Abstract
Primary biliary cirrhosis (PBC) is a progressive cholestatic liver disease characterized by the immune-mediated destruction of biliary epithelial cells in small intrahepatic bile ducts. The disease is characterized by circulating antimitochondrial antibodies (AMAs) as well as disease-specific antinuclear antibodies, cholestatic liver function tests, and characteristic histological features, including granulomas. A variety of organisms are involved in granuloma formation, of which mycobacteria are the most commonly associated. This has led to the hypothesis that mycobacteria may be involved in the pathogenesis of PBC, along with other infectious agents. Additionally, AMAs are found in a subgroup of patients with mycobacterial infections, such as leprosy and pulmonary tuberculosis. Antibodies against species-specific mycobacterial proteins have been reported in patients with PBC, but it is not clear whether these antibodies are specific for the disease. In addition, data in support of the involvement of the role of molecular mimicry between mycobacterial and human mitochondrial antigens as triggers of cross-reactive immune responses leading to the loss of immunological tolerance, and the induction of pathological features have been published. Thus, antibodies against mycobacterial heat shock protein appear to cross-recognize AMA-specific autoantigens, but it is not clear whether these autoantibodies are mycobacterium-species-specific, and whether they are pathogenic or incidental. The view that mycobacteria are infectious triggers of PBC is intriguing, but the data provided so far are not conclusive.
Keywords: Antimitochondrial antibodies, Autoantibody, Autoimmunity, Cholestasis, Heat shock, Infection, Liver disease, Liver failure, Mycobacterium, Tuberculosis
INTRODUCTION
Primary biliary cirrhosis (PBC) is a chronic cholestatic, autoimmune liver disease characterized by inflammatory destruction of the small intrahepatic bile ducts, fibrosis progressing to cirrhosis[1-5], and subsequent liver failure[6,7]. PBC primarily affects middle-aged women, with an increased incidence within families[2,8,9]. The prevalence of the disease is estimated to be less than 1/2000[10]. Despite the variance noted amongst countries/ethnic groups, there is a notion that the incidence of the disease is increasing[10-13].
Several autoantibody profiles have been found to be specific for the disease[14], and aid in the diagnostic workup of PBC. These include antimitochondrial antibodies (AMAs)[15-19], and/or disease-specific antinuclear antibodies (ANAs)[20-22], which are found in both symptomatic and asymptomatic patients[23]. Most common symptoms at presentation are nonspecific and include fatigue, pruritus, Sicca symptomatology and arthralgia. In more severe cases, symptoms relate to portal hypertension and hepatic decompensation (jaundice, ascites or variceal bleeding), which may indicate the need for liver transplantation[2,5,24]. The progression of PBC is generally slow and unpredictable[6,24].
The diagnosis of PBC is based on the presence of serum AMA, biochemical markers of cholestasis and histological features on liver biopsy[2]. The diagnosis of PBC is likely when at least two of these criteria are met[2,4]. Biochemical indices of cholestasis include increased levels of alkaline phosphatase and γ glutamyltransferase (GT). The diagnostic hallmark of PBC is the presence of AMA in high titers, with less than 3%-10% of patients with PBC being negative for AMA[23]. Positivity for AMA appears to be predictive of future PBC development in asymptomatic individuals[17,25]. The prevailing notion is that the presence of AMAs and their titers/concentrations do not correlate with the severity of the disease[23]. AMA seropositivity appears unable to identify patients at risk for faster progression of their liver disease compared to the seronegative cases[19,26]. Disease-specific ANAs[27-30] are also found in PBC, to a lesser degree than AMAs (20%-50% vs 90%-97%)[31-34], but tend to have elevated titers, and more importantly, appear to characterize patients with a more aggressive form of the disease[35-38]. Levels of IgM are also raised in most cases[2,4]. Histologically, PBC features include destruction of biliary epithelial cells and loss of small bile ducts with portal inflammatory cell infiltration, and granuloma formation (see below)[2,4,5].
The antigenic specificity of AMA[15-19] and ANA[20-22] responses have been extensively studied. PBC-specific ANAs recognize either nuclear body proteins such as the speckled protein 100 and the promyelocytic leukemia protein, or the gp210 and nucleoporin 62 nuclear membrane proteins[28,29,39-41].
AMAs in PBC are directed against the 2-oxo-acid dehydrogenase complex family of enzymes, and in particular the E2 subunits of pyruvate dehydrogenase complex (PDC-E2), branched-chain 2-oxo acid dehydrogenase complex (BCOADC) and 2-oxoglutarate dehydrogenase complex (OGDC)[2,42-44]. PDC-E2 reactivity is found in > 95% of patients with PBC, and 70% recognize BCOADC-E2 and/or OGDC-E2[2]. Reactivity to all three antigens occurs in < 50% of patients[19]. The immunodominant antigenic regions recognized by (CD4 and CD8) T lymphocytes[45-47] on PDC-E2[48-50] comprise a region within the inner lipoyl-binding domain of the subunit, spanning amino acids 212-226 (PDC-E2212-226)[51-53]. This region is also the core target of B cell receptors, which are antibodies in their soluble form[54-56].
Medical treatment of PBC includes ursodeoxycholic acid, with the best response seen in patients who initiate treatment early in the disease[2]. These patients often show decreased or even normalized levels of alkaline phosphatase (ALP), γGT and other markers of cholestasis[2,4,6]. Studies reporting findings in large North American and European patient cohorts indicate that the percentage of patients with PBC who require liver transplantation has fallen significantly[2,57].
The cause of PBC remains undetermined[58-61], but it is believed to be the result of a genetic predisposition compounded with several lifetime exposures[62,63], similar to a “multi-hit” model[61,64-67] of disease pathogenesis[50,68,69]. Recent genome-wide association studies[70,71] have identified several HLA[72] and non-HLA[73-76] genes to be associated with PBC. Environmental factors implicated are numerous[77-81], and range from cosmetic products and xenobiotics[82], to estrogen deficiency and infectious organisms[50,58,83-85] including bacteria and viruses[50,54,58,59,63,65,66,68,69]. Mycobacteria have been included in the list of infectious organisms, partially due to the presence of granulomas in the histopathology of PBC, and the association of granulomas with mycobacteria[2,86]. In addition, AMA is found in some patients with mycobacterium-related infections[87,88]. This review will critically analyze the evidence surrounding the role of mycobacteria in the pathogenesis of PBC.
GRANULOMAS IN PBC
Granulomas consist of focal collections of inflammatory cells and cellular debris[89-92]. Their formation occurs when nondegradable products persist, as well as in hypersensitivity reactions or a combination of the two[91]. They form in a complex process involving the interaction of the infectious organism, antigen, macrophages, T cell responses, B cell hyper-reactivity, and circulating mediators[91]. T cells involved in granuloma formation may be of the T helper (Th)1 or Th2 type[91]. Several organisms may initiate granuloma formation, including Mycobacterium, Yersinia, Toxoplasma gondii and Bartonella henselae[93,94]. Zumla et al[91] have noted three categories of granulomatous infections: those due to well recognized organisms; those due to organisms that are detected by molecular techniques but not by conventional microbiological techniques; and those due to organisms that have not been identified, but which are suspected. Mycobacterium tuberculosis (M. tuberculosis) is the most common organism associated with granulomas, and is histologically characterized by epithelioid cells, lymphocytes, histiocytes, Langerhans giant cells and fibroblasts surrounding a core of necrotic debris[91]. The necrotic core is usually caseating, although non-caseating granulomas may also occur[91,92].
The presence of granulomas in liver biopsies has been well documented, with a prevalence ranging from 2% to 15% in some studies, with geographical differences in prevalence rates (Table 1)[95-99]. A German study conducted by Drebber et al[100] examined 12 161 liver biopsies for the presence of granulomas, in addition to determining the etiology of the granuloma through histology, clinicopathological data, and polymerase chain reaction (PCR) for the detection of infectious organisms. Granulomas were found in 442 (3.6%) of the liver biopsies and interestingly, 215 were from PBC patients (1.8% of all biopsies, and 48.7% of all biopsies with granulomas)[100]. PCR demonstrated the presence of infectious organisms in 15 samples (3.4%), with M. tuberculosis being detected in three of the 15 (20%). It was not indicated whether any of the samples with positive PCR results came from PBC patients[100]. Although only 1.8% of liver biopsies contained granulomas, nearly half of these were obtained from patients with PBC. However, if mycobacterial infection with associated granuloma formation is a feature of PBC, a much higher percentage of granulomas would be expected. The preponderance of granulomas in liver biopsies from PBC patients has been demonstrated in other studies as well, which show geographical differences in prevalence rates. In a Northern Irish study, 55% of liver biopsies with granulomas were from PBC patients[96], compared to 23.8% in a United Kingdom study[95]. A Greek study indicates an underlying diagnosis of PBC or overlap syndrome in 62% of liver biopsies with granulomas, followed by viral hepatitis in 7.5%, and autoimmune hepatitis (AIH) in 6%[101]. Despite these findings, it should be noted that only a small percentage of granulomas can be attributed to mycobacteria, with the liver disease in these patients being due to infection and not to PBC. Figure 1 illustrates PBC-related liver granulomas contrasted with liver granulomas seen in conditions not directly related to PBC such as sarcoidosis and schistosomiasis.
Table 1.
Prevalence of granulomas in liver disease n (%)
| Ref. | Origin | Total number liver biopsies | Total number liver biopsies with granulomas | Diagnosis of PBC in granuloma group |
| Drebber et al[100] | Germany | 12 161 | 442 (3.6) | 215 (48.6) |
| Dourakis et al[101] | Greece | 1768 | 66 (3.7) | 41 (62) |
| Gaya et al[95] | United Kingdom | 1662 | 63 (3.8) | 15 (23.8) |
| McCluggage et al[96] | Northern Ireland | 4075 | 163 (4) | 90 (55) |
| Satti et al[99] | Saudi Arabia | 404 | 59 (14.6) | Unknown |
PBC: Primary biliary cirrhosis.
Figure 1.
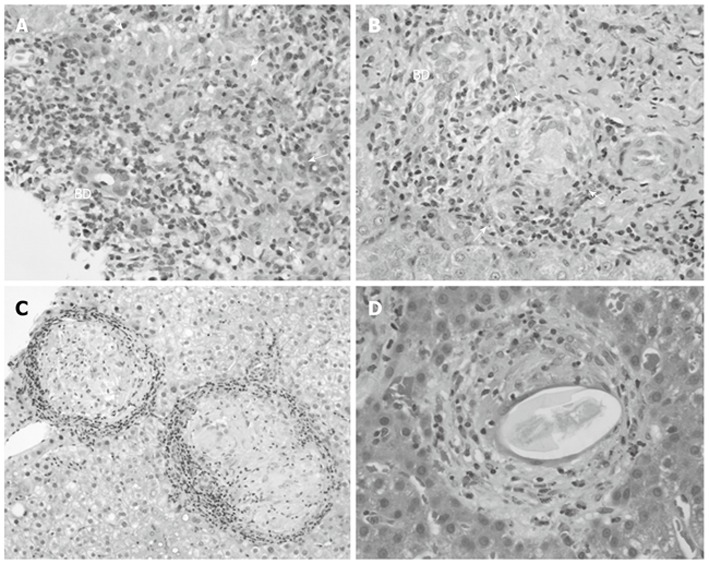
Epithelioid granuloma in liver biopsy. A, B: Liver specimens from patients with primary biliary cirrhosis show granuloma (arrows) close to bile ducts (BD) in portal tracts; C: Granuloma in sarcoidosis is more discrete and large in size; D: Granuloma associated with schistosomiasis contains a large number of eosinophils.
The predominance of PBC as the underling liver disease in most cases of hepatic granulomas and the association of granulomas with mycobacteria, raise the question as to whether mycobacteria play a role in the pathogenesis of PBC[58,86,102]. If mycobacteria play a role in the pathogenesis of PBC (or at least in some cases of PBC), it would be expected that certain PBC-specific features, such as disease-specific AMA positivity, would also be present in patients with mycobacterial infections. Likewise, evidence of mycobacterial infection would be expected in PBC patients. This evidence may comprise more prevalent immune responses against mycobacterial antigens, or detectable mycobacterial DNA in liver tissues of PBC patients, compared to healthy and pathological controls. If mycobacterial infection plays a role in the pathogenesis of PBC, it is likely that it is limited to a triggering event, because PBC has been known to recur following transplantation[103-106] in up to 35% of patients[107]. The recurrence rate ranges from 21% to 37% at 10 years, to 43% at 15 years[108-111]. Immunosuppression with tacrolimus, as well as warm ischemic time, is associated with an increased risk of recurrence[108-112]. The more aggressive course of recurrent disease suggests that the pathological process involved in the development of PBC persists after transplantation[113]. This does not, however, suggest that the triggers of PBC also persist, and may indicate their transient involvement in the pathogenesis of PBC[113]. Neuberger highlights that hepatitis A virus can trigger autoimmune hepatitis after viral clearance, and also indicates there are several similarities between the recurrence of hepatitis C virus and PBC after transplantation, suggesting a possible infectious trigger of PBC in some individuals[113]. Although these theories are plausible, no conclusive evidence exists to demonstrate an infectious agent (including mycobacteria) as the trigger of PBC.
PBC-specific features in patients with mycobacterial infection
In an analysis of sera from 28 patients with active pulmonary tuberculosis (M. tuberculosis infection), Klein and colleagues[88] found that 43% of these sera recognized PDC-E2 by immunoblotting based on purified mitochondrial fraction derived from beef heart mitochondria as an antigenic source. Only 2% of sera from individuals with other viral and bacterial infections [including 25 individuals with Escherichia coli (E. coli)] reacted with PDC-E2, and there was no reaction with sera from healthy controls[88]. The titers of anti-PDC-E2 antibodies were low, with no seropositive cases showing immunofixation of the 70-kDa band corresponding to PDC-E2, at a dilution of < 1/500[88]. Of relevance, none of the anti-PDC-E2 antibody-positive sera (1/10 dilution) gave an immunofluorescent pattern typical of PBC when tested by indirect immunofluorescence based on rat kidney and stomach tissue as substrates[14,16,17,88]. Among the 12 AMA-positive cases with active tuberculosis, six had abnormal levels of γGT and four had slightly elevated levels of alkaline phosphatase; only two of the cases had increased IgM levels[88]. Two of the 12 cases had alcoholic disease and of the remaining 10, none had clinical evidence of chronic liver disease[88].
A variety of autoantibodies have also been reported in the sera of patients with leprosy [caused by Mycobacterium leprae (M. leprae)], including antibodies against mitochondrial antigens[87,114,115]. In one study, the most common autoantibodies found in leprosy patients were antibodies to SS-B and mitochondria, including cardiolipin[115]. Despite the presence of AMA in the sera of leprosy patients, very few develop liver disease. Gilburd and colleagues[87] screened 69 leprosy patients with no clinical or biochemical evidence of liver disease, for the presence of anti-PDC antibodies. Positive controls consisted of three PBC patients and 18 healthy individuals served as negative controls[87]. Twenty-seven (39%) patients were found to have elevated anti-PDC antibodies by enzyme linked immunosorbent assay, but the absorbance values were relatively low; none of the normal controls had detectable AMA reactivity[87]. By immunoblotting, AMA reactivity was directed against the 54-, 41- and 35-kDa PDC subunits, and only two leprosy patients reacted with PDC-E2[87]. Inhibition studies showed that in contrast to PBC sera, none of the AMA-positive sera gave significant PDC enzyme inhibition; the rate of inhibition being similar to that noted in normal controls. Immunofluorescence testing was not performed[87].
Although AMAs are found in patients infected with mycobacteria, the reactivity patterns observed differ, with M. tuberculosis-infected patients showing reactivity to PDC-E2, which has not been observed in those infected with M. leprae[87,88]. The source of the AMAs in either case remains unknown. Gilburd et al[87] have suggested that the AMAs seen in leprosy patients may arise from the presence of bacterial antigens (such as M. leprae), which share sequence homology with the 35-, 41- and 54-kDa subunits. It is not clear whether all bands correspond to PDC subunits (such as PDC-E1 α and β) but may indeed be contaminants of other 2-oxo-acid dehydrogenase complexes (formerly known as M2 antigen) such as BCOADC-E2 and OGDC-E2, but reactivity to these antigens has not been tested[87].
Evidence of mycobacterial infection in PBC
In the search for evidence of antimycobacterial antibody reactivity in patients with PBC, Vilagut et al[86] investigated a cohort of 19 PBC patients from Spain, using membrane extracts from 10 atypical mycobacteria and found that all cases specifically reacted with Mycobacterium gordonae (M. gordonae) antigens of approximately 65 kDa and 55 kDa. It remained elusive why the other nine atypical mycobacteria were not targets of antibody responses. The unreactive mycobacterial membranes were those prepared from Mycobacterium chelonei, Mycobacterium flavescens, Mycobacterium fortuitum (M. fortuitum), Mycobacterium intracellulare, Mycobacterium kansasii (M. kansasii), Mycobacterium malmoense (M. malmoense), Mycobacterium scrofulaceum, Mycobacterium xenopi (M. xenopi), and Mycobacterium terrae[86]. The extraction of the 10 atypical mycobacterial membranes was performed using the same protocol, therefore, Vilagut et al[86] considered that their findings were not a consequence of methodological problems and suggested a biological significance for the role of M. gordonae in the pathogenesis of PBC.
This intriguing finding has initiated a series of subsequent studies from the same investigators as well as from independent groups[116-120]. Following the original Spanish report, a study was conducted at King’s College Hospital in London investigating reactivity to M. gordonae and seven other atypical mycobacteria[119]. O’Donohue et al[119] found that 23 of 26 (88%) PBC sera reacted with a 65-kDa protein in extracts of six of the mycobacterial species tested (M. gordonae, M. kansasii, M. fortuitum, Mycobacterium chelonae, Mycobacterium szulgai and M. malmoense). Also, 15 and nine of these samples reacted with the 65-kDa band in membrane extracts of Mycobacterium avium-intracellulare and M. xenopi, respectively. However, the antibody reactivity to M. gordonae or other atypical mycobacteria was not restricted to PBC, but was also present in a similar prevalence in patients with other chronic liver diseases as well as in normal controls[119].
Several studies have attempted to provide evidence of mycobacterial infection in liver tissues from PBC patients. Broomé and colleagues[121] conducted an immunohistochemical study investigating liver biopsy specimens from 10 PBC cases, 13 from primary sclerosing cholangitis, five chronic hepatitis C, four alcoholic liver disease, and six healthy controls. Samples were studied using a monoclonal antibody specific for mycobacterial heat shock protein (hsp) 65. Positive staining was observed in nine of the 10 PBC cases, all primary sclerosing cholangitis, three chronic hepatitis C, and three alcoholic liver disease cases, but in none of the healthy controls[121]. Both interlobular and septal bile duct staining was observed in the PBC cases, with perinuclear staining in all positive interlobular ductal cells, and perinuclear as well as apical staining in the positive septal cells[121].
Direct evidence of microbial products in an affected tissue is best studied at the molecular level using PCR techniques. Vilagut et al[116] assessed the presence of M. gordonae DNA in liver tissue from PBC patients and controls using PCR based on amplification of a 565-bp fragment of mycobacterial gene coding for 16S rRNA on M. gordonae. They detected M. gordonae in nine of 11 (82%) PBC liver tissues but in none of the six control livers from patients with other liver diseases[116]. Contradictory results were obtained by O’Donohue et al[118] who investigated whether mycobacterial DNA could be detected in archival liver biopsy material from PBC and AIH. Archival material was obtained from 11 PBC and 11 AIH cases, with five lymph nodes from patients with tuberculous lymphadenopathy acting as positive controls[118]. Three of the positive controls also had liver biopsies taken for concurrent tuberculous hepatitis. No mycobacterial DNA was detected in PBC or AIH cases, while four of the five positive controls had detectable mycobacterial DNA[118]. A similar study also utilized a PCR approach for the detection of mycobacterial and other organisms in archived (paraffin embedded) liver tissues from 29 cases of PBC, as well as pathological and healthy controls. Again, no mycobacterial DNA was detected in PBC samples, and only Helicobacter pylori DNA was found in one case of PBC[122].
Mycobacteria and PBC: The role of molecular mimicry
The above studies do raise doubt as to whether mycobacteria play a role in PBC, although it is unclear whether a negative detection test only implies that there is no current active infection or can rule out previous infection as well. However, it has been suggested that mycobacteria and other infectious agents may not be actively present in PBC patients, but rather involved in the initiation of autoimmunity by microbial/self immunological cross-reactivity[55,56,123,124]. We and others have studied the role of molecular mimicry[68,69,123,125-128] and immunological cross-reactivity[129-133] as a mechanism responsible for the induction of autoantigen-specific immune responses in viral-hepatitis-triggered autoimmunity and several autoimmune diseases (including those affecting the liver)[134-138] in susceptible individuals[139,140]. Impairments in T-regulatory functions also appear to be a feature[141,142]. Cross-reactivity between PBC-specific mitochondrial antigens and mycobacterial proteins has been investigated by two groups[86,117]. Vilagut et al[86] have found that antibody responses to the 65-kDa M. gordonae antigen cross-reacted with anti-PDC-E2 antibodies. The same group of investigators went on to demonstrate that the 65-kDa protein was the mycobacterial hsp65, and that preincubation with PBC sera prevented binding of antibodies against hsp65[117]. The apparent cause of this cross-reactivity remained elusive until another group noted a sequence similarity between human PDC-E2 and M. gordonae hsp65[120]. These authors have found that the hexameric motif [GDL(IL)AE)] is shared by M. gordonae hsp6594-99 and the major PBC-specific mitochondrial autoepitopes, namely, the inner lipoyl PDC-E2216-221 and the outer lipoyl domain human PDC-E2102-107[120]. No other sequences were found to be in common with M. gordonae hsp65 and human PDC-E2[120]. A database search analysis has found that, among bacteria, the motif SxGDL[IL]AE is virtually unique to mycobacterial hsp, and the only human sequence containing that motif was the inner lipoyl domain of PDC-E2[120]. This excellent and almost unique match between sequences of the dominant epitope of human PDC-E2 and of mycobacterial hsp65 has led the authors to investigate whether the corresponding sequences were targets of immunological cross-reactivity specifically present in patients with PBC. That study involved testing sera from 40 Spanish and 50 British PBC patients, with antibody reactivity to M. gordonae hsp 6590-104/human PDC-E2212-226 being detected in 47.5% of Spanish PBC patients and in only 4% of British PBC patients[120]. No reactivity was observed in controls. Inhibition studies confirmed that the reactivity to the mimics was due to cross-reactivity. In addition, the affinity of anti-M. gordonae hsp 6590-104 antibodies was higher than that against human PDC-E2212-226, raising the possibility that antibody reactivity to the microbe long precedes that against the human homolog[120]. Table 2 provides a summary of the above evidence for and against the role of mycobacteria in PBC.
Table 2.
Summary of studies in support or against a role of mycobacteria as triggers of primary biliary cirrhosis
| Ref. | Year | Mycobacterium | M2 antigen | In support | Against |
| Klein et al[88] | 1993 | M. tuberculosis | Bovine heart | Anti-M2 AMAs (70 kDa) found in 12/28 (43%) tuberculosis patients | None of the patients had PBC |
| AMA by IIFL were negative | |||||
| Anti-PDC-E2 titers were low | |||||
| Gilburd et al[87] | 1994 | M. leprae | Bovine heart | Anti-M2 against 54-, 41- and 35-kDa bands found in 27/69 (39.1%) leprosy patients | None of the patients had PBC |
| Only 2/27 (1%) sera reacted with the PDC-E2 band | |||||
| Anti-M2 inhibited anti-PDC activity in 19.1% | |||||
| Vilagut et al[86] | 1994 | M. gordonae | Porcine heart | Anti-hsp65 M. gordonae antibodies found in 19/19 (100%) of PBC patients. No reactivity to other atypical mycobacteria was found | |
| Anti-porcine AMAs (anti-PDC-E2 and BCOADC-E2) cross-reacted with anti-hsp65 M. gordonae antibodies and vice versa | |||||
| O’ Donohue et al[119] | 1994 | M. gordonae, M. kansasii, M. fortuitum, M. chelonae, M. szulgai and M. malmoense, M. avium-intracellulare and M. xenopi | 23 of 26 (88%) PBC sera reacted with a 65-kDa protein in extracts of six of the mycobacterial species tested | Reactivity to the atypical mycobacterial mimics was found in non-PBC liver disease patients and controls | |
| Sera from 15 PBC patients reacted with M. avium-intracellulare | |||||
| Sera from 9 PBC patients reacted with M. xenopi | |||||
| Bogdanos et al[54] | 2004 | M. gordonae | Primate liver | The motif GDL(IL)AE is shared by M. gordonae hsp6594-99 and PDC-E2216–221 | Reactivity to M. gordonae hsp6590-104 /human PDC-E2212-226 seen in Spanish (47.5%), but rarely British patients (4%) |
| Broome et al[121] | 1993 | - | - | Immunohistochemistry demonstrated positive mycobacterial hsp65 staining in PBC liver tissue | Positive staining was also observed in pathological controls |
| Vilagut et al[116] | 1996 | M. gordonae | - | M. gordonae genetic material detected in 9 of 11 (82%) PBC liver tissues | |
| O’Donohue et al[118] | 1998 | M. gordonae | - | Mycobacterial DNA not detected in PBC liver tissues, but was detected in positive controls | |
| Tanaka et al[122] | 1999 | Mycobacterial genus specific primers | - | Mycobacterial DNA not detected in PBC liver tissues |
M. tuberculosis: Mycobacterium tuberculosis; M. leprae: Mycobacterium leprae; M. gordonae: Mycobacterium gordonae; M. kansasii: Mycobacterium kansasii; M. fortuitum: Mycobacterium fortuitum; M. chelonae: Mycobacterium chelonae; M. szulgai: Mycobacterium szulgai; M. malmoense: Mycobacterium malmoense; M. avium-intracellulare: Mycobacterium avium-intracellulare; M. xenopi: Mycobacterium xenopi; IIFL: Indirect immunofluorescence; PDC: Pyruvate dehydrogenase complex; PBC: Primary biliary cirrhosis; AMAs: Antimitochondrial antibodies; hsp: Heat shock protein.
It remains unclear whether the differing reactivity patterns noted among PBC patients from differing geographical locations (highly prevalent in Spanish PBC cases, practically absent in British PBC cases), could be explained by differing prevalence exposure rates of certain atypical mycobacteria in geographical locations. It should also be noted that multinational epidemiological studies on PBC have not indicated an association with mycobacterial infection, in contrast to what has been reported for E. coli or other microbes[77,78,80,81].
CONCLUSION
The presence of granulomas in liver biopsies of PBC patients raises the suspicion that bacterial infections are involved in the pathogenesis of PBC. Although infection with several bacterial species is associated with granulomatous disease, mycobacteria are the most common culprits. It is therefore not surprising that mycobacteria have been added to the list of infectious agents implicated in the pathogenesis of PBC. Furthermore, it has been observed that AMAs, which are characteristic of PBC, are also found in many patients with mycobacterial infections but their titers are low, their epitope specificity differs from that seen in PBC, and they do not appear to be detectable by conventional immunofluorescence. Additionally, mycobacteria have not been found in any significant proportion of hepatic granulomas associated with PBC. When found, the liver disease is often attributed to infection and not PBC.
It is unlikely that ongoing mycobacterial infection is a characteristic of PBC, but an early, transient infection with immunological clearance may be capable of inducing cross-reactivity. Genetic studies on PBC are now demonstrating a genetic susceptibility to the disease, and it is likely that a variety of other factors act in an additive fashion towards PBC development. These factors probably not only vary from patient to patient, but also from one geographical location to the next. A correlation between PBC and certain organisms in the context of their geographical prevalence is warranted. In addition, sequence homology between PDC-E2 and mycobacterial epitopes at the T-cell level has not been fully explored, which is of interest given that differing reactivity patterns have been observed between a variety of mycobacteria and the major mitochondrial epitope of PBC. Experimental studies in animal models of PBC involving mycobacteria may provide useful hints as to whether mycobacteria play a role in the induction of PBC. They may also distinguish between mycobacteria being a trigger of the disease, or being epiphenomena secondary to an increased susceptibility to infection in PBC patients.
Footnotes
Peer reviewer: Radan Bruha, MD, PhD, Associate Professor, 4th Department of Internal Medicine, General Teaching Hospital, Charles University, U Nemocnice 2, 128 08 Prague, Czech Republic
S- Editor Cheng JX L- Editor Kerr C E- Editor Zhang DN
References
- 1.Hohenester S, Oude-Elferink RP, Beuers U. Primary biliary cirrhosis. Semin Immunopathol. 2009;31:283–307. doi: 10.1007/s00281-009-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 3.Kumagi T, Heathcote EJ. Primary biliary cirrhosis. Orphanet J Rare Dis. 2008;3:1. doi: 10.1186/1750-1172-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50:291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 5.Neuberger J. Primary biliary cirrhosis. Lancet. 1997;350:875–879. doi: 10.1016/S0140-6736(97)05419-6. [DOI] [PubMed] [Google Scholar]
- 6.Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology. 2000;31:1005–1013. doi: 10.1053/he.2000.5984. [DOI] [PubMed] [Google Scholar]
- 7.Poupon R. Primary biliary cirrhosis: a 2010 update. J Hepatol. 2010;52:745–758. doi: 10.1016/j.jhep.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 8.Jones DE, Palmer JM, Leon MP, Yeaman SJ, Bassendine MF, Diamond AG. T cell responses to tuberculin purified protein derivative in primary biliary cirrhosis: evidence for defective T cell function. Gut. 1997;40:277–283. doi: 10.1136/gut.40.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smyk D, Cholongitas E, Kriese S, Rigopoulou EI, Bogdanos DP. Primary biliary cirrhosis: family stories. Autoimmune Dis. 2011;2011:189585. doi: 10.4061/2011/189585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James OF, Bhopal R, Howel D, Gray J, Burt AD, Metcalf JV. Primary biliary cirrhosis once rare, now common in the United Kingdom? Hepatology. 1999;30:390–394. doi: 10.1002/hep.510300213. [DOI] [PubMed] [Google Scholar]
- 11.Kim WR, Lindor KD, Locke GR, Therneau TM, Homburger HA, Batts KP, Yawn BP, Petz JL, Melton LJ, Dickson ER. Epidemiology and natural history of primary biliary cirrhosis in a US community. Gastroenterology. 2000;119:1631–1636. doi: 10.1053/gast.2000.20197. [DOI] [PubMed] [Google Scholar]
- 12.Sood S, Gow PJ, Christie JM, Angus PW. Epidemiology of primary biliary cirrhosis in Victoria, Australia: high prevalence in migrant populations. Gastroenterology. 2004;127:470–475. doi: 10.1053/j.gastro.2004.04.064. [DOI] [PubMed] [Google Scholar]
- 13.Selmi C, Invernizzi P, Keeffe EB, Coppel RL, Podda M, Rossaro L, Ansari AA, Gershwin ME. Epidemiology and pathogenesis of primary biliary cirrhosis. J Clin Gastroenterol. 2004;38:264–271. doi: 10.1097/00004836-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Bogdanos DP, Mieli-Vergani G, Vergani D. Autoantibodies and their antigens in autoimmune hepatitis. Semin Liver Dis. 2009;29:241–253. doi: 10.1055/s-0029-1233533. [DOI] [PubMed] [Google Scholar]
- 15.Bogdanos DP, Baum H, Vergani D. Antimitochondrial and other autoantibodies. Clin Liver Dis. 2003;7:759–779, vi. doi: 10.1016/s1089-3261(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 16.Bogdanos DP, Invernizzi P, Mackay IR, Vergani D. Autoimmune liver serology: current diagnostic and clinical challenges. World J Gastroenterol. 2008;14:3374–3387. doi: 10.3748/wjg.14.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogdanos DP, Komorowski L. Disease-specific autoantibodies in primary biliary cirrhosis. Clin Chim Acta. 2011;412:502–512. doi: 10.1016/j.cca.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Bogdanos DP, Liaskos C, Rigopoulou EI, Dalekos GN. Anti-mitochondrial antibodies in patients with systemic lupus erythematosus: revealing the unforeseen. Clin Chim Acta. 2006;373:183–184; author reply 185. doi: 10.1016/j.cca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Leung PS, Coppel RL, Ansari A, Munoz S, Gershwin ME. Antimitochondrial antibodies in primary biliary cirrhosis. Semin Liver Dis. 1997;17:61–69. doi: 10.1055/s-2007-1007183. [DOI] [PubMed] [Google Scholar]
- 20.Bogdanos DP, Pares A, Rodés J, Vergani D. Primary biliary cirrhosis specific antinuclear antibodies in patients from Spain. Am J Gastroenterol. 2004;99:763–764; author reply 765. doi: 10.1111/j.1572-0241.2004.04119.x. [DOI] [PubMed] [Google Scholar]
- 21.Bogdanos DP, Vergani D, Muratori P, Muratori L, Bianchi FB. Specificity of anti-sp100 antibody for primary biliary cirrhosis. Scand J Gastroenterol. 2004;39:405–406; author reply 407. doi: 10.1080/00365520310008412. [DOI] [PubMed] [Google Scholar]
- 22.Invernizzi P, Podda M, Battezzati PM, Crosignani A, Zuin M, Hitchman E, Maggioni M, Meroni PL, Penner E, Wesierska-Gadek J. Autoantibodies against nuclear pore complexes are associated with more active and severe liver disease in primary biliary cirrhosis. J Hepatol. 2001;34:366–372. doi: 10.1016/s0168-8278(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 23.Muratori L, Granito A, Muratori P, Pappas G, Bianchi FB. Antimitochondrial antibodies and other antibodies in primary biliary cirrhosis: diagnostic and prognostic value. Clin Liver Dis. 2008;12:261–276; vii. doi: 10.1016/j.cld.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Kumagi T, Onji M. Presentation and diagnosis of primary biliary cirrhosis in the 21st century. Clin Liver Dis. 2008;12:243–259; vii. doi: 10.1016/j.cld.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Metcalf JV, Mitchison HC, Palmer JM, Jones DE, Bassendine MF, James OF. Natural history of early primary biliary cirrhosis. Lancet. 1996;348:1399–1402. doi: 10.1016/S0140-6736(96)04410-8. [DOI] [PubMed] [Google Scholar]
- 26.Invernizzi P, Crosignani A, Battezzati PM, Covini G, De Valle G, Larghi A, Zuin M, Podda M. Comparison of the clinical features and clinical course of antimitochondrial antibody-positive and -negative primary biliary cirrhosis. Hepatology. 1997;25:1090–1095. doi: 10.1002/hep.510250507. [DOI] [PubMed] [Google Scholar]
- 27.Bogdanos DP, Liaskos C, Pares A, Norman G, Rigopoulou EI, Caballeria L, Dalekos GN, Rodes J, Vergani D. Anti-gp210 antibody mirrors disease severity in primary biliary cirrhosis. Hepatology. 2007;45:1583; author reply 1583–1584. doi: 10.1002/hep.21678. [DOI] [PubMed] [Google Scholar]
- 28.Miyachi K, Hankins RW, Matsushima H, Kikuchi F, Inomata T, Horigome T, Shibata M, Onozuka Y, Ueno Y, Hashimoto E, et al. Profile and clinical significance of anti-nuclear envelope antibodies found in patients with primary biliary cirrhosis: a multicenter study. J Autoimmun. 2003;20:247–254. doi: 10.1016/s0896-8411(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 29.Muratori P, Muratori L, Ferrari R, Cassani F, Bianchi G, Lenzi M, Rodrigo L, Linares A, Fuentes D, Bianchi FB. Characterization and clinical impact of antinuclear antibodies in primary biliary cirrhosis. Am J Gastroenterol. 2003;98:431–437. doi: 10.1111/j.1572-0241.2003.07257.x. [DOI] [PubMed] [Google Scholar]
- 30.Rigopoulou EI, Davies ET, Pares A, Zachou K, Liaskos C, Bogdanos DP, Rodes J, Dalekos GN, Vergani D. Prevalence and clinical significance of isotype specific antinuclear antibodies in primary biliary cirrhosis. Gut. 2005;54:528–532. doi: 10.1136/gut.2003.036558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liaskos C, Bogdanos DP, Rigopoulou EI, Dalekos GN. Development of antimitochondrial antibodies in patients with autoimmune hepatitis: art of facts or an artifact? J Gastroenterol Hepatol. 2007;22:454–455. doi: 10.1111/j.1440-1746.2006.04751.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Norman GL, Shums Z, Worman HJ, Krawitt EL, Bizzaro N, Vergani D, Bogdanos DP, Dalekos GN, Milkiewicz P, et al. PBC screen: an IgG/IgA dual isotype ELISA detecting multiple mitochondrial and nuclear autoantibodies specific for primary biliary cirrhosis. J Autoimmun. 2010;35:436–442. doi: 10.1016/j.jaut.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Mytilinaiou MG, Bogdanos DP. Primary biliary cirrhosis-specific autoantibodies in patients with systemic sclerosis. Dig Liver Dis. 2009;41:916; author reply 916–917. doi: 10.1016/j.dld.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Rigopoulou EI, Davies ET, Bogdanos DP, Liaskos C, Mytilinaiou M, Koukoulis GK, Dalekos GN, Vergani D. Antimitochondrial antibodies of immunoglobulin G3 subclass are associated with a more severe disease course in primary biliary cirrhosis. Liver Int. 2007;27:1226–1231. doi: 10.1111/j.1478-3231.2007.01586.x. [DOI] [PubMed] [Google Scholar]
- 35.Dähnrich C, Pares A, Caballeria L, Rosemann A, Schlumberger W, Probst C, Mytilinaiou M, Bogdanos D, Vergani D, Stöcker W, et al. New ELISA for detecting primary biliary cirrhosis-specific antimitochondrial antibodies. Clin Chem. 2009;55:978–985. doi: 10.1373/clinchem.2008.118299. [DOI] [PubMed] [Google Scholar]
- 36.Nakanuma Y, Harada K, Kaji K, Terasaki S, Tsuneyama K, Moteki S, Van de Water J, Leung PS, Gershwin ME. Clinicopathological study of primary biliary cirrhosis negative for antimitochondrial antibodies. Liver. 1997;17:281–287. doi: 10.1111/j.1600-0676.1997.tb01033.x. [DOI] [PubMed] [Google Scholar]
- 37.Van Norstrand MD, Malinchoc M, Lindor KD, Therneau TM, Gershwin ME, Leung PS, Dickson ER, Homburger HA. Quantitative measurement of autoantibodies to recombinant mitochondrial antigens in patients with primary biliary cirrhosis: relationship of levels of autoantibodies to disease progression. Hepatology. 1997;25:6–11. doi: 10.1002/hep.510250103. [DOI] [PubMed] [Google Scholar]
- 38.Wesierska-Gadek J, Penner E, Battezzati PM, Selmi C, Zuin M, Hitchman E, Worman HJ, Gershwin ME, Podda M, Invernizzi P. Correlation of initial autoantibody profile and clinical outcome in primary biliary cirrhosis. Hepatology. 2006;43:1135–1144. doi: 10.1002/hep.21172. [DOI] [PubMed] [Google Scholar]
- 39.Courvalin JC, Worman HJ. Nuclear envelope protein autoantibodies in primary biliary cirrhosis. Semin Liver Dis. 1997;17:79–90. doi: 10.1055/s-2007-1007185. [DOI] [PubMed] [Google Scholar]
- 40.Szostecki C, Guldner HH, Will H. Autoantibodies against “nuclear dots” in primary biliary cirrhosis. Semin Liver Dis. 1997;17:71–78. doi: 10.1055/s-2007-1007184. [DOI] [PubMed] [Google Scholar]
- 41.Lozano F, Parés A, Borche L, Plana M, Gallart T, Rodés J, Vives J. Autoantibodies against nuclear envelope-associated proteins in primary biliary cirrhosis. Hepatology. 1988;8:930–938. doi: 10.1002/hep.1840080438. [DOI] [PubMed] [Google Scholar]
- 42.Van de Water J, Gershwin ME, Leung P, Ansari A, Coppel RL. The autoepitope of the 74-kD mitochondrial autoantigen of primary biliary cirrhosis corresponds to the functional site of dihydrolipoamide acetyltransferase. J Exp Med. 1988;167:1791–1799. doi: 10.1084/jem.167.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeaman SJ, Kirby JA, Jones DE. Autoreactive responses to pyruvate dehydrogenase complex in the pathogenesis of primary biliary cirrhosis. Immunol Rev. 2000;174:238–249. doi: 10.1034/j.1600-0528.2002.00021h.x. [DOI] [PubMed] [Google Scholar]
- 44.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138:3525–3531. [PubMed] [Google Scholar]
- 45.Kita H, Lian ZX, Van de Water J, He XS, Matsumura S, Kaplan M, Luketic V, Coppel RL, Ansari AA, Gershwin ME. Identification of HLA-A2-restricted CD8(+) cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. J Exp Med. 2002;195:113–123. doi: 10.1084/jem.20010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimoda S, Nakamura M, Ishibashi H, Hayashida K, Niho Y. HLA DRB4 0101-restricted immunodominant T cell autoepitope of pyruvate dehydrogenase complex in primary biliary cirrhosis: evidence of molecular mimicry in human autoimmune diseases. J Exp Med. 1995;181:1835–1845. doi: 10.1084/jem.181.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimoda S, Van de Water J, Ansari A, Nakamura M, Ishibashi H, Coppel RL, Lake J, Keeffe EB, Roche TE, Gershwin ME. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102:1831–1840. doi: 10.1172/JCI4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bogdanos DP, Baum H, Gunsar F, Arioli D, Polymeros D, Ma Y, Burroughs AK, Vergani D. Extensive homology between the major immunodominant mitochondrial antigen in primary biliary cirrhosis and Helicobacter pylori does not lead to immunological cross-reactivity. Scand J Gastroenterol. 2004;39:981–987. doi: 10.1080/00365520410003236. [DOI] [PubMed] [Google Scholar]
- 49.Bogdanos DP, Vergani D. Origin of cross-reactive autoimmunity in primary biliary cirrhosis. Liver Int. 2006;26:633–635. doi: 10.1111/j.1478-3231.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- 50.Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: Convenient and inconvenient truths. Hepatology. 2008;47:737–745. doi: 10.1002/hep.22042. [DOI] [PubMed] [Google Scholar]
- 51.Fregeau DR, Prindiville T, Coppel RL, Kaplan M, Dickson ER, Gershwin ME. Inhibition of alpha-ketoglutarate dehydrogenase activity by a distinct population of autoantibodies recognizing dihydrolipoamide succinyltransferase in primary biliary cirrhosis. Hepatology. 1990;11:975–981. doi: 10.1002/hep.1840110611. [DOI] [PubMed] [Google Scholar]
- 52.Tuaillon N, Andre C, Briand JP, Penner E, Muller S. A lipoyl synthetic octadecapeptide of dihydrolipoamide acetyltransferase specifically recognized by anti-M2 autoantibodies in primary biliary cirrhosis. J Immunol. 1992;148:445–450. [PubMed] [Google Scholar]
- 53.Van de Water J, Ansari AA, Surh CD, Coppel R, Roche T, Bonkovsky H, Kaplan M, Gershwin ME. Evidence for the targeting by 2-oxo-dehydrogenase enzymes in the T cell response of primary biliary cirrhosis. J Immunol. 1991;146:89–94. [PubMed] [Google Scholar]
- 54.Bogdanos DP, Baum H, Grasso A, Okamoto M, Butler P, Ma Y, Rigopoulou E, Montalto P, Davies ET, Burroughs AK, et al. Microbial mimics are major targets of crossreactivity with human pyruvate dehydrogenase in primary biliary cirrhosis. J Hepatol. 2004;40:31–39. doi: 10.1016/s0168-8278(03)00501-4. [DOI] [PubMed] [Google Scholar]
- 55.Bogdanos DP, Baum H, Okamoto M, Montalto P, Sharma UC, Rigopoulou EI, Vlachogiannakos J, Ma Y, Burroughs AK, Vergani D. Primary biliary cirrhosis is characterized by IgG3 antibodies cross-reactive with the major mitochondrial autoepitope and its Lactobacillus mimic. Hepatology. 2005;42:458–465. doi: 10.1002/hep.20788. [DOI] [PubMed] [Google Scholar]
- 56.Bogdanos DP, Baum H, Sharma UC, Grasso A, Ma Y, Burroughs AK, Vergani D. Antibodies against homologous microbial caseinolytic proteases P characterise primary biliary cirrhosis. J Hepatol. 2002;36:14–21. doi: 10.1016/s0168-8278(01)00252-5. [DOI] [PubMed] [Google Scholar]
- 57.Neuberger J. Transplantation for primary biliary cirrhosis. Semin Liver Dis. 1997;17:137–146. doi: 10.1055/s-2007-1007192. [DOI] [PubMed] [Google Scholar]
- 58.Bogdanos DP, Vergani D. Bacteria and primary biliary cirrhosis. Clin Rev Allergy Immunol. 2009;36:30–39. doi: 10.1007/s12016-008-8087-9. [DOI] [PubMed] [Google Scholar]
- 59.Gershwin ME, Mackay IR. Primary biliary cirrhosis: paradigm or paradox for autoimmunity. Gastroenterology. 1991;100:822–833. doi: 10.1016/0016-5085(91)80033-6. [DOI] [PubMed] [Google Scholar]
- 60.Jones DE. Pathogenesis of primary biliary cirrhosis. Clin Liver Dis. 2008;12:305–321; viii. doi: 10.1016/j.cld.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Vergani D, Choudhuri K, Bogdanos DP, Mieli-Vergani G. Pathogenesis of autoimmune hepatitis. Clin Liver Dis. 2002;6:727–737. doi: 10.1016/s1089-3261(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 62.Selmi C, Gershwin ME. The role of environmental factors in primary biliary cirrhosis. Trends Immunol. 2009;30:415–420. doi: 10.1016/j.it.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 63.Smyk D, Mytilinaiou MG, Rigopoulou EI, Bogdanos DP. PBC triggers in water reservoirs, coal mining areas and waste disposal sites: from Newcastle to New York. Dis Markers. 2010;29:337–344. doi: 10.3233/DMA-2010-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bogdanos DP, Dalekos GN. Enzymes as target antigens of liver-specific autoimmunity: the case of cytochromes P450s. Curr Med Chem. 2008;15:2285–2292. doi: 10.2174/092986708785747508. [DOI] [PubMed] [Google Scholar]
- 65.Smyk D, Rigopoulou EI, Baum H, Burroughs AK, Vergani D, Bogdanos DP. Autoimmunity and environment: am I at risk? Clin Rev Allergy Immunol. 2012;42:199–212. doi: 10.1007/s12016-011-8259-x. [DOI] [PubMed] [Google Scholar]
- 66.Vergani D, Bogdanos DP, Baum H. Unusual suspects in primary biliary cirrhosis. Hepatology. 2004;39:38–41. doi: 10.1002/hep.20028. [DOI] [PubMed] [Google Scholar]
- 67.Vergani D, Longhi MS, Bogdanos DP, Ma Y, Mieli-Vergani G. Autoimmune hepatitis. Semin Immunopathol. 2009;31:421–435. doi: 10.1007/s00281-009-0170-7. [DOI] [PubMed] [Google Scholar]
- 68.Bogdanos DP, Baum H, Vergani D, Burroughs AK. The role of E. coli infection in the pathogenesis of primary biliary cirrhosis. Dis Markers. 2010;29:301–311. doi: 10.3233/DMA-2010-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haydon GH, Neuberger J. PBC: an infectious disease? Gut. 2000;47:586–588. doi: 10.1136/gut.47.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Invernizzi P, Selmi C, Poli F, Frison S, Floreani A, Alvaro D, Almasio P, Rosina F, Marzioni M, Fabris L, et al. Human leukocyte antigen polymorphisms in Italian primary biliary cirrhosis: a multicenter study of 664 patients and 1992 healthy controls. Hepatology. 2008;48:1906–1912. doi: 10.1002/hep.22567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mells GF, Floyd JA, Morley KI, Cordell HJ, Franklin CS, Shin SY, Heneghan MA, Neuberger JM, Donaldson PT, Day DB, et al. Genome-wide association study identifies 12 new susceptibility loci for primary biliary cirrhosis. Nat Genet. 2011;43:329–332. doi: 10.1038/ng.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu X, Invernizzi P, Lu Y, Kosoy R, Lu Y, Bianchi I, Podda M, Xu C, Xie G, Macciardi F, et al. Genome-wide meta-analyses identify three loci associated with primary biliary cirrhosis. Nat Genet. 2010;42:658–660. doi: 10.1038/ng.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirschfield GM, Liu X, Han Y, Gorlov IP, Lu Y, Xu C, Lu Y, Chen W, Juran BD, Coltescu C, et al. Variants at IRF5-TNPO3, 17q12-21 and MMEL1 are associated with primary biliary cirrhosis. Nat Genet. 2010;42:655–657. doi: 10.1038/ng.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Lu Y, Gu X, Walker EJ, Jing K, Juran BD, et al. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med. 2009;360:2544–2555. doi: 10.1056/NEJMoa0810440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Invernizzi P. Human leukocyte antigen in primary biliary cirrhosis: an old story now reviving. Hepatology. 2011;54:714–723. doi: 10.1002/hep.24414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Invernizzi P, Miozzo M, Battezzati PM, Bianchi I, Grati FR, Simoni G, Selmi C, Watnik M, Gershwin ME, Podda M. Frequency of monosomy X in women with primary biliary cirrhosis. Lancet. 2004;363:533–535. doi: 10.1016/S0140-6736(04)15541-4. [DOI] [PubMed] [Google Scholar]
- 77.Corpechot C, Chrétien Y, Chazouillères O, Poupon R. Demographic, lifestyle, medical and familial factors associated with primary biliary cirrhosis. J Hepatol. 2010;53:162–169. doi: 10.1016/j.jhep.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 78.Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, Lindor KD, Kaplan MM, Vierling JM. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194–1202. doi: 10.1002/hep.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirschfield GM, Heathcote EJ, Gershwin ME. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology. 2010;139:1481–1496. doi: 10.1053/j.gastro.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 80.Parikh-Patel A, Gold EB, Worman H, Krivy KE, Gershwin ME. Risk factors for primary biliary cirrhosis in a cohort of patients from the united states. Hepatology. 2001;33:16–21. doi: 10.1053/jhep.2001.21165. [DOI] [PubMed] [Google Scholar]
- 81.Prince MI, Ducker SJ, James OF. Case-control studies of risk factors for primary biliary cirrhosis in two United Kingdom populations. Gut. 2010;59:508–512. doi: 10.1136/gut.2009.184218. [DOI] [PubMed] [Google Scholar]
- 82.Kimura Y, Selmi C, Leung PS, Mao TK, Schauer J, Watnik M, Kuriyama S, Nishioka M, Ansari AA, Coppel RL, et al. Genetic polymorphisms influencing xenobiotic metabolism and transport in patients with primary biliary cirrhosis. Hepatology. 2005;41:55–63. doi: 10.1002/hep.20516. [DOI] [PubMed] [Google Scholar]
- 83.Bogdanos DP, Koutsoumpas A, Baum H, Vergani D. Borrelia Burgdorferi: a new self-mimicking trigger in primary biliary cirrhosis. Dig Liver Dis. 2006;38:781–782; author reply 782-783. doi: 10.1016/j.dld.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 84.Mayo I, Arizti P, Parés A, Oliva J, Doforno RA, de Sagarra MR, Rodés J, Castaño JG. Antibodies against the COOH-terminal region of E. coli ClpP protease in patients with primary biliary cirrhosis. J Hepatol. 2000;33:528–536. doi: 10.1034/j.1600-0641.2000.033004528.x. [DOI] [PubMed] [Google Scholar]
- 85.Xu L, Shen Z, Guo L, Fodera B, Keogh A, Joplin R, O’Donnell B, Aitken J, Carman W, Neuberger J, et al. Does a betaretrovirus infection trigger primary biliary cirrhosis? Proc Natl Acad Sci USA. 2003;100:8454–8459. doi: 10.1073/pnas.1433063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vilagut L, Vila J, Viñas O, Parés A, Ginés A, Jiménez de Anta MT, Rodés J. Cross-reactivity of anti-Mycobacterium gordonae antibodies with the major mitochondrial autoantigens in primary biliary cirrhosis. J Hepatol. 1994;21:673–677. doi: 10.1016/s0168-8278(94)80117-7. [DOI] [PubMed] [Google Scholar]
- 87.Gilburd B, Ziporen L, Zharhary D, Blank M, Zurgil N, Scheinberg MA, Guedes LH, Gershwin ME, Shoenfeld Y. Antimitochondrial (pyruvate dehydrogenase) antibodies in leprosy. J Clin Immunol. 1994;14:14–19. doi: 10.1007/BF01541171. [DOI] [PubMed] [Google Scholar]
- 88.Klein R, Wiebel M, Engelhart S, Berg PA. Sera from patients with tuberculosis recognize the M2a-epitope (E2-subunit of pyruvate dehydrogenase) specific for primary biliary cirrhosis. Clin Exp Immunol. 1993;92:308–316. doi: 10.1111/j.1365-2249.1993.tb03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Silverman L, Shorter RG. Histogenesis of the multinucleated giant cell. Lab Invest. 1963;12:985–990. [PubMed] [Google Scholar]
- 90.Williams GT, Williams WJ. Granulomatous inflammation--a review. J Clin Pathol. 1983;36:723–733. doi: 10.1136/jcp.36.7.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zumla A, James DG. Granulomatous infections: etiology and classification. Clin Infect Dis. 1996;23:146–158. doi: 10.1093/clinids/23.1.146. [DOI] [PubMed] [Google Scholar]
- 92.Lefkowitch JH. Hepatic granulomas. J Hepatol. 1999;30 Suppl 1:40–45. [PubMed] [Google Scholar]
- 93.Kleiner DE. Granulomas in the liver. Semin Diagn Pathol. 2006;23:161–169. doi: 10.1053/j.semdp.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 94.Mert A, Ozaras R, Bilir M, Tahan V, Cetinkaya A, Yirmibescik S, Ozbay G, Senturk H. The etiology of hepatic granulomas. J Clin Gastroenterol. 2001;32:275–276. doi: 10.1097/00004836-200103000-00026. [DOI] [PubMed] [Google Scholar]
- 95.Gaya DR, Thorburn D, Oien KA, Morris AJ, Stanley AJ. Hepatic granulomas: a 10 year single centre experience. J Clin Pathol. 2003;56:850–853. doi: 10.1136/jcp.56.11.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCluggage WG, Sloan JM. Hepatic granulomas in Northern Ireland: a thirteen year review. Histopathology. 1994;25:219–228. doi: 10.1111/j.1365-2559.1994.tb01321.x. [DOI] [PubMed] [Google Scholar]
- 97.Onal IK, Ersoy O, Aydinli M, Yonem O, Harmanci O, Sokmensuer C, Bayraktar Y. Hepatic granuloma in Turkish adults: a report of 13 cases. Eur J Intern Med. 2008;19:527–530. doi: 10.1016/j.ejim.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 98.Sartin JS, Walker RC. Granulomatous hepatitis: a retrospective review of 88 cases at the Mayo Clinic. Mayo Clin Proc. 1991;66:914–918. doi: 10.1016/s0025-6196(12)61578-x. [DOI] [PubMed] [Google Scholar]
- 99.Satti MB, al-Freihi H, Ibrahim EM, Abu-Melha A, al-Ghassab G, al-Idrissi HY, al-Sohaibani MO. Hepatic granuloma in Saudi Arabia: a clinicopathological study of 59 cases. Am J Gastroenterol. 1990;85:669–674. [PubMed] [Google Scholar]
- 100.Drebber U, Kasper HU, Ratering J, Wedemeyer I, Schirmacher P, Dienes HP, Odenthal M. Hepatic granulomas: histological and molecular pathological approach to differential diagnosis--a study of 442 cases. Liver Int. 2008;28:828–834. doi: 10.1111/j.1478-3231.2008.01695.x. [DOI] [PubMed] [Google Scholar]
- 101.Dourakis SP, Saramadou R, Alexopoulou A, Kafiri G, Deutsch M, Koskinas J, Archimandritis AJ. Hepatic granulomas: a 6-year experience in a single center in Greece. Eur J Gastroenterol Hepatol. 2007;19:101–104. doi: 10.1097/01.meg.0000243882.09820.d2. [DOI] [PubMed] [Google Scholar]
- 102.Smyk DS, Bogdanos DP, Kriese S, Billinis C, Burroughs AK, Rigopoulou EI. Urinary tract infection as a risk factor for autoimmune liver disease: from bench to bedside. Clin Res Hepatol Gastroenterol. 2012;36:110–121. doi: 10.1016/j.clinre.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 103.Christensen E, Neuberger J, Crowe J, Altman DG, Popper H, Portmann B, Doniach D, Ranek L, Tygstrup N, Williams R. Beneficial effect of azathioprine and prediction of prognosis in primary biliary cirrhosis. Final results of an international trial. Gastroenterology. 1985;89:1084–1091. doi: 10.1016/0016-5085(85)90213-6. [DOI] [PubMed] [Google Scholar]
- 104.Mitchison HC, Palmer JM, Bassendine MF, Watson AJ, Record CO, James OF. A controlled trial of prednisolone treatment in primary biliary cirrhosis. Three-year results. J Hepatol. 1992;15:336–344. doi: 10.1016/0168-8278(92)90065-w. [DOI] [PubMed] [Google Scholar]
- 105.Neuberger J, Portmann B, Macdougall BR, Calne RY, Williams R. Recurrence of primary biliary cirrhosis after liver transplantation. N Engl J Med. 1982;306:1–4. doi: 10.1056/NEJM198201073060101. [DOI] [PubMed] [Google Scholar]
- 106.Rautiainen H, Kärkkäinen P, Karvonen AL, Nurmi H, Pikkarainen P, Nuutinen H, Färkkilä M. Budesonide combined with UDCA to improve liver histology in primary biliary cirrhosis: a three-year randomized trial. Hepatology. 2005;41:747–752. doi: 10.1002/hep.20646. [DOI] [PubMed] [Google Scholar]
- 107.Carbone M, Neuberger J. Liver transplantation in PBC and PSC: indications and disease recurrence. Clin Res Hepatol Gastroenterol. 2011;35:446–454. doi: 10.1016/j.clinre.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 108.Charatcharoenwitthaya P, Pimentel S, Talwalkar JA, Enders FT, Lindor KD, Krom RA, Wiesner RH. Long-term survival and impact of ursodeoxycholic acid treatment for recurrent primary biliary cirrhosis after liver transplantation. Liver Transpl. 2007;13:1236–1245. doi: 10.1002/lt.21124. [DOI] [PubMed] [Google Scholar]
- 109.Jacob DA, Neumann UP, Bahra M, Klupp J, Puhl G, Neuhaus R, Langrehr JM. Long-term follow-up after recurrence of primary biliary cirrhosis after liver transplantation in 100 patients. Clin Transplant. 2006;20:211–220. doi: 10.1111/j.1399-0012.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- 110.Liermann Garcia RF, Evangelista Garcia C, McMaster P, Neuberger J. Transplantation for primary biliary cirrhosis: retrospective analysis of 400 patients in a single center. Hepatology. 2001;33:22–27. doi: 10.1053/jhep.2001.20894. [DOI] [PubMed] [Google Scholar]
- 111.Neuberger J, Gunson B, Hubscher S, Nightingale P. Immunosuppression affects the rate of recurrent primary biliary cirrhosis after liver transplantation. Liver Transpl. 2004;10:488–491. doi: 10.1002/lt.20123. [DOI] [PubMed] [Google Scholar]
- 112.Garcia CE, Garcia RF, Gunson B, Christensen E, Neuberger J, McMaster P, Mirza DF. Analysis of marginal donor parameters in liver transplantation for primary biliary cirrhosis. Exp Clin Transplant. 2004;2:183–188. [PubMed] [Google Scholar]
- 113.Neuberger J. Recurrent primary biliary cirrhosis. Liver Transpl. 2003;9:539–546. doi: 10.1053/jlts.2003.50096. [DOI] [PubMed] [Google Scholar]
- 114.Duggan DB, Mackworth-Young C, Kari-Lefvert A, Andre-Schwartz J, Mudd D, McAdam KP, Schwartz RS. Polyspecificity of human monoclonal antibodies reactive with Mycobacterium leprae, mitochondria, ssDNA, cytoskeletal proteins, and the acetylcholine receptor. Clin Immunol Immunopathol. 1988;49:327–340. doi: 10.1016/0090-1229(88)90123-7. [DOI] [PubMed] [Google Scholar]
- 115.Guedes Barbosa LS, Gilbrut B, Shoenfeld Y, Scheinberg MA. Autoantibodies in leprosy sera. Clin Rheumatol. 1996;15:26–28. doi: 10.1007/BF02231680. [DOI] [PubMed] [Google Scholar]
- 116.Vilagut L, Parés A, Rodés J, Vila J, Viñas O, Ginès A, Jìménez de Anta MT. Mycobacteria--related to the aetiopathogenesis of primary biliary cirrhosis? J Hepatol. 1996;24:125. doi: 10.1016/s0168-8278(96)80198-x. [DOI] [PubMed] [Google Scholar]
- 117.Vilagut L, Parés A, Viñas O, Vila J, Jiménez de Anta MT, Rodés J. Antibodies to mycobacterial 65-kD heat shock protein cross-react with the main mitochondrial antigens in patients with primary biliary cirrhosis. Eur J Clin Invest. 1997;27:667–672. doi: 10.1046/j.1365-2362.1997.1690724.x. [DOI] [PubMed] [Google Scholar]
- 118.O’Donohue J, Fidler H, Garcia-Barcelo M, Nouri-Aria K, Williams R, McFadden J. Mycobacterial DNA not detected in liver sections from patients with primary biliary cirrhosis. J Hepatol. 1998;28:433–438. doi: 10.1016/s0168-8278(98)80317-6. [DOI] [PubMed] [Google Scholar]
- 119.O’Donohue J, McFarlane B, Bomford A, Yates M, Williams R. Antibodies to atypical mycobacteria in primary biliary cirrhosis. J Hepatol. 1994;21:887–889. doi: 10.1016/s0168-8278(94)80255-6. [DOI] [PubMed] [Google Scholar]
- 120.Bogdanos DP, Pares A, Baum H, Caballeria L, Rigopoulou EI, Ma Y, Burroughs AK, Rodes J, Vergani D. Disease-specific cross-reactivity between mimicking peptides of heat shock protein of Mycobacterium gordonae and dominant epitope of E2 subunit of pyruvate dehydrogenase is common in Spanish but not British patients with primary biliary cirrhosis. J Autoimmun. 2004;22:353–362. doi: 10.1016/j.jaut.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 121.Broomé U, Scheynius A, Hultcrantz R. Induced expression of heat-shock protein on biliary epithelium in patients with primary sclerosing cholangitis and primary biliary cirrhosis. Hepatology. 1993;18:298–303. doi: 10.1002/hep.1840180212. [DOI] [PubMed] [Google Scholar]
- 122.Tanaka A, Prindiville TP, Gish R, Solnick JV, Coppel RL, Keeffe EB, Ansari A, Gershwin ME. Are infectious agents involved in primary biliary cirrhosis? A PCR approach. J Hepatol. 1999;31:664–671. doi: 10.1016/s0168-8278(99)80346-8. [DOI] [PubMed] [Google Scholar]
- 123.Van de Water J, Ishibashi H, Coppel RL, Gershwin ME. Molecular mimicry and primary biliary cirrhosis: premises not promises. Hepatology. 2001;33:771–775. doi: 10.1053/jhep.2001.23902. [DOI] [PubMed] [Google Scholar]
- 124.Bogdanos D, Pusl T, Rust C, Vergani D, Beuers U. Primary biliary cirrhosis following Lactobacillus vaccination for recurrent vaginitis. J Hepatol. 2008;49:466–473. doi: 10.1016/j.jhep.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 125.Bogdanos DP, Baum H, Butler P, Rigopoulou EI, Davies ET, Ma Y, Burroughs AK, Vergani D. Association between the primary biliary cirrhosis specific anti-sp100 antibodies and recurrent urinary tract infection. Dig Liver Dis. 2003;35:801–805. doi: 10.1016/s1590-8658(03)00466-3. [DOI] [PubMed] [Google Scholar]
- 126.Koutsoumpas A, Polymeros D, Tsiamoulos Z, Smyk D, Karamanolis G, Triantafyllou K, Rigopoulou EI, Forbes A, Vergani D, Bogdanos DP, et al. Peculiar antibody reactivity to human connexin 37 and its microbial mimics in patients with Crohn’s disease. J Crohns Colitis. 2011;5:101–109. doi: 10.1016/j.crohns.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 127.Gregorio GV, Choudhuri K, Ma Y, Pensati P, Iorio R, Grant P, Garson J, Bogdanos DP, Vegnente A, Mieli-Vergani G, et al. Mimicry between the hepatitis C virus polyprotein and antigenic targets of nuclear and smooth muscle antibodies in chronic hepatitis C virus infection. Clin Exp Immunol. 2003;133:404–413. doi: 10.1046/j.1365-2249.2003.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van de Water J, Coppel RL, Kaplan MM, Gershwin ME. Analysis of TCR antagonism and molecular mimicry of an HLA-A0201-restricted CTL epitope in primary biliary cirrhosis. Hepatology. 2002;36:918–926. doi: 10.1053/jhep.2002.35616. [DOI] [PubMed] [Google Scholar]
- 129.Polymeros D, Bogdanos DP, Day R, Arioli D, Vergani D, Forbes A. Does cross-reactivity between mycobacterium avium paratuberculosis and human intestinal antigens characterize Crohn’s disease? Gastroenterology. 2006;131:85–96. doi: 10.1053/j.gastro.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 130.Baum H, Bogdanos DP, Vergani D. Antibodies to Clp protease in primary biliary cirrhosis: possible role of a mimicking T-cell epitope. J Hepatol. 2001;34:785–787. doi: 10.1016/s0168-8278(01)00059-9. [DOI] [PubMed] [Google Scholar]
- 131.Kerkar N, Choudhuri K, Ma Y, Mahmoud A, Bogdanos DP, Muratori L, Bianchi F, Williams R, Mieli-Vergani G, Vergani D. Cytochrome P4502D6(193-212): a new immunodominant epitope and target of virus/self cross-reactivity in liver kidney microsomal autoantibody type 1-positive liver disease. J Immunol. 2003;170:1481–1489. doi: 10.4049/jimmunol.170.3.1481. [DOI] [PubMed] [Google Scholar]
- 132.Koutsoumpas A, Mytilinaiou M, Polymeros D, Dalekos GN, Bogdanos DP. Anti-Helicobacter pylori antibody responses specific for VacA do not trigger primary biliary cirrhosis-specific antimitochondrial antibodies. Eur J Gastroenterol Hepatol. 2009;21:1220. doi: 10.1097/MEG.0b013e32831a4807. [DOI] [PubMed] [Google Scholar]
- 133.Longhi MS, Hussain MJ, Bogdanos DP, Quaglia A, Mieli-Vergani G, Ma Y, Vergani D. Cytochrome P450IID6-specific CD8 T cell immune responses mirror disease activity in autoimmune hepatitis type 2. Hepatology. 2007;46:472–484. doi: 10.1002/hep.21658. [DOI] [PubMed] [Google Scholar]
- 134.Hannam S, Bogdanos DP, Davies ET, Hussain MJ, Portmann BC, Mieli-Vergani G, Vergani D. Neonatal liver disease associated with placental transfer of anti-mitochondrial antibodies. Autoimmunity. 2002;35:545–550. doi: 10.1080/0891693021000054057. [DOI] [PubMed] [Google Scholar]
- 135.Ma Y, Bogdanos DP, Hussain MJ, Underhill J, Bansal S, Longhi MS, Cheeseman P, Mieli-Vergani G, Vergani D. Polyclonal T-cell responses to cytochrome P450IID6 are associated with disease activity in autoimmune hepatitis type 2. Gastroenterology. 2006;130:868–882. doi: 10.1053/j.gastro.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 136.Ma Y, Meregalli M, Hodges S, Davies N, Bogdanos DP, Fargion S, Fiorelli G, Vergani D. Alcohol dehydrogenase: an autoantibody target in patients with alcoholic liver disease. Int J Immunopathol Pharmacol. 2005;18:173–182. doi: 10.1177/039463200501800118. [DOI] [PubMed] [Google Scholar]
- 137.Ma Y, Okamoto M, Thomas MG, Bogdanos DP, Lopes AR, Portmann B, Underhill J, Dürr R, Mieli-Vergani G, Vergani D. Antibodies to conformational epitopes of soluble liver antigen define a severe form of autoimmune liver disease. Hepatology. 2002;35:658–664. doi: 10.1053/jhep.2002.32092. [DOI] [PubMed] [Google Scholar]
- 138.Ma Y, Thomas MG, Okamoto M, Bogdanos DP, Nagl S, Kerkar N, Lopes AR, Muratori L, Lenzi M, Bianchi FB, et al. Key residues of a major cytochrome P4502D6 epitope are located on the surface of the molecule. J Immunol. 2002;169:277–285. doi: 10.4049/jimmunol.169.1.277. [DOI] [PubMed] [Google Scholar]
- 139.Muratori L, Bogdanos DP, Muratori P, Lenzi M, Granito A, Ma Y, Mieli-Vergani G, Bianchi FB, Vergani D. Susceptibility to thyroid disorders in hepatitis C. Clin Gastroenterol Hepatol. 2005;3:595–603. doi: 10.1016/s1542-3565(05)00018-2. [DOI] [PubMed] [Google Scholar]
- 140.Wen L, Ma Y, Bogdanos DP, Wong FS, Demaine A, Mieli-Vergani G, Vergani D. Pediatric autoimmune liver diseases: the molecular basis of humoral and cellular immunity. Curr Mol Med. 2001;1:379–389. doi: 10.2174/1566524013363672. [DOI] [PubMed] [Google Scholar]
- 141.Longhi MS, Ma Y, Bogdanos DP, Cheeseman P, Mieli-Vergani G, Vergani D. Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. J Hepatol. 2004;41:31–37. doi: 10.1016/j.jhep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 142.Longhi MS, Ma Y, Mitry RR, Bogdanos DP, Heneghan M, Cheeseman P, Mieli-Vergani G, Vergani D. Effect of CD4+ CD25+ regulatory T-cells on CD8 T-cell function in patients with autoimmune hepatitis. J Autoimmun. 2005;25:63–71. doi: 10.1016/j.jaut.2005.05.001. [DOI] [PubMed] [Google Scholar]


