Abstract
Mounting evidence indicates that T2-weighted images can be used not only to measure the area at risk but also to refine our understanding of gadoliniumenhanced myocardium in the setting of acute myocardial infarction, as well as the pathophysiology of acute myocardial infarction in general.
Introduction
T2-weighted imaging in the assessment of magnetic resonance (MR) imaging of myocardial area at risk has recently generated a great deal of interest but has also caused substantial controversy. The area at risk (1) represents the amount of myocardium that is hypoperfused during coronary occlusion (Fig 1). Since the infarct is a subset of the area at risk, the difference between the two measures is the amount of myocardium salvaged, which is a measure of therapeutic efficacy.
Figure 1:
Diagram shows that an image of the area at risk (black) and infarct size (white) can be used to determine the amount of myocardial salvage (blue). Red circle represents short axis of left ventricle, and gray circle represents blood within the cavity. (Adapted and reprinted, with permission, from reference 1.)
The purpose of this article is to discuss the role of T2-weighted MR imaging in assessment of the area at risk in patients with acute myocardial infarction. Some investigators have raised doubts about the veracity and feasibility of using T2-weighted images to measure the area at risk. However, mounting evidence indicates that T2-weighted images can be used not only to measure the area at risk but also to refine our understanding of gadolinium-enhanced myocardium in the setting of acute myocardial infarction, as well as the pathophysiology of acute myocardial infarction in general.
Motivation for Finding an MR Imaging Method to Determine Area at Risk and Myocardial Salvage
While there are a number of clinical cardiac applications that benefit from T2-weighted imaging, the concept that T2-weighted images could delineate the area at risk associated with acute myocardial infarction stimulated a remarkable academic response. The work of Aletras et al (2) has now been cited more than 156 times in slightly more than 5 years since its publication, as shown by a Web of Science citation search in November 2011. The relationship between edema and abnormal tissue T2 was first reported in 1975 (3). Although investigators recognized the association between acute myocardial infarction and T2 abnormalities in the myocardium as early as 1983 (4), the purpose of much of the early work was to find nonenhanced MR imaging methods suitable for use in the diagnosis of acute myocardial infarction. Most of that work did not incorporate measurement of the area at risk (5). The link between T2-weighted MR imaging and the area at risk was described with ex vivo imaging in 1993 (6) and with in vivo imaging in 2006 (2).
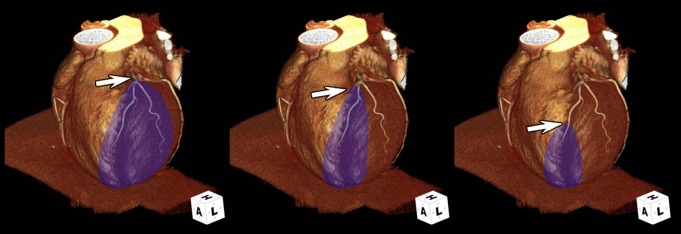
There is great interest in finding more effective ways to treat acute myocardial infarction (7). Testing efficacy of cardioprotective agents in animal studies generally requires measurement of infarct size and area at risk to calculate the amount of myocardial salvage (Fig 1). The area at risk is the major determinant of the upper size limit of the myocardial infarction. Ultimately, the infarct will be no larger than the amount of myocardium that is downstream from a coronary occlusion; however, the infarct might be much smaller if treatment is able to salvage myocardium. As shown in Figure 2, there is substantial variability in the size of the area at risk due to the location of the occlusion within the infarct-related artery (8). Other studies have highlighted the importance of collateral blood flow and residual coronary blood flow in modulating infarct size (9).
Figure 2:
Location of thrombosis is a major determinant of area at risk (purple) and maximal possible infarct size. Three-dimensional computed tomographic (CT) coronary angiograms show the location of coronary occlusion (arrows) relative to major side branches has a substantial effect on area at risk size.
Nuclear perfusion imaging has been used to measure infarct size in more than 30 clinical trials, but it is less commonly used to measure both infarct size and area at risk (10). Thus, in most clinical trials in which nuclear imaging is used, researchers have not determined myocardial salvage. In the Acute Myocardial Infarction Study of Adenosine (AMISTAD) trial, which was the pilot study for one of the largest cardioprotection clinical trials to date, researchers attempted to use a single photon emission computed tomography (SPECT) study performed in the emergency department to measure area at risk and a second SPECT study to measure infarct size in a trial of adenosine as a cardioprotective agent in patients with acute myocardial infarction (11). SPECT area at risk measurement was not feasible in the pilot study, so the investigators chose to use only infarct size as an outcome measure in the main AMISTAD II clinical trial (12). In the AMISTAD II study, researchers failed to detect a 37% relative reduction in infarct size (27% of the left ventricle in the control group vs 17% of the left ventricle in the pooled adenosine group, P = .074) (12). One can only wonder if accurate measurement of infarct size and area at risk could have helped detect a therapeutic benefit of adenosine in this cardioprotection trial.
Since MR imaging is already widely accepted as the highest-spatial-resolution measure of infarct size and is feasible in many patients with acute myocardial infarction (13), there is substantial motivation to find an MR imaging method that can be used to measure area at risk. Area at risk measurement would make possible a clinical equivalent to the basic scientists’ way of determining myocardial salvage. Ultimately, we cannot currently afford to conduct large acute myocardial infarction clinical trials without sufficient and promising preliminary data. Perhaps pilot clinical trials in which myocardial salvage is detected could become an evaluation criterion prior to funding major clinical trials in this area.
Pathophysiologic Basis of Myocardial Edema in the Area at Risk and the Relationship to Hyperintense Signal on T2-weighted Images
Myocardial edema is a fundamental response to ischemia reperfusion injury in the heart (14,15). Inflammatory responses are a second common mechanism for tissue edema. The inflammatory mechanism tends to peak in the first 48–72 hours depending on the presence or absence of ongoing inciting mechanisms. Intramyocardial hemorrhage also leads to tissue swelling. Thus, at least three factors contribute to myocardial edema after acute myocardial infarction. Higgins et al (4) related their experience with myocardial T2 measured by using MR imaging versus infarct-related myocardial edema measured by using the wet-dry weight ratio.
Myocardial edema occurs early in the course of myocardial ischemia (16), a fact that was misquoted in an editorial by Klem and Kim (17) that was critical of imaging the area at risk. Ischemia-related edema is visible with MR imaging in stunned myocardium in the absence of myocardial necrosis (18). Bragadeesh et al (19) concluded that myocardial edema could be an important contributor to the mechanism of myocardial stunning if the spacing of the contractile elements increased due to intracellular edema and provided ultrastructural analysis to support these findings. Thus, the relationship between ischemia and myocardial edema is well documented by a number of laboratories in which a variety of methods were used.
Edema has higher signal intensity on T2-weighted images than does normal myocardium (Fig 3). However, intramyocardial hemorrhage causes decreased signal intensity on T2*- and T2-weighted images (20). Thus, the core of an infarct may have lower signal intensity than the other portions of the area at risk. From a signal intensity perspective, a darker core within a brighter hyperintense T2 abnormality is analogous to the way microvascular obstruction appears dark within the core of a bright infarct on late gadolinium-enhanced images. Clinicians using either T2-weighted images or gadolinium-enhanced images of myocardial infarction need to be aware of factors that modulate signal intensity on either type of image and correct for these factors in quantitative analyses.
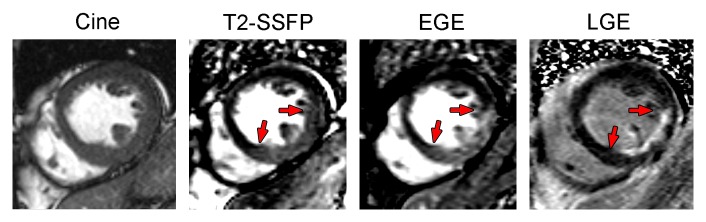
Figure 3:
Examples of the relationship between T2-weighted area at risk, zone of early gadolinium enhancement (EGE), and infarct size, as determined with late gadolinium enhancement (LGE) in a patient with an acute inferior ST-elevation myocardial infarction treated successfully with percutaneous intervention. The door to balloon time was 1.6 hours, peak troponin I level was 115 μg/L, and MR imaging was performed 3 days after myocardial infarction. MR images were acquired with the following protocols: steady state free-precession (SSFP) cine sequence (repetition time msec/echo time msec, 2.96/1.23); T2-prepared steady state free-precession sequence (60-msec T2 preparation, 3.2/1.6); early gadolinium-enhanced inversion recovery single-shot steady state free-precession sequence (2.9/1.46); late gadolinium-enhanced inversion-recovery gradient-echo sequence (8.7/4.2).
Methods to Assess the Accuracy or Effectiveness of Imaging the Area at Risk
The following three pathologic analysis techniques are commonly used to measure area at risk and serve as reference standards: (a) tissue-staining dyes injected at the end of an experiment during reocclusion of the coronary artery, (b) microparticles injected during reocclusion, and (c) 15-μm microspheres injected during occlusion. We have used all three of these methods in our experiments (2,5,21,22), but we favor injection of microspheres for the following reasons: Dyes injected in vivo at the end of an experiment can alter tissue color enough to interfere with some postmortem histopathologic analyses, and they tend to obliterate details near the edges of the perfusion defect. For example, Reimer and Jennings (14) used some slices to assess area at risk and used other slices for histopathologic analysis and specifically excluded the edge sectors “to avoid cross-contamination of ischemic and nonischemic samples.” The over- and underestimation of perfused dye infusions depends on the level of residual perfusion and whether all coronary arteries were perfused under equal and physiologic pressure. The osmolality of the dye solutions must be carefully controlled to avoid inducing edema or drawing water out of the tissue.
Fluorescent microparticles that are large enough to be seen on cut slices of the heart can cause microinfarcts and may have indistinct borders around the area at risk (22,23). The indistinct edges are the result of low relative density of particles. These edge effects are visible in the green-yellow portions of images acquired with this technique (22,23). Fluorescent microspheres must be injected at the end of an experiment during reocclusion; therefore, precise repositioning of the occluding device is required to accurately delineate the area at risk.
When large numbers (1–5 million microspheres for dogs weighing 20 kg) of 15-μm microspheres are injected, myocardial blood flow and area at risk can be quantified down to subgram regions of interest (half gram sections in a 0.5-g heart provide a reference standard equivalent to 1% of the myocardium) (2,24). Furthermore, microspheres enable a reference standard measurement of the severity of occlusion (25), the amount of collateral flow, and the adequacy of reperfusion (assuming injections are performed before, during, and after occlusion). Microspheres do not interfere with histologic analysis. However, interpretation of microspheres in chronic experiments must account for the amount of edema and tissue swelling that occurs during the postinfarct period (14).
Human studies of area at risk are currently limited to SPECT, angiography, and MR imaging. Injection of sestamibi during coronary occlusion provides an excellent measure of the area at risk, as long as the coronary artery is completely occluded and the patient can undergo imaging within 4–6 hours after injection. Angiographic measures of the area at risk generally are based on splitting the coronary artery tree into different segments based on the location of a lesion along the artery and the number and/or size of side branches. These angiographic estimates of area at risk make sense but require assumptions and modeling to translate to the coronary anatomy into grams of myocardium in the perfusion bed. Nonetheless, the angiographic measures are feasible since a high fraction of patients with acute ST-segment elevation myocardial infarction (STEMI) undergo percutaneous coronary intervention. Other measures, such as Thrombolysis in Myocardial Infarction Trial flow grade (26) and currently available serum biomarkers, cannot be used to measure area at risk.
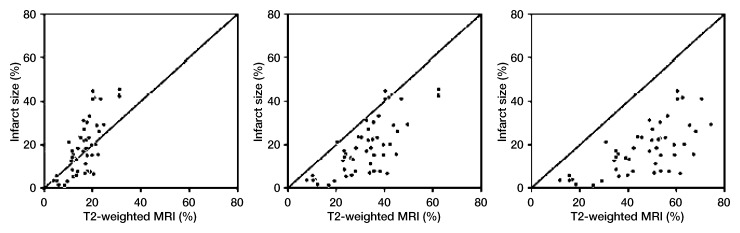
Beyond correlation with a reference standard, there are measures of internal consistency that provide much insight with regard to the validity of an area-at-risk measurement. As depicted in Figure 4, the relationship between an estimate of area at risk and infarct size has specific patterns associated with accurate estimation of area at risk, as well as over- or underestimation of area at risk. If T2-weighted imaging led to underestimation of the area at risk, many infarcts would be larger than the line of identity on this type of plot. If T2-weighted imaging led to overestimation of the area at risk, no transmural myocardial infarctions would be plotted above the line of identity, and there would be a gap separating the most complete infarcts from the line of identity. In a case where the area at risk is accurately measured, the most transmural infarcts should approach the line of identity as seen in the actual clinical trial.
Figure 4:
Scatterplots show patterns expected when a measure of area at risk results in underestimation (left), overestimation (right), or correct estimation (middle) of the size of the area at risk, with area at risk as a constraint of the upper limit of infarct size. These images represent the relationship between infarct size and the T2-weighted MR imaging estimate of area at risk. Line of identity is plotted to depict the case where the infarct filled the entire area at risk. Cases where there is some myocardial salvage result in a point below the line of identity (infarct is smaller than area at risk). There is no physiologic case where the infarct is expected to be larger than the area at risk (points above the line of identity). These data are replotted from Berry et al (27), scaling the actual area at risk data to represent 50% over- or underestimation of area at risk. Note that degrees of over- and underestimation of area at risk were scaled based on the results of Mewton et al (28).
Evidence That T2-weighted Images Portray the Area at Risk
Garcia-Dorado et al (6) concluded that T2-weighted images represent the area at risk and confirmed a tight relationship between myocardial T2 and tissue water content. Aletras et al (2) showed that high-quality in vivo T2-weighted images enabled measurement of the area at risk and compared well with microspheres. Furthermore, myocardial salvage was associated with partial recovery of function, as expected for subendocardial infarcts. One of the most important realizations was that MR imaging performed approximately 2 days after infarction could depict the area at risk, infarct size, and myocardial salvage.
In consideration of the fact that the extent of edema might be substantially altered based on the presence or absence of reperfusion, Tilak et al (5) reported that T2-weighted images correspond to the area at risk in a canine model of nonreperfused infarcts by using first-pass perfusion images obtained during coronary occlusion as a reference standard. More recently, Ugander et al (29) validated precontrast T1- and T2-weighted images as measures of the area at risk against whole-heart microsphere reference standards. In that study, the approximately 50-g left ventricles were divided into approximately 160 sectors for analysis, thereby yielding microsphere flow information in sectors equivalent to about 0.3-g tissue samples for a finer perfusion resolution than in any of our prior studies.
In humans, a key prior study noted that T2-weighted images could be used to differentiate acute from chronic myocardial infarction (30). Furthermore, Berry et al (27) showed that T2-weighted images of human acute myocardial infarctions had hyperintense regions in the distribution of the culprit coronary artery and that the hyperintense zones were typically transmural in extent. Thus, the T2-weighted hyperintense zones were distinctly different from at least the subendocardial late gadolinium enhancement patterns. Friedrich et al (31) recognized that T2-weighted images could be used to image salvaged myocardium in humans. Carlsson et al (32) used sestamibi injected prior to acute percutaneous coronary intervention and imaged with SPECT to validate T2-weighted images with regard to determination of area at risk and found excellent correlation. They also determined that the T2-weighted area at risk measurement was similar on days 1–7 after acute myocardial infarction but then decreased with time over the next 6 months.
Berry et al (27) compared new bright-blood T2-weighted methods with angiographic area at risk indexes in 50 patients with recent acute myocardial infarction. T2 area at risk correlated with the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (or APPROACH) score (33), with a bias of about 2.5% at Bland-Altman analysis. Neither Duke Jeopardy score nor ST elevation score performed as well. Thrombolysis in Myocardial Infarction (26) flow grade at the end of angiography or percutaneous coronary intervention was predictive of MR imaging–determined myocardial salvage. T2-weighted area at risk was predictive of the upper limit of infarct size, as expected for a measure of area at risk. Thus, this study looked at a wide range of independent clinical measures that should relate to the area at risk and had multiple positive findings.
Myocardial Edema Imaging Is Revising Our Understanding of Gadolinium-enhanced Imaging of Acute Myocardial Infarction
While detractors have suggested that use of T2-weighted images of area at risk is a risky business (34), in reality, area-at-risk research with T2-weighted imaging has helped refine our understanding of gadolinium enhancement of the heart. The field has benefited from but also been hampered by a decade-old thesis about gadolinium enhancement of the heart that proclaims, “Bright is dead!” The myocardium that appears bright on late gadolinium-enhanced images is infarcted, while the myocardium that is nulled or dark is interpreted to represent viable myocardium. Thus, the late gadolinium-enhanced images depict both viable and nonviable myocardium based on simple signal intensity differences. This theme was substantiated by a series of high-quality publications (35–43) that changed the field and put gadolinium-enhanced viability and myocardial infarct imaging at the forefront of imaging. However, gadolinium kinetics were neglected. Recent research on MR imaging of the area at risk (44) has raised new concerns about how and when we should perform late gadolinium-enhanced imaging of the heart (45). Thus, the new concept is that T2-weighted images have been instrumental in understanding why early gadolinium-enhanced images represent the area at risk while late gadolinium-enhanced images can represent the size of the myocardial infarction.
History provides many clues about the importance of kinetics in the interpretation of gadolinium-enhanced imaging of the heart. For example, Kim et al (46) found that there is a period of time where the rim around the infarct enhanced to a greater degree than the remote normal myocardium or the core of the infarct. Other groups observed that what we now recognize as microvascular obstruction was a more important predictor of the recovery of function after myocardial infarction than simple gadolinium enhancement (47). Microvascular obstruction appears as a dark zone in the core of the infarct and is caused by severe residual perfusion defects. Incidentally, the images were obtained early after injection of contrast material and would now be described as early gadolinium enhancement, not late gadolinium enhancement. Thus, it is not surprising that such images might lead to overestimation of infarct size and incorrect prediction of recovery of function.
Oshinski et al (48) found that gadolinium enhancement led to overestimation of infarct size for 20–30 minutes in a rat model of acute myocardial infarction. However, enthusiasm for this work was suppressed by an eloquent but ultimately incorrect editorial (49). Judd and Kim (49) summarized the rat study with the following crushing comment: “The changes in size of the hyperenhanced regions observed by Oshinski et al, however, were likely caused by an incorrect implementation of the MRI technique.”
It is time to get beyond the editorial sword and recognize that kinetics are important in imaging acute myocardial infarction and that research on area at risk imaging is helping us understand the pathophysiology of early versus late gadolinium enhancement. Matsumoto et al (44) found that early gadolinium-enhanced images highlight the area at risk, while late gadolinium-enhanced images highlight the infarct. While the image quality was fair, the data analysis was clear. We were impressed that we could immediately confirm the results on images we obtained (Fig 3). Also in support of these conclusions, cine imaging with a combination of T2 and T1 weighting early after gadolinium enhancement can depict the area at risk (50). How did these authors prove that the early gadolinium-enhanced images represented area at risk rather than infarct size? They used T2-weighted images to correlate with the early gadolinium-enhanced images. They also showed that the late gadolinium-enhanced images had much smaller hyperintense zones than did the early gadolinium-enhanced images, as one would expect for area-at-risk measures compared with infarct size images. For T2-weighted imaging to help readjust our understanding of what had been a dogmatic teaching about gadolinium enhancement is an impressive testament that the T2-weighted images contain important pathophysiologic information about myocardial edema.
Critical Reading of the Literature Regarding T2-weighted Estimates of the Area at Risk
While many groups have found positive results related to imaging the area at risk, others have raised concerns about many aspects of this imaging. One should not underestimate the difficulties associated with this type of imaging, as there are relatively subtle differences in signal intensity between the area at risk and the remote myocardium. At the same time, some of the questions raised in recent editorials and reviews are off target or based on imprecise or incorrect summary of the existing validation studies. To highlight some of these issues, a number of questions are posed and addressed in the following section.
Is a Plot of Infarct Size versus Area at Risk a Legitimate Validation?
Conceptually, the relationship between area at risk and infarct size is fundamental—by definition, the area at risk must be larger than the infarct size. Friedrich et al (51) editorialize with negative overtones about this method and analysis. However, the comparison of area at risk and infarct size is a method that is sensitive to both over- and underestimation of area at risk by using data from a recent clinical trial (Fig 4) (27). If an experimental measure results in underestimation of the area at risk, some infarcts will be larger than the line of identity. If the experimental image results in overestimation of the area at risk, there will be a gap with no infarcts between the line of identity and the individual data points. As shown by this plot, the data of Berry et al (27) satisfy this important criterion in addition to the many other positive findings summarized earlier.
Is Endocardial Surface Area a Better Measure of the Area at Risk than T2?
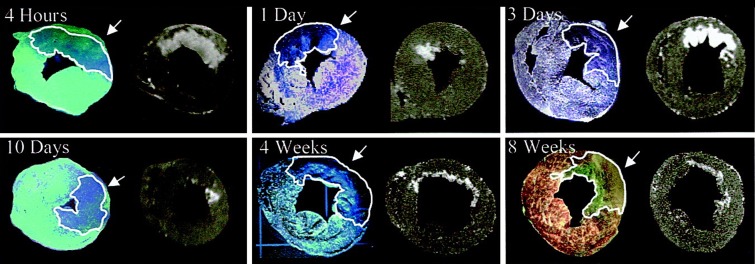
The relationship between the transmural extent of infarction and the wavefront of ischemia that starts in the endocardium serves as the basis for using the endocardial surface area (ESA) of infarction to estimate the area at risk (1,52,53). In the case of extensive and nearly transmural myocardial infarction, the ESA subtends the area at risk. However, in the case of small myocardial infarcts, the ESA clearly leads to underestimation of the area at risk. Fieno et al (23) showed images obtained in seven experiments, and ESA would obviously lead to underestimation of area at risk in three (Fig 5). Ubachs et al (54) found that ESA led to underestimation of area at risk in humans against a reference standard SPECT study. In a head-to-head comparison, T2 correlated better with angiographic indexes of area at risk than did ESA versus the angiographic area at risk (55). T2 also performed better than ESA and angiographic indexes in a study by Versteylen et al (56). Thus, while the ESA performs well in studies dominated by nearly transmural myocardial infarctions, it is a method that can lead to underestimation of the cases of most dramatic salvage—a bias that could influence the outcome of a clinical trial. One cannot afford to miss the best cases of salvage.
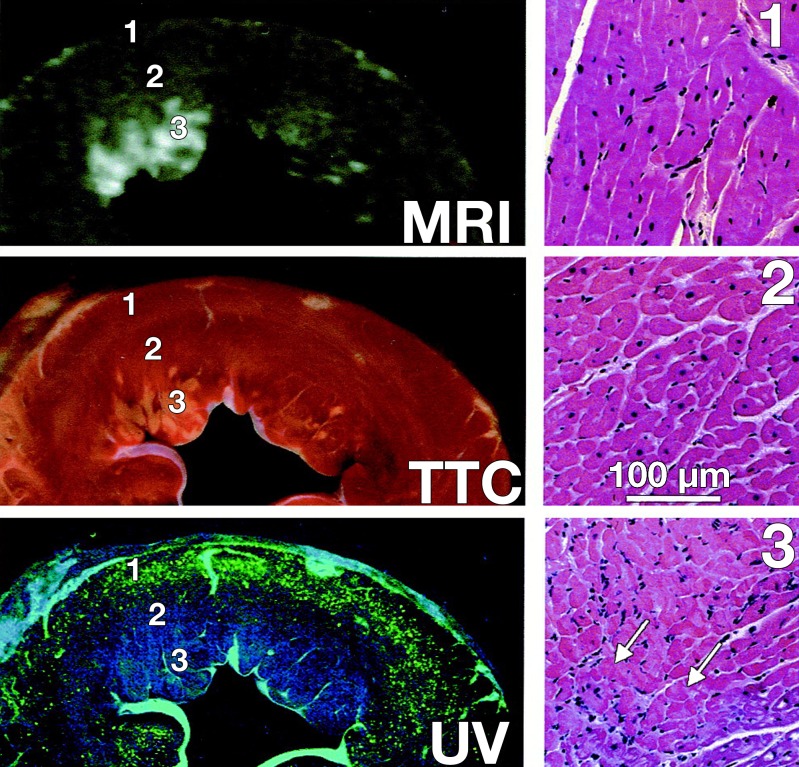
Figure 5a:
Endocardial extent of infarction does not delineate the extent of area at risk in three of the seven examples of Fieno et al (23). (a) Fluorescence images (left) paired with corresponding late gadolinium-enhanced images (right) selected to represent different time points after myocardial infarction. Regions of interest (white outlines) and arrows indicate area at risk. Note the marked discrepancies between area at risk and endocardial extent of infarction at 1 day and 10 days. (b) Left: Comparison of MR imaging hyperenhancement (top), triphenol tetrazolium chloride (TTC) staining (middle) and myocardium at risk (bottom) (region without fluorescent microparticles) in an animal with a 1-day-old reperfused infarction. UV = ultraviolet. Right: Light microscopy views of regions 1 (not at risk, not infarcted), 2 (at risk but not infarcted), and 3 (infarcted) are shown on the right panels. Arrows = contraction bands. MR imaging infarct size and negative triphenol tetrazolium chloride staining zone have a smaller endocardial extent than does the area at risk depicted by pale blue patch on the fluorescent microparticles. (Reprinted, with permission, from reference 23.)
Figure 5b:
Endocardial extent of infarction does not delineate the extent of area at risk in three of the seven examples of Fieno et al (23). (a) Fluorescence images (left) paired with corresponding late gadolinium-enhanced images (right) selected to represent different time points after myocardial infarction. Regions of interest (white outlines) and arrows indicate area at risk. Note the marked discrepancies between area at risk and endocardial extent of infarction at 1 day and 10 days. (b) Left: Comparison of MR imaging hyperenhancement (top), triphenol tetrazolium chloride (TTC) staining (middle) and myocardium at risk (bottom) (region without fluorescent microparticles) in an animal with a 1-day-old reperfused infarction. UV = ultraviolet. Right: Light microscopy views of regions 1 (not at risk, not infarcted), 2 (at risk but not infarcted), and 3 (infarcted) are shown on the right panels. Arrows = contraction bands. MR imaging infarct size and negative triphenol tetrazolium chloride staining zone have a smaller endocardial extent than does the area at risk depicted by pale blue patch on the fluorescent microparticles. (Reprinted, with permission, from reference 23.)
Inaccurate Critique of Published Work
Friedrich et al (51) misquoted Berry et al and inappropriately discredited their work when they wrote, “Therefore it is puzzling that Berry et al, with bright blood T2 techniques, report similar amounts of substantial salvage for both transmural and nontransmural infarction.” It would seem that Friedrichet al (51) confused salvage associated with STEMI versus that not associated with STEMI (table 3 in reference 46) as transmural versus nontransmural. The amount of myocardial salvage is determined not with the echocagrdiographic characterization of infarction but with the duration of ischemia (53). One can see dramatic salvage with early intervention for STEMI. Importantly, STEMI is not the same as transmural myocardial infarction. The pattern of misquotation and misrepresentation of recent literature is disturbing.
There Are Problems and Limitations of Current Standard Deviation–based Thresholds Relative to Normal or Remote Myocardium
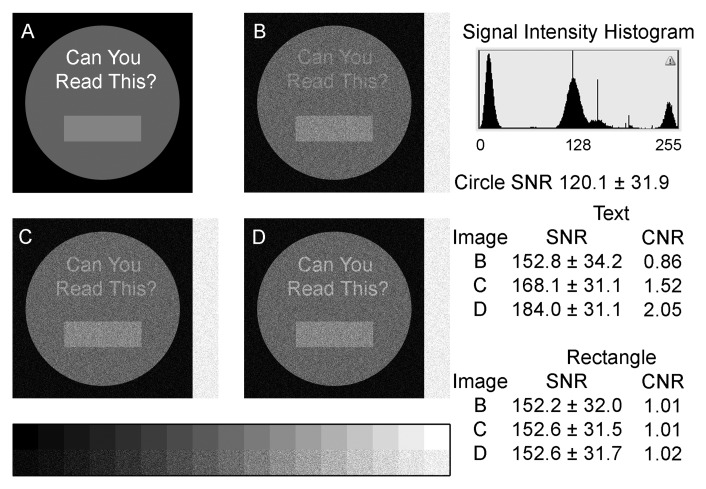
There are real and important problems with respect to how to quantify area at risk on T2-weighted images. However, real information can be perceived on images where the contrast-to-noise ratio is within 1–1.5 standard deviations of background signal intensity (Fig 6b, 6c) depending on some factors, such as the size of the defect. It is already well documented (Fig 7) that similar problems in late-gadolinium-enhancement imaging lead to overestimation of infarct size in animal and human MR imaging studies (57,58). The teaching point is that conspicuity of real details becomes possible well below the commonly used threshold of two standard deviations. These effects can lead to visual overestimation of the extent of hyperintense regions on an image. Studies that rely on human planimetry tend to lead to overestimation of the size of regions of interest because of the confluence of many contiguous pixels of similar signal intensity that are slightly brighter than the background or surrounding tissue. These effects become quite large at the edges of regions of interest and can become important problems when partial volume effects come into play. The conspicuity of abnormalities is also dependent on the size of the object.
Figure 6:
It can be relatively easy to visualize regions that fall within 2 standard deviations of background noise. This depends on the size of an apparently abnormal region and the quality of diagnostic display. A, We used four pure signal intensities ranging from pure black in the background to pure white text as the starting image. Gaussian noise was added, and signal intensity difference between the text and the circle were adjusted to achieve approximate differences between the text and circle of, B, 1 standard deviation; B, 1.5 standard deviations; and, C, 2 standard deviations. In all cases, the text is readable on a high-quality computer monitor capable of displaying the 17-step gray scale. CNR = contrast-to-noise ratio, SNR = signal-to-noise ratio.
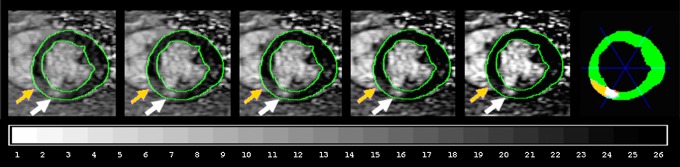
Figure 7:
Left: MR images show human infarct displayed at five brightness and contrast levels to show difficulties with visual interpretation of intermediate gray patches. A gray patch of infarcted myocardium (yellow arrows) is brighter than normal myocardium but is not bright enough to exceed full-width half-maximum threshold (white arrows) determined with the Feature Analysis and Combined Thresholding computer algorithm (57,58). Right: Color diagram shows pixel agreement of human and computer measurements. Green = normal for both, white = infarcted for both; yellow = infarcted for human but not computer measurement; orange = infarcted for computer but not human measurement. Discrepancy between human and computer contouring represents a large fraction of a typical myocardial sector (blue lines). To properly visualize this figure, the computer monitor should be adjusted to exhibit 26 shades on the gray-scale bar.
At the same time, the use of thresholds that are too high or too low could lead to inaccurate estimation of the area at risk. Limited numbers of pixels with the remote region of interest can lead to inaccurate estimates of the standard deviation. The accuracy of determining a statistical measure is dependent on the sample size. A standard deviation estimate based on too few pixels in the remote myocardium might not represent the true standard deviation of remote myocardium and might lead to thresholds that are too high or too low. Inadvertent inclusion of some pixels with residual blood signal or artifacts could also lend bias to the standard deviation estimate. This topic is beyond the scope of this article, as the more established late gadolinium-enhanced images still are not quantified in a consistent and uniform way. However, it is important to recognize that apparently objective or quantitative methods can be applied in ways that could over- or underestimate the size of the area at risk.
Recent Work Provides the Strongest Challenge to the Hypothesis That T2 Represents the Area at Risk
Mewton et al (28) recently compared pre- and postreperfusion measures of area at risk, including CT perfusion during coronary occlusion, two different T2-weighted postreperfusion evaluations, endocardial surface length on MR infarct images, and pathologic evaluation by using dyes infused during reocclusion of the coronary artery. They concluded that “post-reperfusion imaging methods overestimated the AAR [area at risk] likely due to the presence of edema outside of the boundaries of the AAR. Pre-reperfusion arterial enhanced MDCT [multidetector CT] showed the greatest accuracy for the assessment of the AAR” (28). They defined postreperfusion imaging methods as T2-weighted short inversion-recovery turbo spin-echo imaging (59); bright-blood T2-weighted hybrid turbo spin-echo and steady-state free precession imaging (60); and measurement of ESL (54,61,62).
At first glance, comparison of the methods used by Mewton et al (28) to assess area at risk seems quite convincing and carefully performed. However, the pig model is one of the most sensitive to infarction and ischemia. The ischemic time to develop a 50% transmural extent of infarction averages 37 minutes in pigs, 41 minutes in rats, 181 minutes in dogs, and 288 minutes in humans (63). Thus, transient occlusions caused by the angioplasty guide catheter and during placement of the balloon may have added up to a substantial fraction of the occlusion time.
Do the data from Mewton et al (28) support any of these possibilities? First, if one accepts the CT and pathologic measures of area at risk at face value, then the extent of infarction is much greater than expected for a 40-minute occlusion. Second, if one recognizes the possibility of additional ischemic episodes or even more extensive ischemic territories due to the catheter-based procedure as described in the prior paragraph, then it is intriguing that the bright-blood T2-weighted hybrid turbo spin-echo and steady-state free precession imaging area at risk and the ESL method yield physiologic measures that were concordant within 1% of the left ventricle myocardium. Third, the agreement between CT perfusion and pathologic analysis findings is consistent with these critiques, as the CT- and dye-based area at risk were assessed with the same balloon location in the coronary artery but would not have been assessed when the whole coronary artery was occluded by a guide catheter or during one or more inflations in alternative locations. Finally, if T2-weighted images resulted in approximately 50% overestimation of the area at risk, as suggested by Mewton et al (28), this should have been easily detected by Berry et al in humans (Fig 4) (27).
Thus, there are rational explanations that can explain the discrepancies between different AAR measures observed in the pig model of Mewton et al (28). Fortunately, the much slower time to infarct development in humans means that catheter-based ischemic episodes are less likely to explain overestimation of area at risk with T2 except when iatrogenic occlusions add perhaps 30 or more minutes of ischemia.
Best Practices and Recommendations
We currently favor bright-blood T2-prepared steady state free-precession methods that eliminate artifacts from the dark-blood preparation. Acquisition for Cardiac Unified T2 Edema (or ACUT2E) (60) is an alternative method developed in our laboratory, but it had some artifacts that limited our investigation of this otherwise promising sequence.
T2 mapping sequences (64) appear promising and rely on MR physics similar to those in the bright-blood T2-prepared steady state free-precession method. The main benefit of T2 mapping is providing an objective measure of T2 that could be used to objectively threshold the image.
Use of black-blood–prepared T2-weighted images should be reserved for patients with a heart rate of less than 85 beats per minute (65) and should use either the body coil with thick sections or the phased-array coils with proton-density-weighted surface coil correction methods.
Use of early gadolinium-enhanced imaging to image area at risk needs additional validation studies to determine the correct imaging time after contrast material administration.
The ESA measured from late-gadolinium-enhancement images is an excellent second choice that is available in all patients who undergo late gadolinium-enhanced imaging. Its second place status is derived from the potential for underestimation of the area at risk in patients with dramatic myocardial salvage.
Disclosures of Potential Conflicts of Interest: A.E.A. Financial activities related to the present article: U.S. Government Cooperative Research and Development (CRADA) Agreement with Siemens. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. S.L. No potential conflicts of interest to disclose. P.K. No potential conflicts of interest to disclose.
Received November 21, 2011; revision requested December 27; revision received January 23, 2012; accepted March 29; final version accepted April 23.
Funding: This research was supported by the National Institutes of Health (grants ZO1-HL004607-12).
See also the article by Croisille et al in this issue.
References
- 1.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation 2001;104(24):2981–2989 [DOI] [PubMed] [Google Scholar]
- 2.Aletras AH, Tilak GS, Natanzon A, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation 2006;113(15):1865–1870 [DOI] [PubMed] [Google Scholar]
- 3.Kiricuta IC, Jr, Simplăceanu V. Tissue water content and nuclear magnetic resonance in normal and tumor tissues. Cancer Res 1975;35(5):1164–1167 [PubMed] [Google Scholar]
- 4.Higgins CB, Herfkens R, Lipton MJ, et al. Nuclear magnetic resonance imaging of acute myocardial infarction in dogs: alterations in magnetic relaxation times. Am J Cardiol 1983;52(1):184–188 [DOI] [PubMed] [Google Scholar]
- 5.Tilak GS, Hsu LY, Hoyt RF, Jr, Arai AE, Aletras AH. In vivo T2-weighted magnetic resonance imaging can accurately determine the ischemic area at risk for 2-day-old nonreperfused myocardial infarction. Invest Radiol 2008;43(1):7–15 [DOI] [PubMed] [Google Scholar]
- 6.García-Dorado D, Oliveras J, Gili J, et al. Analysis of myocardial oedema by magnetic resonance imaging early after coronary artery occlusion with or without reperfusion. Cardiovasc Res 1993;27(8):1462–1469 [DOI] [PubMed] [Google Scholar]
- 7.Schwartz Longacre L, Kloner RA, Arai AE, et al. New horizons in cardioprotection: recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation 2011;124(10):1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klarich KW, Christian TF, Higano ST, Gibbons RJ. Variability of myocardium at risk for acute myocardial infarction. Am J Cardiol 1999;83(8):1191–1195 [DOI] [PubMed] [Google Scholar]
- 9.Christian TF, Gibbons RJ, Clements IP, Berger PB, Selvester RH, Wagner GS. Estimates of myocardium at risk and collateral flow in acute myocardial infarction using electrocardiographic indexes with comparison to radionuclide and angiographic measures. J Am Coll Cardiol 1995;26(2):388–393 [DOI] [PubMed] [Google Scholar]
- 10.Gibbons RJ. Tc-99m SPECT sestamibi for the measurement of infarct size. J Cardiovasc Pharmacol Ther 2011;16(3-4):321–331 [DOI] [PubMed] [Google Scholar]
- 11.Mahaffey KW, Puma JA, Barbagelata NA, et al. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol 1999;34(6):1711–1720 [DOI] [PubMed] [Google Scholar]
- 12.Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW; AMISTAD-II Investigators. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J Am Coll Cardiol 2005;45(11):1775–1780 [DOI] [PubMed] [Google Scholar]
- 13.Kim RJ, Albert TS, Wible JH, et al. Performance of delayed-enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: an international, multicenter, double-blinded, randomized trial. Circulation 2008;117(5):629–637 [DOI] [PubMed] [Google Scholar]
- 14.Reimer KA, Jennings RB. The changing anatomic reference base of evolving myocardial infarction: underestimation of myocardial collateral blood flow and overestimation of experimental anatomic infarct size due to tissue edema, hemorrhage and acute inflammation. Circulation 1979;60(4):866–876 [DOI] [PubMed] [Google Scholar]
- 15.Buja LM, Willerson JT. Abnormalities of volume regulation and membrane integrity in myocardial tissue slices after early ischemic injury in the dog: effects of mannitol, polyethylene glycol, and propranolol. Am J Pathol 1981;103(1):79–95 [PMC free article] [PubMed] [Google Scholar]
- 16.Jennings RB, Schaper J, Hill ML, Steenbergen C, Jr, Reimer KA. Effect of reperfusion late in the phase of reversible ischemic injury: changes in cell volume, electrolytes, metabolites, and ultrastructure. Circ Res 1985;56(2):262–278 [DOI] [PubMed] [Google Scholar]
- 17.Klem I, Kim RJ. Assessment of microvascular injury after acute myocardial infarction: importance of the area at risk. Nat Clin Pract Cardiovasc Med 2008;5(12):756–757 [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Aty H, Cocker M, Meek C, Tyberg JV, Friedrich MG. Edema as a very early marker for acute myocardial ischemia: a cardiovascular magnetic resonance study. J Am Coll Cardiol 2009;53(14):1194–1201 [DOI] [PubMed] [Google Scholar]
- 19.Bragadeesh T, Jayaweera AR, Pascotto M, et al. Post-ischaemic myocardial dysfunction (stunning) results from myofibrillar oedema. Heart 2008;94(2):166–171 [DOI] [PubMed] [Google Scholar]
- 20.Payne AR, Berry C, Kellman P, et al. Bright-blood T(2)-weighted MRI has high diagnostic accuracy for myocardial hemorrhage in myocardial infarction: a preclinical validation study in swine. Circ Cardiovasc Imaging 2011;4(6):738–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arai AE, Pantely GA, Anselone CG, Bristow J, Bristow JD. Active downregulation of myocardial energy requirements during prolonged moderate ischemia in swine. Circ Res 1991;69(6):1458–1469 [DOI] [PubMed] [Google Scholar]
- 22.Natanzon A, Aletras AH, Hsu LY, Arai AE. Determining canine myocardial area at risk with manganese-enhanced MR imaging. Radiology 2005;236(3):859–866 [DOI] [PubMed] [Google Scholar]
- 23.Fieno DS, Kim RJ, Chen EL, Lomasney JW, Klocke FJ, Judd RM. Contrast-enhanced magnetic resonance imaging of myocardium at risk: distinction between reversible and irreversible injury throughout infarct healing. J Am Coll Cardiol 2000;36(6):1985–1991 [DOI] [PubMed] [Google Scholar]
- 24.Christian TF, Aletras AH, Arai AE. Estimation of absolute myocardial blood flow during first-pass MR perfusion imaging using a dual-bolus injection technique: comparison to single-bolus injection method. J Magn Reson Imaging 2008;27(6):1271–1277 [DOI] [PubMed] [Google Scholar]
- 25.Christian TF, O'Connor MK, Schwartz RS, Gibbons RJ, Ritman EL. Technetium-99m MIBI to assess coronary collateral flow during acute myocardial infarction in two closed-chest animal models. J Nucl Med 1997;38(12):1840–1846 [PubMed] [Google Scholar]
- 26.The Thrombolysis in Myocardial Infarction (TIMI) trial Phase I findings. TIMI Study Group. N Engl J Med 1985;312(14):932–936 [DOI] [PubMed] [Google Scholar]
- 27.Berry C, Kellman P, Mancini C, et al. Magnetic resonance imaging delineates the ischemic area at risk and myocardial salvage in patients with acute myocardial infarction. Circ Cardiovasc Imaging 2010;3(5):527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mewton N, Rapacchi S, Augeul L, et al. Determination of the myocardial area at risk with pre- versus post-reperfusion imaging techniques in the pig model. Basic Res Cardiol 2011;106(6):1247–1257 [DOI] [PubMed] [Google Scholar]
- 29.Ugander MBP, Oki AJ, Chen B, et al. Quantitative T1-maps delineate myocardium at risk as accurately as T2-maps: experimental validation with microspheres. In: 2011 Scientific Sessions of the Society of Cardiovascular Magnetic Resonance and the EuroCMR. Nice, France, 2010 [Google Scholar]
- 30.Abdel-Aty H, Zagrosek A, Schulz-Menger J, et al. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation 2004;109(20):2411–2416 [DOI] [PubMed] [Google Scholar]
- 31.Friedrich MG, Abdel-Aty H, Taylor A, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol 2008;51(16):1581–1587 [DOI] [PubMed] [Google Scholar]
- 32.Carlsson M, Ubachs JF, Hedström E, Heiberg E, Jovinge S, Arheden H. Myocardium at risk after acute infarction in humans on cardiac magnetic resonance: quantitative assessment during follow-up and validation with single-photon emission computed tomography. JACC Cardiovasc Imaging 2009;2(5):569–576 [DOI] [PubMed] [Google Scholar]
- 33.Graham MM, Faris PD, Ghali WA, et al. Validation of three myocardial jeopardy scores in a population-based cardiac catheterization cohort. Am Heart J 2001;142(2):254–261 [DOI] [PubMed] [Google Scholar]
- 34.Wince WB, Kim RJ. Molecular imaging: T2-weighted CMR of the area at risk: a risky business? Nat Rev Cardiol 2010;7(10):547–549 [DOI] [PubMed] [Google Scholar]
- 35.Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100(19):1992–2002 [DOI] [PubMed] [Google Scholar]
- 36.Kim RJ, Judd RM, Chen EL, Fieno DS, Parrish TB, Lima JA. Relationship of elevated 23Na magnetic resonance image intensity to infarct size after acute reperfused myocardial infarction. Circulation 1999;100(2):185–192 [DOI] [PubMed] [Google Scholar]
- 37.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343(20):1445–1453 [DOI] [PubMed] [Google Scholar]
- 38.Choi KM, Kim RJ, Gubernikoff G, Vargas JD, Parker M, Judd RM. Transmural extent of acute myocardial infarction predicts long-term improvement in contractile function. Circulation 2001;104(10):1101–1107 [DOI] [PubMed] [Google Scholar]
- 39.Ricciardi MJ, Wu E, Davidson CJ, et al. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation 2001;103(23):2780–2783 [DOI] [PubMed] [Google Scholar]
- 40.Mahrholdt H, Wagner A, Holly TA, et al. Reproducibility of chronic infarct size measurement by contrast-enhanced magnetic resonance imaging. Circulation 2002;106(18):2322–2327 [DOI] [PubMed] [Google Scholar]
- 41.Rehwald WG, Fieno DS, Chen EL, Kim RJ, Judd RM. Myocardial magnetic resonance imaging contrast agent concentrations after reversible and irreversible ischemic injury. Circulation 2002;105(2):224–229 [DOI] [PubMed] [Google Scholar]
- 42.Mahrholdt H, Wagner A, Parker M, et al. Relationship of contractile function to transmural extent of infarction in patients with chronic coronary artery disease. J Am Coll Cardiol 2003;42(3):505–512 [DOI] [PubMed] [Google Scholar]
- 43.Wagner A, Mahrholdt H, Holly TA, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 2003;361(9355):374–379 [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto H, Matsuda T, Miyamoto K, Shimada T, Mikuri M, Hiraoka Y. Peri-infarct zone on early contrast-enhanced CMR imaging in patients with acute myocardial infarction. JACC Cardiovasc Imaging 2011;4(6):610–618 [DOI] [PubMed] [Google Scholar]
- 45.Arai AE. Gadolinium can depict area at risk and myocardial infarction: a double-edged sword? JACC Cardiovasc Imaging 2011;4(6):619–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim RJ, Chen EL, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation 1996;94(12):3318–3326 [DOI] [PubMed] [Google Scholar]
- 47.Kramer CM, Rogers WJ, Jr, Mankad S, Theobald TM, Pakstis DL, Hu YL. Contractile reserve and contrast uptake pattern by magnetic resonance imaging and functional recovery after reperfused myocardial infarction. J Am Coll Cardiol 2000;36(6):1835–1840 [DOI] [PubMed] [Google Scholar]
- 48.Oshinski JN, Yang Z, Jones JR, Mata JF, French BA. Imaging time after Gd-DTPA injection is critical in using delayed enhancement to determine infarct size accurately with magnetic resonance imaging. Circulation 2001;104(23):2838–2842 [DOI] [PubMed] [Google Scholar]
- 49.Judd RM, Kim RJ. Imaging time after Gd-DTPA injection is critical in using delayed enhancement to determine infarct size accurately with magnetic resonance imaging. Circulation 2002;106(2):e6; author reply e6 [DOI] [PubMed] [Google Scholar]
- 50.Sörensson P, Heiberg E, Saleh N, et al. Assessment of myocardium at risk with contrast enhanced steady-state free precession cine cardiovascular magnetic resonance compared to single-photon emission computed tomography. J Cardiovasc Magn Reson 2010;12(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedrich MG, Kim HW, Kim RJ. T2-weighted imaging to assess post-infarct myocardium at risk. JACC Cardiovasc Imaging 2011;4(9):1014–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reimer KA, Jennings RB. The "wavefront phenomenon" of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest 1979;40(6):633–644 [PubMed] [Google Scholar]
- 53.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation 1977;56(5):786–794 [DOI] [PubMed] [Google Scholar]
- 54.Ubachs JF, Engblom H, Erlinge D, et al. Cardiovascular magnetic resonance of the myocardium at risk in acute reperfused myocardial infarction: comparison of T2-weighted imaging versus the circumferential endocardial extent of late gadolinium enhancement with transmural projection. J Cardiovasc Magn Reson 2010;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuernau G, Eitel I, Franke V, et al. Myocardium at risk in ST-segment elevation myocardial infarction comparison of T2-weighted edema imaging with the MR-assessed endocardial surface area and validation against angiographic scoring. JACC Cardiovasc Imaging 2011;4(9):967–976 [DOI] [PubMed] [Google Scholar]
- 56.Versteylen MO, Bekkers SC, Smulders MW, et al. Performance of angiographic, electrocardiographic and MRI methods to assess the area at risk in acute myocardial infarction. Heart 2012;98(2):109–115 [DOI] [PubMed] [Google Scholar]
- 57.Hsu LY, Ingkanisorn WP, Kellman P, Aletras AH, Arai AE. Quantitative myocardial infarction on delayed enhancement MRI. II. Clinical application of an automated feature analysis and combined thresholding infarct sizing algorithm. J Magn Reson Imaging 2006;23(3):309–314 [DOI] [PubMed] [Google Scholar]
- 58.Hsu LY, Natanzon A, Kellman P, Hirsch GA, Aletras AH, Arai AE. Quantitative myocardial infarction on delayed enhancement MRI. I. Animal validation of an automated feature analysis and combined thresholding infarct sizing algorithm. J Magn Reson Imaging 2006;23(3):298–308 [DOI] [PubMed] [Google Scholar]
- 59.Simonetti OP, Finn JP, White RD, Laub G, Henry DA. "Black blood" T2-weighted inversion-recovery MR imaging of the heart. Radiology 1996;199(1):49–57 [DOI] [PubMed] [Google Scholar]
- 60.Aletras AH, Kellman P, Derbyshire JA, Arai AE. ACUT2E TSE-SSFP: a hybrid method for T2-weighted imaging of edema in the heart. Magn Reson Med 2008;59(2):229–235 [DOI] [PubMed] [Google Scholar]
- 61.Ortiz-Pérez JT, Meyers SN, Lee DC, et al. Angiographic estimates of myocardium at risk during acute myocardial infarction: validation study using cardiac magnetic resonance imaging. Eur Heart J 2007;28(14):1750–1758 [DOI] [PubMed] [Google Scholar]
- 62.Wright J, Adriaenssens T, Dymarkowski S, Desmet W, Bogaert J. Quantification of myocardial area at risk with T2-weighted CMR: comparison with contrast-enhanced CMR and coronary angiography. JACC Cardiovasc Imaging 2009;2(7):825–831 [DOI] [PubMed] [Google Scholar]
- 63.Hedström E, Engblom H, Frogner F, et al. Infarct evolution in man studied in patients with first-time coronary occlusion in comparison to different species: implications for assessment of myocardial salvage. J Cardiovasc Magn Reson 2009;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giri S, Chung YC, Merchant A, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson 2009;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arai AE, Epstein FH, Bove KE, Wolff SD. Visualization of aortic valve leaflets using black blood MRI. J Magn Reson Imaging 1999;10(5):771–777 [DOI] [PubMed] [Google Scholar]