Abstract
Objective
To examine and describe vascular depression epidemiology in the United States.
Methods
Cross-sectional data from a national probability sample of household resident adults (18-years and older; N = 16,423) living in the 48 coterminous United States were analyzed to calculate prevalence estimates of vascular depression, associated disability and treatment rates. In this study, vascular depression was defined as the presence of cardiovascular and cerebrovascular disease (CVD) and CVD major risk factors (e.g., diabetes, hypertension, heart disease, and obesity) among adults 50-years and older who also met 12-month DSM-IV major depression criteria.
Results
We estimated that about 3.4% or approximately 2.64 million American adults 50-years and older met our criteria for vascular depression. Among adults who met criteria for lifetime major depression, over one-in-five (22.1%) were considered to have the vascular depression subtype. Secondly, vascular depression was associated with significantly increased functional impairment relative to the non-depressed population and adults meeting criteria for major depression alone. Although depression care use was significantly higher among vascular depression respondents relative to those with major depression alone, practice guideline concordant therapy use was not.
Conclusions
Vascular depression appears to be an important public health problem that affects a large portion of the U.S. adult population with major depression, and that it is associated with excess functional impairment without concomitant better depression care.
Keywords: Major depressive disorder, Depression, Vascular depression, Epidemiology, Cardiovascular disease, Stroke
1. Introduction
In the coming decades, unipolar depression has been projected to become the second leading cause of disability worldwide and the leading cause of disability in high-income nations, including the United States (Mathers and Loncar, 2006). Major depression is known to co-exist with multiple chronic health problems associated with vascular disease (Krishnan, 2000). The vascular depression hypothesis suggests that a large subgroup of people with major depression have concomitant occult neurologic disorders with vascular etiologies (i.e., stroke and/or vascular disease risk factors) (Alexopoulos et al., 1997). Neuroradiological and neuropsychological reports have implicated subcortical structures and related projections as responsible for the executive cognitive problems and psychomotor retardation observed in patients with vascular depression (Sheline et al., 2010). Among adults ages 50-years and older, about half of individuals with major depression evinced subclinical or so-called silent brain infarcts in the form of subcortical lacunes (Vermeer et al., 2007). Such findings hint that a large subgroup of patients with vascular depression may experience excess disease burden relative to non-depressed adults and those with major depression alone.
In this study, we examined the prevalence and epidemiology of vascular depression in the United States. The vascular depression hypothesis suggests that a “large subpopulation” of persons with major depression would be considered to have this subtype of major depression (Alexopoulos et al., 1997, p.915). Secondly, excess vascular depression disease burden would be expected in comparison to non-depressed adults and adults with major depression alone. Thirdly, if vascular depression was associated with higher disease burden as the hypothesis suggests, then increased rates of depression care would be expected relative to persons with major depression alone. To achieve these study goals, we use available data from a nationally representative psychiatric survey.
2. Methods
2.1. Data
The National Institute of Mental Health Collaborative Psychiatric Epidemiology Surveys (CPES) data were collected between February 2001 and November 2003. Interviewers used face-to-face computer-assisted technology to collect data from national household probability samples of non-institutionalized adults. The CPES sample combines data from three national surveys including the National Comorbidity Survey Replication (NCS-R), the National Study of American Life (NSAL) and the National Latino and Asian American Study of Mental Health (NLAAS). The three studies shared a common objective of collecting information about the prevalence of psychiatric disorders, associated impairments, and treatment patterns of adult populations in the United States, and were based on common sampling methods and procedures originating at the University of Michigan’s Survey Research Center. CPES statisticians reconfigured the initial individual sampling methods to generate a common sample design with over 20,000 observations allowing investigators to conduct nationally representative analysis, with sufficient sample sizes and power, for multiple diagnostic subpopulations. The resulting CPES sample is based on a complex multi-stage area probability design and includes sampling weights to correct for unequal probability of selection and non-response bias. The methods used to integrate the individual CPES studies are discussed at length in Heeringa et al. (2004), and a detailed treatment of the development and implementation of the CEPS and its component studies is provided by Pennell et al. (2004) (Hartley, 1974; Heeringa et al., 2004). The overall CPES response rate was 72.3%.
2.2. Analysis of subpopulations
We examined the distribution and correlates of vascular depression in the US population age 50-years and older. Appropriate methods for subpopulation analyses of complex sample survey data were used for estimating descriptive parameters and in analytic models. Analysis of this de-identified, publicly available dataset was reviewed and approved by the Wayne State University institutional review board.
2.3. Measures
2.3.1. Vascular depression
Vascular depression was operationalized using Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV), World Mental Health (WMH) Composite International Diagnostic Interview (CIDI) criteria for a lifetime major depressive episode and the absence or presence of vascular disease or multiple cerebrovascular risk factors (CVRF) or stroke, conditional on the depressive episode occurring at or after the age of 49-years. Conditioning depression recency on age 49-years allows us to capture sample respondents aged 50-years with an ongoing or recent (i.e. within the past 12-month) depressive episode. The risk factors for cerebrovascular disease included self-reported medical diagnoses of diabetes, hypertension, heart disease, and obesity. In each case, except for obesity, respondents were asked to indicate whether a doctor or health professional has ever told them that they have the condition. Obesity was measured using a recode of the Body Mass Index based on respondents’ reported height (in inches) and weight (in pounds). A BMI value of 30 or more was considered obese. Respondents were classified as having a vascular depression if they met criteria for a depressive episode occurring after the age of 49-years and reported 1) more than one CVRF or 2) a medical diagnosis of stroke. Respondents that met criteria for lifetime depression with a reported depression occurring before the age of 49-years, or a report of no medical diagnosis of stroke and no more than one risk factor for cerebrovascular disease were grouped into a second, other depression, category. Finally, respondents that did not meet DSM-IV, WHM-CIDI criteria for lifetime depression were grouped into a third, non-depressed, category.
2.3.2. Days of functional impairment
We used the World Health Organization Disability Assessment Scale (WHODAS-II) to measure 30-days impairment in five domains of functioning: 1) overall role impairment; 2) cognition; 3) mobility; 4) self-care; and 5) social impairment (WHO, 2001). Individuals indicating that they had no such problems in the 30-days, preceding the interview, were assigned the value zero on the respective indicator. All other respondents were assigned their self-reported number of days outof-role for the specific WHODAS-II functional domains. The common metric for the WHODAS-II outcomes is the total number of the past 30-days of functional impairment in the aforementioned domains.
2.3.3. Depression care
A three category indicator based on respondents’ use of either pharmacotherapy or psychotherapy was generated to distinguish between 1) non-users, 2) users, and 3) adequate depression care users. Assessment of pharmacotherapy use was based on 1) interview records of pill bottles of respondents’ antidepressant (generic and trade name) prescriptions, and 2) self-reports of prescription source and duration of use. Drug coding was done consequent to prescription names review and verification by two board certified psychiatrists and a psychiatric nurse specialist. To assess psychotherapy, we used respondents’ self-reports of 1) past-year number of visits made to mental health professionals, including psychiatrists, psychologists, counselors, and social workers, and 2) the duration of these visits in minutes.
We distinguished between depression care users and adequate users by following Practice Guidelines for the Treatment of Patients with Major Depressive Disorder. Respondents with supervised anti-depressant use, by a psychiatrist or other prescribing clinician for at least four visits in the past year, lasting for 45 days or more were coded as meeting criteria for adequate pharmacotherapy. Respondents with at least 4 visits to a mental health professional each lasting on average at least 30-min were classified as meeting criteria for adequate psychotherapy.
2.3.4. Other variables
When appropriate, we account for age and sex as covariates. Age was included as a continuous indicator. Sex was a dichotomous indicator (0 = Female; 1 = Male).
2.3.5. Statistical approach
Procedures designed for the analysis of complex sample survey data in the Stata software package (Version 11.1) were used to perform all study analyses. More specifically, all estimates accounted for CPES sampling weights that adjusted for unequal probabilities of selection, non-response, and additional post-stratification to ensure adequate representation of the U.S. population. In addition, design-based analyses, accounting for the complex multistage clustered structure of the CPES samples, specifically a Taylor Series Linearization approach to variance estimation, were used to estimate correct standard errors.
Our analyses were conducted in four steps. First, we provided sample estimates describing demographic characteristics. Second, we calculated the prevalence rates for vascular depression. Third, we estimated the prevalence of depression treatment by type for the two depression groups of interest. To test for statistically significant differences we calculated design adjusted tests of differences in proportions, and computed a design corrected chi-squared test of overall independence between treatment type and depression grouping. Fourth, we modeled impairment rates as a function of depression grouping controlling for age and sex. Considering the count nature of the outcomes of interest and to guard against the stringent assumption of equality between conditional variance and conditional mean imposed by a Poisson distribution, and account for the skewed nature of our impairment indicators, (Cameron and Trivedi, 2010) negative binomial regression models were fitted to estimate impairment days, adjusting for sex and age, in the five impairment domains. The negative binomial distribution presents a more general fit in applied work where indicators commonly violate the equidispersion condition characteristic of the Poisson distribution (Cameron and Trivedi, 2010). Considering the difficulty of model interpretation based on the coefficients resulting from a negative binomial regression, where incremental change in the outcome is not constant but rather depends on the values of the model covariates, we generated incidence rate ratios, which can be roughly interpreted as odds ratios, to facilitate interpretation.
Incidence rate ratios focus on the ratio of the change in the outcome of interest as a function of a unit increase in an associated predictor or covariate. Unlike negative binomial coefficients, incidence rate ratios are constant and independent of the particular values of the covariates. For instance, an incidence rate ratio of 1.5 implies that the prevalence of the outcome increases by a factor of 1.5 with a unit increase in an associated covariate (Hardin and Hilbe, 2007).
3. Results
3.1. Prevalence
CPES sample characteristics are provided in Table 1. We estimate that about 15.6% (SE = 0.8) of U.S adults ages 50-years and older met criteria for DSM-IV lifetime major depression. Of the vascular diseases and risk factors we examined, we found that 15.4% (SE = 1.3) of respondents reported a diagnosis of heart disease, 44.4% (SE = 0.9) reported hypertension, 14.3% (SE = 0.8) reported diabetes, and 26.5% (SE = 1.0) had a BMI of 30 or more. Finally, 5.2% (SE = 0.6) of respondents reported a medical diagnosis of stroke.
Table 1.
Demographic characteristics of respondents 50-years and older. Results are from the collaborative psychiatric epidemiology study.
| Na | %b | SEc | |
|---|---|---|---|
| Ethnicity/race | |||
| Asians | 594 | 3.3 | 0.4 |
| Latinos | 825 | 6.7 | 0.6 |
| Blacks | 1721 | 9.1 | 0.7 |
| Non-Latino Whites and All others | 1997 | 80.9 | 1.3 |
| Age (years) | |||
| 50–64 | 3263 | 56.6 | 1.9 |
| 65+ | 1874 | 43.4 | 1.9 |
| Sex | |||
| Female | 3059 | 55.0 | 1.8 |
| Male | 2078 | 45.0 | 1.8 |
| Education (years) | |||
| Less than high school | 1524 | 22.9 | 1.2 |
| High school | 1438 | 31.6 | 1.2 |
| Some college | 1088 | 23.3 | 1.2 |
| College or more | 1087 | 22.2 | 1.3 |
| Annual household income (US Dollars) | |||
| $0–$17,999 | 1632 | 23.3 | 1.2 |
| $18,000–$31,999 | 993 | 18.0 | 0.9 |
| $32,000–$54,999 | 1010 | 21.7 | 1.1 |
| $55,000 and over | 1502 | 37.1 | 1.6 |
Unweighted sample numbers.
Weighted percentages.
Survey design adjusted standard errors.
3.2. Depression groups
In the adult population 50-years and older, 12.2% of respondents were classified as having a non-vascular depression, and 3.4% met criteria for vascular depression (Table 2). Over one-fifth (22.1%; SE = 1.8) of respondents who met criteria for DSM-IV lifetime major depression also met our criteria for vascular depression. Vascular depression was more common among men (25.5%; SE = 3.9) than women (20.5%; SE = 1.8); however, the difference did not reach statistical significance (P = 0.22).
Table 2.
Prevalence of vascular depression among respondents 50-years and older in the U.S. Results are from the collaborative psychiatric epidemiology surveys.
| Total population
|
Major depression
|
|||
|---|---|---|---|---|
| %c | SEd | %c | SEd | |
| Major depression alonea | 12.2 | 0.7 | 77.9 | 1.8 |
| Vascular depressionb | 3.4 | 0.4 | 22.1 | 1.8 |
Meeting criteria for lifetime major depression based on World Mental Health Composite International Diagnostic Interviews.
Meeting criteria for lifetime major depression based on World Mental Health Composite International Diagnostic Interviews with two or more reported cerebrovascular risk factors or a report of a medical diagnosis of stroke and a depressive episode occurring after the age of 49-years.
Weighted prevalence rates.
Survey design adjusted standard errors.
3.3. Depression care
About two-fifths of respondents meeting criteria for vascular depression (40.1%) were treated for depression (Table 3). The non-vascular depression group presented a similar overall treatment rate. Overall, treatment type was statistically independent from depression grouping.
Table 3.
Prevalence of depression treatment among respondents 50-years and older in the U.S. Results are from the collaborative psychiatric epidemiology surveys.
| Depression treatmenta
|
|||||
|---|---|---|---|---|---|
| Major depressionb
|
Vascular depressionc
|
Tests of proportions
|
|||
| %d | SEe | %d | SEe | ||
| Non-users | 62.0 | 2.4 | 60.0 | 5.4 | P = 0.742 |
| Users | 28.2 | 2.5 | 29.1 | 4.5 | P = 0.865 |
| Adequate users | 9.9 | 1.2 | 11.0 | 2.8 | P = 0.723 |
| Design-based F(1.87, 282.99) = 0.085; P = 0.908 | |||||
Depression treatment is based on reports of use of either pharmacotherapy or psychotherapy. Distinction between users and adequate users is based on practice guidelines for the treatment of patients with major depressive disorder. Adequate users have at least 45 days of supervised, based on at least four visits to a professional in the past year, antidepressant drug use, or at least four visits to a mental health professional each lasting on 30-min on average.
Meeting criteria for lifetime major depression based on World Mental Health Composite International Diagnostic Interviews, with less than 2 reported cerebrovascular risk factors and no reported stroke.
Meeting criteria for lifetime major depression based on World Mental Health Composite International Diagnostic Interviews with 2 or more reported cerebrovascular risk factors or a report of a medical diagnosis of stroke and a depressive episode occurring after the age of 49-years.
Weighted prevalence rates.
Survey design adjusted standard errors.
3.4. Days of functional impairment
Our negative binomial regression model results indicated that across the five impairment domains, and controlling for age and sex, both major depression, but especially, vascular depression had significantly higher incidence rate ratios (IRR) of impairment relative to the non-depressed group (Table 4). More specifically, the IRR among respondents meeting criteria for vascular depression compared to respondents with no depression was more than 18 times higher for days out of role, 6.4 times higher for cognition, 3.7 times higher for mobility, 4.7 times higher for self-care and 7.1 times higher for social interaction. Moreover, the IRRs for vascular depression were significantly higher than the non-vascular depression group in impairment days out of role, cognition, mobility and social interactions. The IRRs for vascular depression were not statistically distinguishable from the non-vascular depression group for self-care (P = 0.133).
Table 4.
World health organization disability assessment scales-II past 30-day impairment ratings among respondents 50-years and older. Results are from multivariate negative binomial regressions using data from the collaborative psychiatric epidemiology surveys.
| Out of rolea
|
Cognitiona
|
Mobilitya
|
Self-carea
|
Social interactiona
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IRRf | 95% CI | IRRf | 95% CI | IRRf | 95% CI | IRRf | 95% CI | IRRf | 95% CI | |
| Nob | ref | n/a | ref | n/a | ref | n/a | ref | n/a | ref | n/a |
| Yesc,e | 5.83g | 3.02–11.24 | 4.12g | 3.00–5.64 | 1.88g | 1.47–2.39 | 2.58g | 1.42–4.68 | 3.66g | 2.39–5.62 |
| Vasculard,e | 18.31g | 6.71–49.95 | 6.40g | 4.48–9.12 | 3.74g | 2.89–4.83 | 4.71g | 2.74–8.08 | 7.11g | 4.20–12.03 |
Models are age and sex adjusted.
Not meeting criteria for DSM-IV lifetime major depression based on World Mental Health Composite International Diagnostic Interviews (Reference category).
Meeting criteria for lifetime major depression based on World Mental Health Composite International Diagnostic Interviews, with less than 2 reported cerebrovascular risk factors and no reported stroke.
Meeting criteria for lifetime major depression based on World Mental Health Composite International Diagnostic Interviews with 2 or more reported cerebrovascular risk factors or a report of a medical diagnosis of stroke and a depressive episode occurring after the age of 49-years.
The IRRs for vascular and uncomplicated depression were significantly different except in the case of self-care were differences were not statistically distinguishable.
Incidence rate ratios.
P < 0.01.
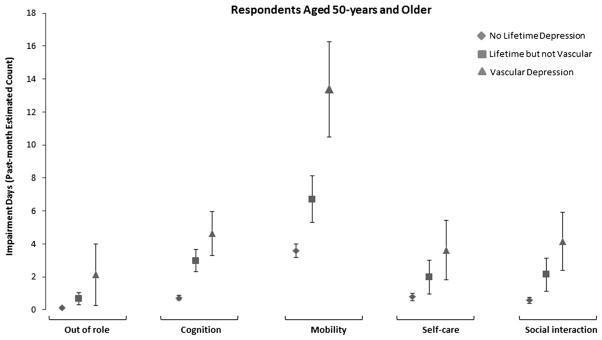
To further facilitate the interpretability of the incidence rates ratios, and quantify the burden of disease in each of the considered depression groups, we estimate the number of impairment days in each WHODAS II domain and graph these predicted values in Fig. 1. Compared to respondents with no major depression each of the considered depression groups presented a higher predicted impairment average. Impairment differentials were most evident with the cognition and mobility domains.
Fig. 1.
Average predicted counts of functional impairment using World Health Organization Disability Assessment Scales-II past 30-day (WHODAS-II) ratings among respondents 50-years and older by depression subtype. Results are from the Collaborative Psychiatric Epidemiology Surveys.
The average predicted cognition impairment days were 2.98 (SE = 0.66; P < 0.01) for the non-vascular depression group, and 4.63 (SE = 1.34; P < 0.01) for those meeting criteria for vascular depression, compared to 0.72 (SE = 0.16; P < 0.01) days for individuals not meeting criteria for major depression. When mobility was considered, the predicted averages for the non-vascular depression group and for those meeting criteria for vascular depression were 6.71 (SE = 1.41; P < 0.01) and 13.37 (SE = 2.89; P < 0.01) days, respectively, compared to 3.58 (SE = 0.42; P < 0.01) days for non-depressed individuals.
4. Discussion
In this study, we reported the first national prevalence estimates of vascular depression in the United States. We estimated that about 3.4% of the U.S. population or approximately 2.6 million American adults ages 50-years and older met our criteria for vascular depression. Among middle-aged and older American adults who met criteria for major depression, over one in five (22.1%) also reported cardiovascular disease comorbidity, which suggests a vascular basis for their depression (i.e., vascular depression). Secondly, we found that vascular depression was associated with increased disease burden relative to the non-depressed and major depression alone populations. Our findings provide population-level information that vascular depression (i.e., major depression and comorbid CVD) is a relatively common and disabling condition (Alexopoulos et al., 1997; Sheline et al., 2010). Clinical and self-management of vascular disease (e.g., smoking cessation) in the presence of major depression may present extra challenges to care providers and patients.
We found the highest disease burden and functional impairment among respondents meeting criteria for the vascular depression. While cognitive and psychomotor problems are considered symptomatic of major depression it is reasonable to infer that this excess morbidity may be associated with some biological etiology. For vascular depression, it may be that the microvascular insults and frank strokes are likely contributory factors to the impairment problems reported by respondents in this study. For example, about 10% of healthy adults in their 40’s evince so-called silent strokes on MRI studies (Vermeer et al., 2007). Furthermore, the proportion of silent strokes jumps to over 40% among persons with one or more vascular disease risk factors and notably 46% among persons with major depression (Vermeer et al., 2007; Bokura et al., 2008). The association we observed may simply be related to the additional burden of having comorbid CVD, however, other problems could explain this additional morbidity. The etiology of mobility and cognitive impairments in vascular depression could be understood to be sequelae of the subcortical insults commonly reported in silent stroke studies (Yakushiji et al., 2008; Putaala et al., 2009). Specifically, apathy and slower information processing, which may have been interpreted and reported by study respondents as disinterest and psychomotor slowing, and may play an important role in the excess disease burden we observed among respondents meeting our criteria for the vascular depression subtype (Butters et al., 2004; Levy et al., 1998). Thus, the vascular depression hypothesis used to explain the clinical manifestations among patients with major depression and comorbid CVD may be demonstrable at the population-level and indicative of increased disease burden. Additional research from non-human models and epidemiologic work examining neuropsychological performance, brain structure and functioning may address important remaining questions about the vascular depression hypothesis and the extent to which it contributes to burden of disability.
The findings herein indicate that CVD and risk factors may commonly occur among persons meeting major depression criteria. For clinicians, our findings suggest that one of every five patients with major depression would also have comorbid CVD. While several studies have examined depression therapies for patients with vascular depression, consensus and treatment guideline do not exist specifically for this depression subtype (Sheline et al., 2010; Lichtman et al., 2009; Jorge et al., 2008). We found that depression therapy use and guideline concordant use was similar between vascular depression and major depression alone (APA, 2000). We anticipated that depression care would be higher among persons meeting our criteria of vascular depression owing to its increased disease burden; however, this was not the case. Secondly, previous work has demonstrated that depression care use is higher among people with major depression and comorbid medical conditions (e.g., diabetes) (González et al., 2008, 2009). While it is not clear from this study why depression care was not higher among respondents with comorbid CVD, it is clear that monitoring depression in the context of comorbid CVD and risk factors presents a more complicated clinical scenario.
There are several caveats that should be considered when evaluating our findings. First, we relied on survey data for our national estimates. In-depth clinical and medical information, such as neuroimaging, were not available. Thus, we can only infer that survey respondents provided accurate information for medical conditions (e.g., stroke). Nevertheless, with nearly half of persons 50-years and older with depression evincing silent strokes on MRI and similarly high rates of subacute infarctions among persons with diabetes and hypertension, it is highly probable that persons meeting criteria for vascular depression in this study would also have some brain changes. Secondly, the CPES excluded homeless or institutionalized persons, which could produce prevalence underestimations. Thirdly, the WMH-CIDI has a modest sensitivity and high specificity for accurately detecting “true” psychiatric disorders (e.g., major depression) among respondents (Williams et al., 2007; Kessler et al., 2003). Thus, it is possible that some cases with “true” psychiatric disorders were missed, which could artificially inflate the proportion of respondents not meeting major depression criteria. WMH-CIDI major depression diagnostic test performance could be biased when comorbid CVD was present. If persons with CVD were less likely to meet criteria for MDD, our estimates of association of vascular depression with disability would have been biased toward the null because the reference group would include some persons with major depression. Fourthly, survey respondents reported impairment days in global domains. It is not clear from this study whether self-reported impairment measures are consistent with performance-based measures, such as standardized neuropsychological testing. Finally, we defined vascular depression as the major depression co-occurring with CVD and risk factors associated with stroke, and it is possible that we have merely reported the excess disease burden associated with the co-occurrence of major depression and CVD and risks.
From a public health perspective, our findings are particularly significant given current U.S. population trends. The U.S. and world population is growing older and prevalence of vascular disease is increasing at alarming rates and at earlier ages (Census, 2008; Danaei et al., 2011; Finucane et al., 2011). Furthermore, few Ameri-cans are treated adequately for major depression (González et al., 2010). Under an unabated scenario, vascular disease risks, and by extension vascular depression, would be expected to increase in coming decades. Furthermore, ethnic/racial minorities who are at higher risk for vascular disease and depression undertreatment would be at increased risk compared to non-Latino Whites (Flegal et al., 2010). Assuming mid-century U.S. population projections are reasonably accurate and stable, current U.S. figures may underestimate the future impact of major depression disease burden by not considering the intimate interaction between vascular disease and depression (Mathers and Loncar, 2006).
5. Conclusions
Our findings indicate that vascular depression is a relatively common and disabling condition among U.S. adults with major depression. The findings reported herein may instruct future clinical and epidemiologic psychiatric research aimed at reducing disease burden associated with major depression and comorbid CVD.
Acknowledgments
Role of funding source: This work was supported by the National Institutes of Health, National Institute of Mental Health, National Heart Lung Blood Institute and the National Institute on Aging. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Drs. González, Tarraf and Whitfield are supported by the NIMH (R01) MH 84994; Drs. González and Tarraf also receive support from NHLBI HC 65233; Dr. Gallo is supported by R01 MH 65539 and K24 MH 70407.
The authors would like to express their sincere thanks to W. Ladson Hinton, MD and Jürgen Unützer, MD, for the valuable expertise used to implement depression care guidelines.
Footnotes
Conflict of interest
The authors report no conflicts of interest that could inappropriately influence, or be perceived to influence, this work.
Contribution
Dr. González was responsible for conceiving, directing and funding the study, and manuscript.
Dr. Tarraf was responsible for the analyses and drafting the manuscript.
Dr. Whitfield contributed to the manuscript preparation.
Dr. Gallo was responsible for directing the development of the manuscript and its writing.
References
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Archives of General Psychiatry. 1997 Oct;54(10):915–22. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- APA. Practice guideline for the treatment of patients with major depressive disorder. Washington, DC: American Psychiatric Association; 2000. [PubMed] [Google Scholar]
- Bokura H, Yamaguchi S, Iijima K, Nagai A, Oguro H. Metabolic syndrome is associated with silent ischemic brain lesions. Stroke. 2008;39(5):1607–9. doi: 10.1161/STROKEAHA.107.508630. [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, et al. The nature and determinants of neuropsychological functioning in late-life depression. Archives of General Psychiatry. 2004;61(6):587–95. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Cameron CA, Trivedi PK. Microeconometrics using stata. Stata Press; 2010. Revised ed. [Google Scholar]
- Census. Current population series. Vol. 15. Washington, DC: US Government Printing Office; 2008. [Google Scholar]
- Danaei D, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5. 4 million participants. The Lancet. 2011;377(9765):568–77. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorak CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9. 1 million participants. The Lancet. 2011;377(9765):557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA: The Journal of the American Medical Association. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- González HM, Croghan T, West B, Williams DR, Nesse R, Tarraf W, et al. Antidepressant use in black and white populations in the United States. Psychiatric Services. 2008 Oct;59(10):1131–8. doi: 10.1176/appi.ps.59.10.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González HM, Tarraf W, West BT, Croghan TW, Bowen ME, Cao Z, et al. Antidepressant use in a nationally representative sample of community-dwelling US Latinos with and without depressive and anxiety disorders. Depress Anxiety. 2009;26(7):674–81. doi: 10.1002/da.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González HM, Vega WA, Williams DR, Tarraf W, West BT, Neighbors HW. Depression care in the United States: too little for too few. Archives of General Psychiatry. 2010;67(1):37–46. doi: 10.1001/archgenpsychiatry.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin JW, Hilbe JM. Generalized linear models and extensions. 2. Stata Press; 2007. [Google Scholar]
- Hartley HO. Multiple frame methodology and selected applications. Sankhya. 1974;(3):99–118. Series C. [Google Scholar]
- Heeringa SG, Wagner J, Torres M, Duan N, Adams T, Berglund P. Sample designs and sampling methods for the collaborative psychiatric epidemiology studies (CPES) International Journal of Methods in Psychiatric Research. 2004;13(4):221–40. doi: 10.1002/mpr.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge RE, Moser DJ, Acion L, Robinson RG. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Archives of General Psychiatry. 2008;65(3):268–76. doi: 10.1001/archgenpsychiatry.2007.45. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the national comorbidity survey replication (NCS-R) The Journal of the American Medical Association. 2003 Jun 18;289(23):3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Krishnan KR. Depression as a contributing factor in cerebrovascular disease. American Heart Journal. 2000 Oct;140(4 Suppl):70–6. doi: 10.1067/mhj.2000.109980. [DOI] [PubMed] [Google Scholar]
- Levy ML, Cummings JL, Fairbanks LA, Masterman D, Miller BL, Craig AH, et al. Apathy is not depression. Journal of Neuropsychiatry and Clinical Neurosciences. 1998;10(3):314–9. doi: 10.1176/jnp.10.3.314. [DOI] [PubMed] [Google Scholar]
- Lichtman JH, Bigger JT, Jr, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lespérance F, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American heart association prevention committee of the council on cardiovascular nursing, council on clinical cardiology, council on epidemiology and prevention, and interdisciplinary council on quality of care and outcomes research: endorsed by the American psychiatric association. Focus. 2009;7(3):406–13. doi: 10.1111/j.1751-7117.2009.00028.x. [DOI] [PubMed] [Google Scholar]
- Mathers C, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell BE, Bowers A, Carr D, Chardoul S, Cheung G, Dinkelmann K, et al. The development and implementation of the national comorbidity survey replication, the National Survey of American Life, and the National Latino and Asian American Survey. International Journal of Methods in Psychiatric Research. 2004;13(4):241–69. doi: 10.1002/mpr.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putaala J, Kurkinen M, Tarvos V, Salonen O, Kaste M, Tatlisumak T. Silent brain infarcts and leukoaraiosis in young adults with first-ever ischemic stroke. Neurology. 2009;72(21):1823–9. doi: 10.1212/WNL.0b013e3181a711df. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Pieper CF, Barch DM, McKinstry RC, MacFall JR, D’Angelo G, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Archives of General Psychiatry. 2010;67(3):277–85. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurology. 2007 Jul;6(7):611–9. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- WHO. World health organization disability assessment schedule II (WHODAS II) Geneva: World Health Organization; 2001. [Google Scholar]
- Williams DR, González HM, Neighbors H, Nesse R, Abelson JM, Sweetman J, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: results from the National Survey of American Life. Archives of General Psychiatry. 2007 Mar;64(3):305–15. doi: 10.1001/archpsyc.64.3.305. [DOI] [PubMed] [Google Scholar]
- Yakushiji Y, Nishiyama M, Yakushiji S, Hirotsu T, Uchino A, Nakajima J, et al. Brain microbleeds and global cognitive function in adults without neurological disorder. Stroke. 2008;39(12):3323–8. doi: 10.1161/STROKEAHA.108.516112. [DOI] [PubMed] [Google Scholar]