Our preliminary phantom and in vivo animal model studies demonstrate that simultaneously administered pairs of contrast media with complementary x-ray attenuation ratios can be readily differentiated with current commercially available dual-energy CT scanners.
Abstract
Purpose:
To evaluate the feasibility of using a commercially available clinical dual-energy computed tomographic (CT) scanner to differentiate the in vivo enhancement due to two simultaneously administered contrast media with complementary x-ray attenuation ratios.
Materials and Methods:
Approval from the institutional animal care and use committee was obtained, and National Institutes of Health guidelines for the care and use of laboratory animals were observed. Dual-energy CT was performed in a set of iodine and tungsten solution phantoms and in a rabbit in which iodinated intravenous and bismuth subsalicylate oral contrast media were administered. In addition, a second rabbit was studied after intravenous administration of iodinated and tungsten cluster contrast media. Images were processed to produce virtual monochromatic images that simulated the appearance of conventional single-energy scans, as well as material decomposition images that separate the attenuation due to each contrast medium.
Results:
Clear separation of each of the contrast media pairs was seen in the phantom and in both in vivo animal models. Separation of bowel lumen from vascular contrast medium allowed visualization of bowel wall enhancement that was obscured by intraluminal bowel contrast medium on conventional CT scans. Separation of two vascular contrast media in different vascular phases enabled acquisition of a perfectly coregistered CT angiogram and venous phase–enhanced CT scan simultaneously in a single examination.
Conclusion:
Commercially available clinical dual-energy CT scanners can help differentiate the enhancement of selected pairs of complementary contrast media in vivo.
© RSNA, 2012
Introduction
Recent improvements in clinical dual-energy computed tomographic (CT) scanners have enabled the rapid and simultaneous acquisition of images at two different tube potentials (kilovolt peaks) in a single scan (1). These scanners use either two separate x-ray tube-detector arrays or a single x-ray tube that rapidly switches potential. A powerful benefit of dual-energy CT is the ability to determine the material composition of imaged objects. Individual materials have fixed ratios of their x-ray attenuation coefficients between high and low tube potentials (eg, 140 and 80 kVp), which is referred to herein as the attenuation ratio. In particular, soft tissue or water is readily differentiated from an iodinated contrast medium owing to the marked difference in their attenuation ratios.
Dual-energy material decomposition has been exploited for several single-agent iodinated contrast medium–enhanced clinical applications, including pulmonary perfusion imaging for improved detection of pulmonary emboli (2), dual-energy CT angiography to differentiate the lumina of vessels from adjacent densely calcified plaques that might have similar attenuation values (Hounsfield units) at conventional CT (3), and virtual unenhanced imaging performed by removing the attenuation due to a contrast medium (4). Dual-energy CT has also been used to differentiate uric acid deposits from calcium and to determine the composition of urinary stones (5,6).
A natural extension of this technology is the use of dual-energy CT to differentiate two or more simultaneously administered contrast media. This has been demonstrated in separate compartments in a phantom (7) and in a preserved mouse carcass with use of an experimental desktop spectroscopic scanner (8) but has not been shown in vivo with a clinical scanner. Notably, current clinically available contrast media for CT are based on iodine or barium, which are difficult to differentiate with clinical dual-energy CT scanners owing to the similarity in the attenuation ratios of these agents (8). However, other contrast medium combinations are amenable to differentiation. The purpose of our study was to evaluate the feasibility of using a commercially available clinical dual-energy CT scanner to differentiate the in vivo enhancement due to two simultaneously administered contrast media with complementary x-ray attenuation ratios.
Materials and Methods
B.M.Y. is a consultant for GE Healthcare; J.M., who has no direct relationship with GE Healthcare, had access to all data and control over which data were included in this study.
Contrast Media
Iodinated contrast media were used for all experiments. Contrast media with complementary attenuation ratios were chosen to satisfy the requirement of having a substantially different attenuation ratio from that of iodine (so as to minimize noise in material decomposition [9]) and, for in vivo experiments, if they were previously demonstrated to have an acceptable toxicity profile at enteric or intravenous administration.
Phantoms
Phantoms consisting of five different proportions (0:1, 1:3, 1:1, 3:1, 1:0 vol/vol) of two contrast medium solutions, 2% iohexol (Omnipaque 350; GE Healthcare, Princeton, NJ) and 58 mmol/L sodium tungstate solution (dihydrate, 99%; Sigma-Aldrich, St Louis, Mo), were prepared in 1.5-mL polypropylene tubes. These concentrations were selected to generate equal CT attenuation at (monoenergetic) 80 keV.
Animals
Approval from the institutional animal care and use committee was obtained, and National Institutes of Health guidelines for the care and use of laboratory animals were observed.
Two New Zealand white rabbits (weight, 3.7 and 4.6 kg) were anesthetized with 35 mg/kg ketamine (Ketaset, Fort Dodge Animal Health, Fort Dodge, Iowa) and 3 mg/kg xylazine hydrochloride (Spectrum, New Brunswick, NJ) and maintained under anesthesia with inhaled isoflurane (Summit Anesthesia Equipment, Foster City, Calif). Auricular vein catheters were placed. In the first rabbit, a 12-F pediatric feeding tube was passed into the stomach, approximately 120 mL of bismuth subsalicylate (Pepto-Bismol; Procter & Gamble, Cincinnati, Ohio) was injected as a bolus through the tube, and the tube was removed; approximately 45 minutes elapsed before imaging to allow time for transit of contrast medium into the small bowel. For the second rabbit, a tungsten cluster contrast medium was initially prepared according to the method of Bino et al (10). To improve solubility, the Na+ counter ion was replaced by a N-methyl-d-glucamine (NMG) ion with use of cationic ion-exchange chromatography, yielding the product NMG+[(W3O2)(acetylate)9]−. This was formulated as an injectable solution of 150 mg of tungsten per milliliter and sterile filtered (0.2 μm). The tungsten cluster contrast medium was hand injected intravenously over approximately 5 seconds approximately 10 seconds before iohexol injection so that the tungsten agent would be in the venous phase and the iodinated iohexol in the arterial phase at the time of subsequent CT. In both rabbits, iohexol (600 mg of iodine per kilogram body weight) was hand injected intravenously over approximately 5 seconds immediately before CT.
Imaging
Imaging of phantoms and animals was performed with a Discovery CT750 HD unit (GE Healthcare, Milwaukee, Wis) in dual-energy (GSI) mode. Phantoms were scanned by using protocol GSI-6 (medium filter, 1-second gantry rotation time, 40-mm collimation). The first rabbit was imaged with protocol GSI-11 (medium filter, 0.8-second rotation time, 40-mm collimation), and the second rabbit was imaged with protocol GSI-28 (medium filter, 0.8-second rotation time, 40-mm collimation). Scan data were postprocessed by using software (GSI Viewer, GE Healthcare). The “MD Analysis” application of this software was used to select the appropriate monochromatic energy level and material decomposition basis pair and generate virtual monochromatic images and material density maps. The current version of this software is limited to working with basis material definitions that do not have a k-edge between 40 and 140 keV (Saad Sirohey, PhD [GE Healthcare], written communication, June 2012). Because the attenuation ratio of tungsten is very similar to that of water, the water and/or iodine material decomposition basis was used for tungsten-iodine systems. For bismuth-iodine systems, an empirically determined pseudo-bismuth material with the same attenuation ratio as that of bismuth but with no k-edge was defined in the software and water and/or pseudo-bismuth was used as the decomposition basis. These substitute decomposition bases would cause systematic error in quantitative measurements of tungsten or bismuth concentration but do not substantially affect the appearance of the material density maps.
Results
Dual-energy CT images of the phantom vials containing different concentrations of tungsten and iodine are shown in Figure 1. Although the attenuation of the vials is similar on virtual monochromatic CT images, which simulate the appearance of conventional CT scans, the material density maps from the dual-energy CT scan demonstrate that each vial has a different concentration of tungsten and iodine.
Figure 1:
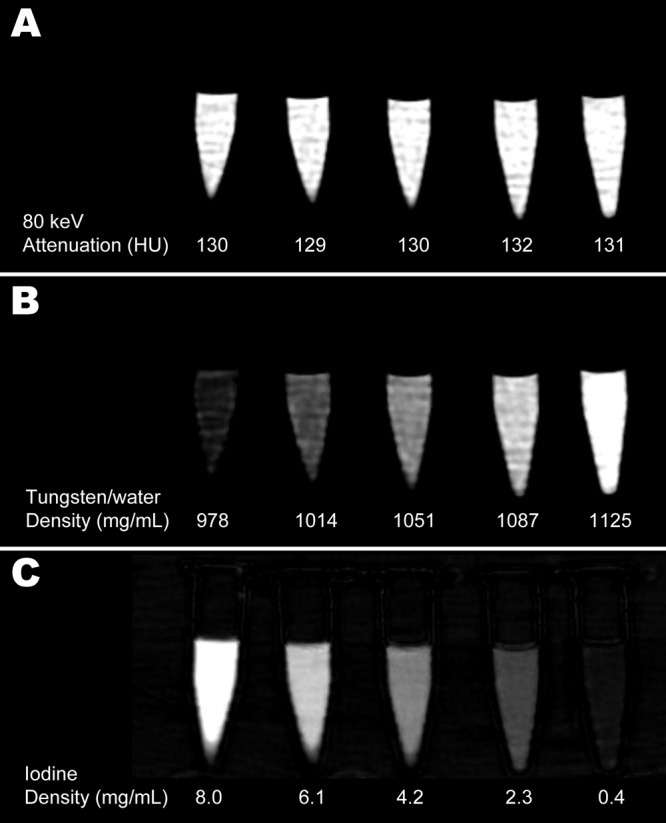
Images of tungsten-iodine solution phantoms rendered as, A, 80-keV virtual monochromatic scan, B, water and tungsten density map, and, C, iodine density map. The uniform CT attenuation across all phantoms seen on virtual monochromatic CT scan (A) is resolved at dual-energy CT into opposing gradients of tungsten and iodine contrast with a distinctly different attenuation in each phantom. Quantitative data are derived from dual-energy CT and represent mean attenuation at 80 keV (A), mean concentration of water that would produce attenuation equivalent to that due to water and tungsten in each phantom (B), and mean concentration of iodine in each phantom (C).
The in vivo dual-energy contrast-enhanced bowel and/or vascular CT images are shown in Figure 2. The virtual monochromatic image shows the appearance of a typical enteric and vascular contrast-enhanced study at conventional CT: High-attenuation contrast media opacify both the bowel lumen and the vasculature, and the two contrast media are difficult to differentiate except by inference and context. Notably, the extent of bowel wall enhancement due to iodinated intravascular contrast medium is obscured by enteric contrast medium where it has filled the bowel lumen. At dual-energy CT, the contrast media can be separated into iodine (intravascular) and bismuth (enteric intraluminal) material density maps. This separation enables bowel wall enhancement to be clearly identified despite the presence of the positive intraluminal bowel contrast medium.
Figure 2:
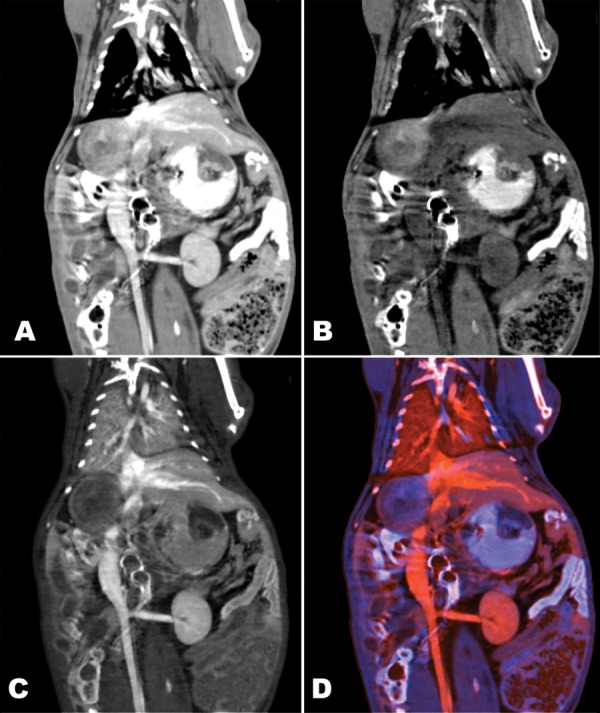
Coronal dual-energy thoracoabdominal images obtained in rabbit after administration of luminal enteric bismuth subsalicylate and iodinated vascular contrast material. Images are rendered as, A, 70-keV virtual monochromatic image, B, bismuth density map, C, iodine density map, and D, color composite of bismuth and iodine density maps. Note enhancement of small bowel wall visible on C and D but obscured by luminal contrast medium on A.
Dual intravascular contrast is depicted in Figure 3. The virtual monochromatic image shows contrast medium throughout the vasculature. The material density maps demonstrate separation of the two contrast media used for scanning. Tungsten contrast medium, which was injected first, provides a venous phase–enhanced image, opacifying both the veins and arteries. Iodinated contrast medium, which is in the arterial phase but has not yet enhanced the visceral parenchyma or veins, provides an uncontaminated angiogram. There is near-perfect coregistration between the angiogram and the venous phase image, as evidenced on the color composite image (Fig 3, D) by the absence of color fringing at the edges of structures such as the bones that appear on both material density maps.
Figure 3:
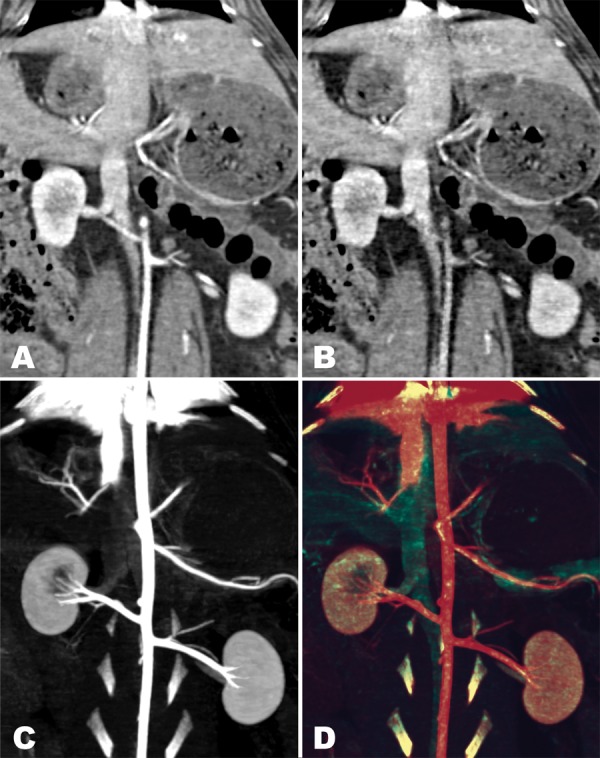
Coronal dual-energy abdominal CT images in rabbit examined simultaneously in the arterial phase with iodinated contrast medium and in the venous phase with a tungsten-based vascular contrast medium. Images are rendered as, A, 140-keV virtual monochromatic image, B, water and tungsten density map, C, maximum intensity projection iodine density map, and, D, color composite of maximum intensity projection tungsten and iodine density maps. Contrast media cannot be readily differentiated on image simulating conventional CT scan (A) but are clearly resolved as being in venous phase on B and in angiographic phase on C. Color composite image (D) demonstrates excellent registration between images in the two simultaneously acquired phases.
Discussion
Our preliminary phantom and in vivo animal model studies demonstrate that simultaneously administered pairs of contrast media with complementary x-ray attenuation ratios can be readily differentiated with current commercially available dual-energy CT scanners. As proof of principle, our in vivo studies showed that a contrast medium in the bowel lumen can be differentiated from an intravascular agent, as may be desired for CT enterography applications. We also showed that dual-energy CT may be used to image a pair of intravascular agents injected at different times so that they are in different phases at the time of scanning.
Two primary advantages of performing multiphase dual-contrast dual-energy CT rather than traditional single-contrast multiple-scan CT are reduced radiation dose and perfect image registration. Although a single dual-energy CT scan necessitates a slightly greater radiation dose than does a single conventional CT scan of equivalent image quality, it typically employs substantially less radiation than two scans (11), yielding a net reduction in radiation. A further drawback of conventional thoracoabdominal multiphase CT studies is that peristalsis and slight differences in positioning due to different depths of inspiration with breath holds result in visceral organs being in slightly different locations between the phases. Such differences complicate image registration, particularly for fine details. If multiple phases of enhancement are obtained simultaneously with dual-contrast dual-energy CT, such difficulties are eliminated.
The use of multiple simultaneously administered contrast media is a common practice with conventional CT but poses several problems. In particular, many radiologists are moving away from the simultaneous administration of positive oral and intravenous contrast media because oral contrast media obscure crucial assessments of bowel wall enhancement (12) and prevent meaningful volume-rendered or three-dimensional rendering of the abdominopelvic vasculature. However, dual-energy CT with an appropriately selected pair of contrast media preserves the diagnostic benefit of a positive bowel contrast medium without sacrificing evaluation of the vasculature and bowel mural enhancement. The ability to differentiate enteric from intravenous contrast media may also have a particular application in trauma imaging, where definitive identification of any free contrast medium as enteric or intravenous enables unequivocal diagnosis of hemorrhage or bowel perforation.
The key property of the contrast media pairs we selected is that they are distinguishable at dual-energy CT. This is determined by the difference between the ratios of the media’s high and low energy attenuation coefficients; the greater the difference, the more readily separable the materials are. The x-ray attenuation properties of a contrast medium are determined almost entirely by the heavy atom it incorporates. Three heavy atoms are used in contrast media in common clinical use: iodine, barium, and gadolinium. Iodine and barium have high, nearly identical, attenuation ratios and, therefore, cannot be effectively distinguished from each other. Gadolinium has a substantially lower attenuation ratio and can be differentiated from iodine or barium, but the x-ray attenuation of clinical formulations of gadolinium contrast media is substantially lower than that of iodinated media, so high doses are necessary for equivalent opacification. High-dose gadolinium is an unattractive option owing to the cost and increased risk of nephrogenic systemic fibrosis (13).
The heavy atoms used in this investigation were selected because they have low attenuation ratios; thus, they are readily separable from barium and iodine. Most heavy atoms have relatively high attenuation ratios, similar to that of iodine, because they have monotonically decreasing attenuation across the diagnostic range of x-ray energies (approximately 40–140 keV). The exceptions are elements that have a k-edge in this range. Because attenuation increases abruptly as energy increases above the k-edge, these elements have relatively increased attenuation at higher energy, resulting in lower attenuation ratios (14). From the candidates with appropriate attenuation properties, bismuth and tungsten were selected for their additional properties of low toxicity compared with other heavy metals (15) and low cost. Other investigators are developing tantalum-based contrast media (14,16) with x-ray attenuation characteristics that are very similar to those of tungsten.
Our proof of principle study demonstrates the simplest cases of multiple-contrast-media dual-energy CT: two contrast media with visual separation of the dual-energy CT scan data into two material density maps. Future studies may determine the accuracy of contrast medium quantification and whether dual-energy CT or spectral CT may provide reliable images of more than two contrast media (17).
Our study has several limitations. This was an exploratory study to show that in vivo dual-energy CT with two contrast media is feasible with a clinical dual-energy CT scanner. As such, the sample size was small. In addition, we did not attempt to determine the quantitative accuracy of dual-energy CT measurements of contrast media. The accuracy of dual-energy CT for contrast medium quantification remains under study. However, the vast majority of clinical CT focuses on qualitative rather than quantitative evaluations of contrast enhancement and anatomy, and so quantitative accuracy may not be crucial for clinical translation. Another limitation is that human subjects were not studied. Because the imaging required for the methods described herein can be performed with any of the several available models of clinical dual-energy CT scanners, the principal challenge in bringing these methods into clinical use in humans is further development of the necessary contrast media. Currently, no U.S. Food and Drug Administration–approved low-attenuation-ratio contrast media are available for human use.
In conclusion, our study shows that dual-energy CT with two contrast media can be used in vivo to provide high-spatial-resolution, near perfectly registered images of two distinct phases or locations of enhancement of thoracoabdominal visceral anatomy in a single scan. We believe that the results presented herein justify continued study and development of low-attenuation-ratio contrast media, and we are working to improve the toxicity profile of bismuth and tungsten-based contrast media to carry them forward into clinical usage.
Advances in Knowledge.
• Clinical dual-energy CT scanners can help differentiate two simultaneously administered contrast media with complementary x-ray attenuation coefficient ratios in vivo.
• Dual-energy CT with complementary oral and intravenous contrast media can depict bowel wall enhancement without interference from intraluminal bowel contrast material.
• Dual-energy CT with serially injected complementary intravenous contrast media can be used to acquire multiphase studies in a single scan.
Implication for Patient Care.
• Further development and U.S. Food and Drug Administration approval of contrast media with complementary x-ray attenuation ratios may enable x-ray dose reduction in multiphase studies and improved diagnosis in positive intraluminal bowel contrast studies.
Disclosures of Potential Conflicts of Interest: J.M. No potential conflicts of interest to disclose. S.R. No potential conflicts of interest to disclose. Y.F. No potential conflicts of interest to disclose. R.W. No potential conflicts of interest to disclose. E.F.J. No potential conflicts of interest to disclose. D.W.G. No potential conflicts of interest to disclose. B.M.Y. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a paid consultant for GE Healthcare and Siemens Medical Systems; institution received grants or has grants pending from GE Healthcare and Medrad; received payment for lectures including service on speakers bureaus from GE Healthcare. Other relationships: none to disclose.
Acknowledgments
The authors are grateful for the assistance of Hing Hung, AS, for assistance with CT scanner operation.
Received March 23, 2012; revision requested May 11; revision received June 3; accepted June 7; final version accepted June 12.
Funding: This research was supported by the National Institutes of Health (grants R01CA122257 and R21EB013816). J.M. is supported by NIBIB T32 training grant 1 T32 EB001631. S.R. is a Howard Hughes Medical Institute Medical Research Fellow.
References
- 1.Fleischmann D, Boas FE. Computed tomography—old ideas and new technology. Eur Radiol 2011;21(3):510–517 [DOI] [PubMed] [Google Scholar]
- 2.Lu GM, Wu SY, Yeh BM, Zhang LJ. Dual-energy computed tomography in pulmonary embolism. Br J Radiol 2010;83(992):707–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran DN, Straka M, Roos JE, Napel S, Fleischmann D. Dual-energy CT discrimination of iodine and calcium: experimental results and implications for lower extremity CT angiography. Acad Radiol 2009;16(2):160–171 [DOI] [PubMed] [Google Scholar]
- 4.Yeh BM, Shepherd JA, Wang ZJ, Teh HS, Hartman RP, Prevrhal S. Dual-energy and low-kVp CT in the abdomen. AJR Am J Roentgenol 2009;193(1):47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitcheson HD, Zamenhof RG, Bankoff MS, Prien EL. Determination of the chemical composition of urinary calculi by computerized tomography. J Urol 1983;130(4):814–819 [DOI] [PubMed] [Google Scholar]
- 6.Graser A, Johnson TR, Bader M, et al. Dual energy CT characterization of urinary calculi: initial in vitro and clinical experience. Invest Radiol 2008;43(2):112–119 [DOI] [PubMed] [Google Scholar]
- 7.Qu M, Fletcher J, Huprich J, et al. Towards bi-phasic CT oral contrast: material classification of luminal BISMUTH and mural iodine in a small bowel phantom at dual-energy CT (DECT) [abstr]. In: Radiological Society of North America Scientific Assembly and Annual Meeting Program. Oak Brook, Ill: Radiological Society of North America, 2010; 311 [Google Scholar]
- 8.Anderson NG, Butler AP, Scott NJ, et al. Spectroscopic (multi-energy) CT distinguishes iodine and barium contrast material in MICE. Eur Radiol 2010;20(9):2126–2134 [DOI] [PubMed] [Google Scholar]
- 9.Kelcz F, Joseph PM, Hilal SK. Noise considerations in dual energy CT scanning. Med Phys 1979;6(5):418–425 [DOI] [PubMed] [Google Scholar]
- 10.Bino A, Cotton FA, Dori Z, et al. A new class of trinuclear tungsten (IV) cluster compounds with tungsten-tungsten single bonds. Inorg Chem 1978;17(11):3245–3253 [Google Scholar]
- 11.Li B, Yadava G, Hsieh J. Quantification of head and body CTDI(VOL) of dual-energy x-ray CT with fast-kVp switching. Med Phys 2011;38(5):2595–2601 [DOI] [PubMed] [Google Scholar]
- 12.Jancelewicz T, Vu LT, Shawo AE, Yeh B, Gasper WJ, Harris HW. Predicting strangulated small bowel obstruction: an old problem revisited. J Gastrointest Surg 2009;13(1):93–99 [DOI] [PubMed] [Google Scholar]
- 13.Sieber MA, Pietsch H, Walter J, Haider W, Frenzel T, Weinmann HJ. A preclinical study to investigate the development of nephrogenic systemic fibrosis: a possible role for gadolinium-based contrast media. Invest Radiol 2008;43(1):65–75 [DOI] [PubMed] [Google Scholar]
- 14.Bonitatibus PJ, Torres AS, Goddard GD, FitzGerald PF, Kulkarni AM. Synthesis, characterization, and computed tomography imaging of a tantalum oxide nanoparticle imaging agent. Chem Commun (Camb) 2010;46(47):8956–8958 [DOI] [PubMed] [Google Scholar]
- 15.Koutsospyros A, Braida W, Christodoulatos C, Dermatas D, Strigul N. A review of tungsten: from environmental obscurity to scrutiny. J Hazard Mater 2006;136(1):1–19 [DOI] [PubMed] [Google Scholar]
- 16.Oh MH, Lee N, Kim H, et al. Large-scale synthesis of bioinert tantalum oxide nanoparticles for x-ray computed tomography imaging and bimodal image–guided sentinel lymph node mapping. J Am Chem Soc 2011;133(14):5508–5515 [DOI] [PubMed] [Google Scholar]
- 17.Mendonça PR, Bhotika R, Maddah M, et al. Multi-material decomposition of spectral CT images. Proc SPIE 2010;7622:76221W [Google Scholar]


