Abstract
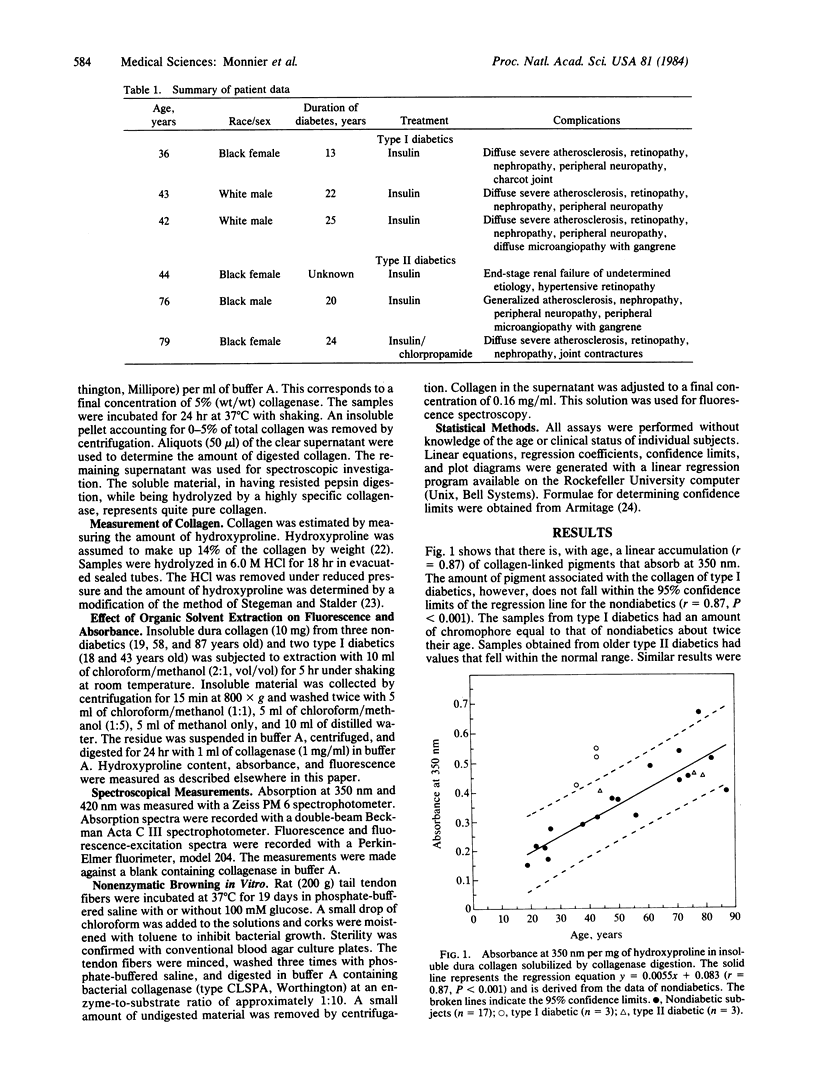
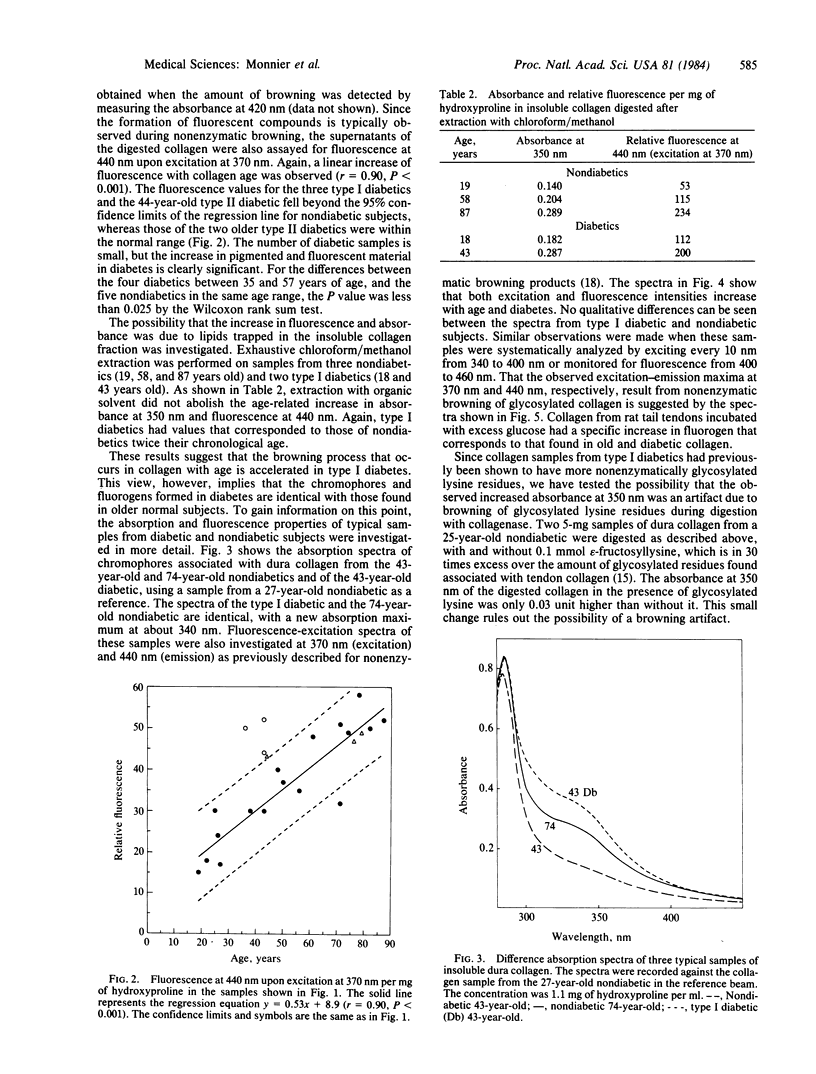
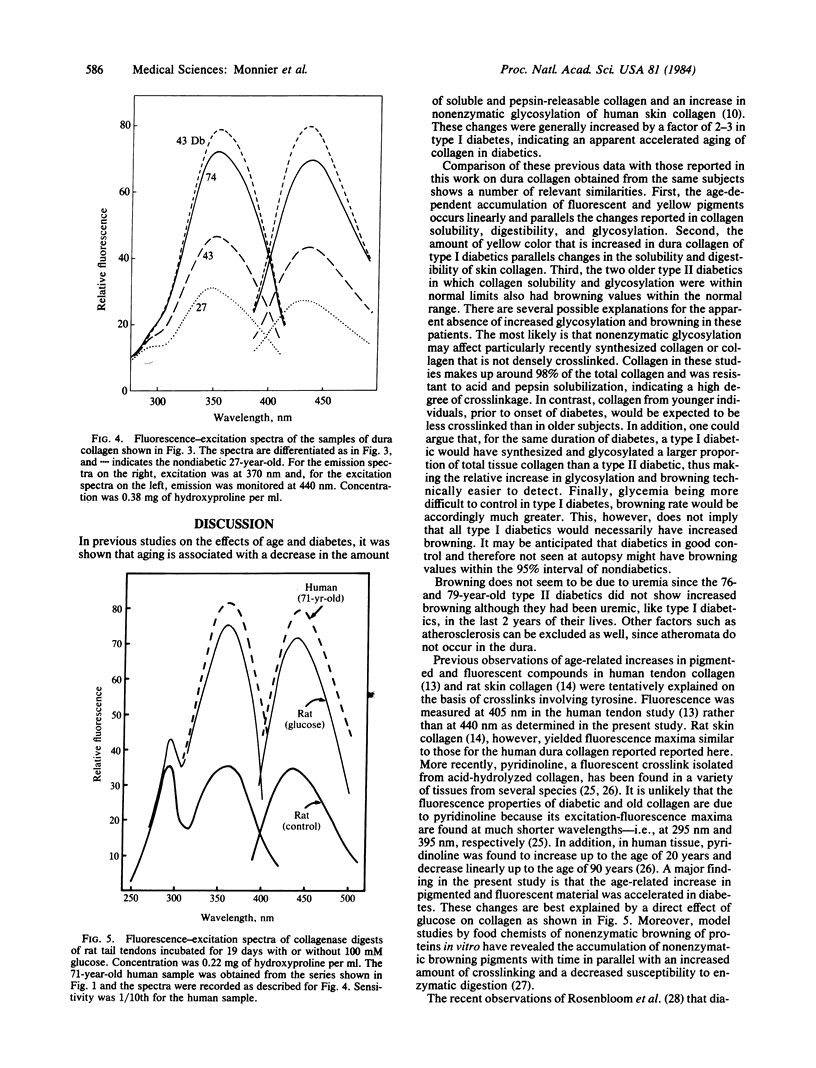
The nonenzymatic glycosylation reaction that is accelerated in diabetes is the first step of the Maillard or nonenzymatic browning reaction that occurs in stored food. The glucose-protein adduct rearranges and dehydrates to form brown and fluorescent pigments, which can act as crosslinks, resulting in decreased protein solubility and altered mechanical properties. Evidence suggesting that this process occurs in vivo has been found in lens crystallins. The observation that nonenzymatic glycosylation and insolubility increases in collagen with age and diabetes led us to investigate the possible browning of human collagen. Insoluble human dura mater collagen was digested with collagenase. Absorbance at 350 nm and fluorescence at 440 nm (excitation at 370 nm) of the solubilized material was measured. A linear increase in the amounts of yellow and fluorescent material was observed with age. Samples obtained at autopsy from three type I diabetics and a young type II diabetic showed increased fluorescence and had absorbance values that corresponded to the amount of chromophore found in nondiabetics twice their age (P less than 0.025). The collagen adducts from aged and diabetic individuals had absorption and fluorescence spectra identical to those of collagen samples that underwent nonenzymatic browning with glucose in vitro. The structure of these collagen adducts is unknown. However, their likely occurrence throughout the body could explain the correlation between arterial stiffening, decreased joint mobility, and the severity of microvascular complications in type I diabetics.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKERMAN S. Quantitative extraction of acid-soluble human skin collagen with age. Nature. 1962 Oct 27;196:375–376. doi: 10.1038/196375a0. [DOI] [PubMed] [Google Scholar]
- Chang K., Uitto J., Rowold E. A., Grant G. A., Kilo C., Williamson J. R. Increased collagen cross-linkages in experimental diabetes: reversal by beta-aminopropionitrile and D-penicillamine. Diabetes. 1980 Oct;29(10):778–781. doi: 10.2337/diacare.20.10.778. [DOI] [PubMed] [Google Scholar]
- Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979 Dec;28(12):1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- De Bats A., Rhodes E. L. Letter: Innate fluorescence in diabetic and aged kidneys. Lancet. 1974 Jan 26;1(7848):137–138. doi: 10.1016/s0140-6736(74)92375-7. [DOI] [PubMed] [Google Scholar]
- Deyl Z., Sulcová H., Praus R., Goldman J. N. A fluorescent compound in collagen and its relation to the age of the animal. Exp Gerontol. 1970 Apr;5(1):57–62. doi: 10.1016/0531-5565(70)90029-x. [DOI] [PubMed] [Google Scholar]
- Fujimoto D., Moriguchi T. Pyridinoline, a non-reducible crosslink of collagen. Quantitative determination, distribution, and isolation of a crosslinked peptide. J Biochem. 1978 Mar;83(3):863–867. doi: 10.1093/oxfordjournals.jbchem.a131983. [DOI] [PubMed] [Google Scholar]
- Grgic A., Rosenbloom A. L., Weber F. T., Giordano B., Malone J. I. Letter: Joint contracture in childhood diabetes. N Engl J Med. 1975 Feb 13;292(7):372–372. doi: 10.1056/nejm197502132920720. [DOI] [PubMed] [Google Scholar]
- Hamlin C. R., Kohn R. R. Evidence for progressive, age-related structural changes in post-mature human collagen. Biochim Biophys Acta. 1971 May 25;236(2):458–467. doi: 10.1016/0005-2795(71)90226-1. [DOI] [PubMed] [Google Scholar]
- Kannel W. B., McGee D. L. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979 May 11;241(19):2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- Klein L., Butcher D. L., Sudilovsky O., Kikkawa R., Miller M. Quantification of collagen in renal glomeruli isolated from human nondiabetic and diabetic kidneys. Diabetes. 1975 Dec;24(12):1057–1065. doi: 10.2337/diab.24.12.1057. [DOI] [PubMed] [Google Scholar]
- Kohn R. R. Effects of age and diabetes mellitus on cyanogen bromide digestion of human dura mater collagen. Connect Tissue Res. 1983;11(2-3):169–173. doi: 10.3109/03008208309004852. [DOI] [PubMed] [Google Scholar]
- LABELLA F. S. STRUCTURE OF COLLAGEN FROM HUMAN TENDON AS INFLUENCED BY AGE AND SEX. J Gerontol. 1965 Jan;20:54–59. doi: 10.1093/geronj/20.1.54. [DOI] [PubMed] [Google Scholar]
- Maekawa T., Rathinasamy T. K., Altman K. I., Forbes W. F. Changes in collagen with age. I. The extraction of acid soluble collagens from the skin of mice. Exp Gerontol. 1970 Jul;5(2):177–186. doi: 10.1016/0531-5565(70)90007-0. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Cerami A. Non-enzymatic glycosylation and browning of proteins in diabetes. Clin Endocrinol Metab. 1982 Jul;11(2):431–452. doi: 10.1016/s0300-595x(82)80023-6. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981 Jan 30;211(4481):491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- Moriguchi T., Fujimoto D. Age-related changes in the content of the collagen crosslink, pyridinoline. J Biochem. 1978 Oct;84(4):933–935. doi: 10.1093/oxfordjournals.jbchem.a132206. [DOI] [PubMed] [Google Scholar]
- Pillsbury H. C., 3rd, Hung W., Kyle M. C., Freis E. D. Arterial pulse waves and velocity and systolic time intervals in diabetic children. Am Heart J. 1974 Jun;87(6):783–790. doi: 10.1016/0002-8703(74)90427-x. [DOI] [PubMed] [Google Scholar]
- Reynolds T. M. Chemistry of nonenzymic browning. II. Adv Food Res. 1965;14:167–283. doi: 10.1016/s0065-2628(08)60149-4. [DOI] [PubMed] [Google Scholar]
- Rosenbloom A. L., Silverstein J. H., Lezotte D. C., Richardson K., McCallum M. Limited joint mobility in childhood diabetes mellitus indicates increased risk for microvascular disease. N Engl J Med. 1981 Jul 23;305(4):191–194. doi: 10.1056/NEJM198107233050403. [DOI] [PubMed] [Google Scholar]
- Schnider S. L., Kohn R. R. Effects of age and diabetes mellitus on the solubility and nonenzymatic glucosylation of human skin collagen. J Clin Invest. 1981 Jun;67(6):1630–1635. doi: 10.1172/JCI110198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnider S. L., Kohn R. R. Glucosylation of human collagen in aging and diabetes mellitus. J Clin Invest. 1980 Nov;66(5):1179–1181. doi: 10.1172/JCI109950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler M. R., Niewoehner D. E., Inkley S. R., Kohn R. Abnormal lung elasticity in juvenile diabetes mellitus. Am Rev Respir Dis. 1976 Jan;113(1):37–41. doi: 10.1164/arrd.1976.113.1.37. [DOI] [PubMed] [Google Scholar]
- Tanzer M. L., Fairweather R., Gallop P. M. Collagen crosslinks: isolation of reduced N -hexosylhydroxylysine from borohydride-reduced calf skin insoluble collagen. Arch Biochem Biophys. 1972 Jul;151(1):137–141. doi: 10.1016/0003-9861(72)90482-1. [DOI] [PubMed] [Google Scholar]
- Vogt B. W., Schleicher E. D., Wieland O. H. epsilon-Amino-lysine-bound glucose in human tissues obtained at autopsy. Increase in diabetes mellitus. Diabetes. 1982 Dec;31(12):1123–1127. doi: 10.2337/diacare.31.12.1123. [DOI] [PubMed] [Google Scholar]
- Vracko R., Thorning D., Huang T. W. Basal lamina of alveolar epithelium and capillaries: quantitative changes with aging and in diabetes mellitus. Am Rev Respir Dis. 1979 Nov;120(5):973–983. doi: 10.1164/arrd.1979.120.5.973. [DOI] [PubMed] [Google Scholar]
- WAINE H., NEVINNY D., ROSENTHAL J., JOFFE I. B. Association of osteoarthritis and diabetes mellitus. Tufts Folia Med. 1961 Jan-Mar;7:13–19. [PubMed] [Google Scholar]



