] [Google Scholar]
- Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J Comp Neurol. 2009;517:69–86. doi: 10.1002/cne.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leybaert L, Braet K, Vandamme W, Cabooter L, Martin PE, Evans WH. Connexin channels, connexin mimetic peptides and ATP release. Cell Commun Adhes. 2003;10:251–257. doi: 10.1080/cac.10.4-6.251.257. [DOI] [PubMed] [Google Scholar]
- Lorier AR, Huxtable AG, Robinson DM, Lipski J, Housley GD, Funk GD. P2Y1 receptor modulation of the pre-Bötzinger complex inspiratory rhythm generating network in vitro. J Neurosci. 2007;27:993–1005. doi: 10.1523/JNEUROSCI.3948-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorier AR, Lipski J, Housley GD, Greer JJ, Funk GD. ATP sensitivity of preBötzinger complex neurones in neonatal rat in vitro: mechanism underlying a P2 receptor-mediated increase in inspiratory frequency. J Physiol. 2008;586:1429–1446. doi: 10.1113/jphysiol.2007.143024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WT. Supraoptic neurones of rat hypothalamus are osmosensitive. Nature. 1980;287:154–157. doi: 10.1038/287154a0. [DOI] [PubMed] [Google Scholar]
- Moreira TS, Takakura AC, Colombari E, Guyenet PG. Central chemoreceptors and sympathetic vasomotor outflow. J Physiol. 2006;577:369–386. doi: 10.1113/jphysiol.2006.115600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J Neurosci. 2006;26:7230–7233. doi: 10.1523/JNEUROSCI.1696-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Wenker IC. Astrocyte chemoreceptors: mechanisms of H+ sensing by astrocytes in the retrotrapezoid nucleus and their possible contribution to respiratory drive. Exp Physiol. 2011;96:400–406. doi: 10.1113/expphysiol.2010.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Fung ML, Li A, St John WM. Responses of respiratory modulated and tonic units in the retrotrapezoid nucleus to CO2. Respir Physiol. 1993;94:35–50. doi: 10.1016/0034-5687(93)90055-f. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. Postsynpatic mechanisms of CO2 responses in parafacial respiratory neurons of newborn rats. J Physiol. 2012;590:1615–1624. doi: 10.1113/jphysiol.2011.222687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signaling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci. 2008;28:4967–4973. doi: 10.1523/JNEUROSCI.5572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CD, Sercombe R. Calcium, magnesium and the electrical activity of guinea-pig olfactory coex in vitro. J Physiol. 1970;211:571–584. doi: 10.1113/jphysiol.1970.sp009294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus. Am J Physiol Regul Integr Comp Physiol. 2005;289:R851–R861. doi: 10.1152/ajpregu.00132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta. 2005;1711:215–224. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Colombari E, Menani JV, Moreira TS. Ventrolateral medulla mechanisms involved in cardiorespiratory responses to central chemoreceptor activation in rats. Am J Physiol Regul Integr Comp Physiol. 2011;300:R501–R510. doi: 10.1152/ajpregu.00220.2010. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS. Contribution of excitatory amino acid receptors of the retrotrapezoid nucleus to the sympathetic chemoreflex in rats. Exp Physiol. 2011;96:989–999. doi: 10.1113/expphysiol.2011.058842. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol. 2008;586:2975–2991. doi: 10.1113/jphysiol.2008.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Ralevic CA, Gadd CA, Spyer KM. Central CO2 chemoreception: a mechansim involving P2 purinoceptors localized in the ventrolateral medulla of the anaesthetized rat. J Physiol. 1999;517:899–905. doi: 10.1111/j.1469-7793.1999.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Spyer KM. ATP as a mediator of mammalian central CO2 chemoreception. J Physiol. 2000;523:441–447. doi: 10.1111/j.1469-7793.2000.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Tucker SJ, Gourine AV. Respiratory responses to hypercapnia and hypoxia in mice with genetic ablation of Kir5.1 (Kcnj16) Exp Physiol. 2011;96:451–459. doi: 10.1113/expphysiol.2010.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiunas V, Manthey D, Vogel R, Willecke K, Weingart R. Biophysical properties of mouse connexin30 gap junction channels studied in transfected human HeLa cells. J Physiol. 1999;519:631–644. doi: 10.1111/j.1469-7793.1999.0631n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Kreneisz O, Nishiyama A, Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol. 2010;104:3042–3052. doi: 10.1152/jn.00544.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston MC, Stornetta RL, Guyenet PG. Glutamatergic neuronal projections from the marginal layer of the rostral ventral medulla to the respiratory centers in rats. J Comp Neurol. 2004;473:73–85. doi: 10.1002/cne.20076. [DOI] [PubMed] [Google Scholar]
- Zhou M, Schools GP, Kimelberg HK. Development of GLAST(+) astrocytes and NG2(+) glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. J Neurophysiol. 2006;95:134–143. doi: 10.1152/jn.00570.2005. [DOI] [PubMed] [Google Scholar]


