Abstract
The purpose of this pilot study was to evaluate lymph node enhancement with an indirect magnetic resonance (MR) lymphography technique using 2 different contrast agents in the head and neck region of healthy dogs. Five dogs were imaged at various times after intradermal injection of gadoversetamide and Gadofluorine M (minimum of 1 week apart) in the right and left mandibular, temporal, and lateral neck regions. We observed consistent progressive enhancement with time in the mandibular, retropharyngeal, and superficial cervical lymph nodes. The node enhancement was comparable for both contrast agents. Contrast enhancement of the parotid lymph nodes was not seen. We conclude that this technique of indirect MR lymphography using either agent could be used to identify those lymph nodes at highest risk of metastatic disease in dogs with cancer, and to guide staging and treatment.
Résumé
Lymphographie par résonance magnétique indirecte de la tête et du cou des chiens en utilisant Gadofluorine M et un agent de contraste conventionnel au gadolinium : étude pilote. Le but de cette étude pilote était d’évaluer le contraste des ganglions lymphatiques à l’aide d’une technique de lymphographie par résonance magnétique (RM) indirecte en utilisant 2 agents de contraste différents dans la région de la tête et du cou des chiens en santé. L’imagerie de 5 chiens a été réalisée à divers moments après l’injection intradermique de gadoversétamide et de Gadofluorine M (avec un intervalle minimum de 1 semaine) dans les régions mandibulaires droite et gauche, temporale et du cou. Nous avons observé une augmentation progressive constante dans le temps dans les ganglions mandibulaires, rétropharyngiens et cervicaux superficiels. Le contraste des ganglions était comparable pour les deux agents. L’augmentation de contraste des ganglions lymphatiques parotidiens n’a pas été vue. Nous concluons que cette technique de lymphographie par RM indirecte, en utilisant l’un ou l’autre des agents, pourrait être utilisée pour identifier les ganglions lymphatiques les plus à risque de maladie métastasique chez les chiens atteints de cancer et pour guider la détermination des stades et le traitement.
(Traduit par Isabelle Vallières)
Introduction
Clinical staging and the selection of treatment of many malignant neoplasms is enhanced by the identification of the regional lymph nodes draining the anatomical site of the tumor. Indirect magnetic resonance (MR) lymphography involving interstitial injection of a contrast agent has been used to identify the lymphatic drainage pattern from specific anatomical sites in dogs and humans, as well as other species (1–3). Various novel lymphotropic contrast agents have been developed for interstitial lymphography, including the micelle-forming agent, Gadofluorine M (Bayer Schering Pharma AG, Berlin, Germany), which has been shown to accumulate in regional lymph nodes after intradermal injection in dogs (1). Misselwitz et al (1) demonstrated a pronounced increase in signal intensity in the popliteal and para-aortal lymph nodes after intradermal injection of Gadofluorine M into the pelvic limbs of dogs. However, more recent papers have suggested that interstitial administration of conventional contrast agents without specific lymphotropic properties also effectively identifies the lymphatic drainage of injected sites (3,4). Intradermal injection of a conventional MR contrast agent into the pelvic limb paw of dogs provided visualization of the popliteal and inguinal lymph nodes (4).
To the authors’ knowledge, indirect MR lymphography of the head and neck region has not been reported in dogs. As well, enhancement using a conventional contrast agent has not been compared with enhancement using Gadofluorine M in the same canine subjects. The purpose of this pilot study was to compare the degree of enhancement of regional lymph nodes of the head and neck following the intradermal administration of Gadoflourine M and a conventional contrast agent, gadoversetamide (Optimark; Mallinckrodt Imaging, Hazelwood, Missouri, USA), in normal dogs. This ultimately may be a potential method for identifying metastatic nodes in the lymphatic drainage region of head and neck neoplasms. Findings in dogs would relate to human medicine, because physical distances from injection sites to lymph nodes and lymph node anatomy in the head and neck region are comparable between the 2 species (5–7).
Materials and methods
This work was approved by the University of Saskatchewan’s Animal Research Ethics Board, and adhered to the Canadian Council on Animal Care guidelines for humane animal use. Five healthy research dogs, median age 3 y (range: 2 to 7 y), and median weight 24 kg (range: 18.5 to 31 kg), were studied. Each dog was imaged twice, once with each contrast agent, with a minimum of 7 d between imaging studies. Prior to the second MR examination there was no residual enhancement of the injection sites and draining lymphatic pathways (sufficient washout).
The contrast agents were injected intradermally using a 25-gauge 5/8-inch needle in the right and left mandibular, temporal, and lateral neck regions, using palpable and visible landmarks. The injections and imaging were performed under the same anesthetic episode. The dogs were anesthetized using premedication with acepromazine (Acepromazine; Atravet, Boehringer Ingelheim, Burlington, Ontario), 0.02 mg/kg body weight (BW), IM, diazepam (Diazepam Injection; Sandoz, Quebec City, Quebec), 0.2 mg/kg BW, IV, and fentanyl (Fentanyl Injection; Sandoz), 5 μg/kg BW, IV, induction with propofol (Diprivan; AstraZeneca, Mississauga, Ontario), 4 mg/kg BW, IV and maintenance with inhaled sevoflurane (2% to 3% in O2 to effect). Normosol-R (Hospira, Montreal, Quebec), 10 mL/kg BW per hour, IV was administered during the anesthesia. The mandibular injections were given 1 cm ventral to the lower lip at the level of the third premolar, and the temporal injections were given midway between the zygomatic arch and the external sagittal crest at the caudal aspect of the zygomatic arch. The lateral neck injections were given over the transverse process of cervical vertebra 2. Injection sites were massaged for 30 s immediately after injection to facilitate movement of contrast into the lymphatic vessels. A total of 1 mL of gadoversetamide was used per site, divided into 2 injections (i.e., for the right temporal site, 2 injections of 0.5 mL each were administered). The volume of injection was based on a previous study using indirect MR lymphography with a conventional contrast agent in dogs (3). A dose of 5 μmol/kg BW of Gadofluorine M was injected at each site, divided into 2 injections per site (1). The median volume per injection of Gadofluorine M was 0.55 mL. The Gadofluorine M was provided by Bayer Schering Pharma AG. The osmolality of gadoversetamide is 1100 mOsm/kg water, while that of Gadofluorine M is 4 mOsm/kg water (e-mail communication, Misselwitz B., 2011; Bayer Pharma AG, Berlin) (8).
The MRI technique included transverse and dorsal T1-weighted TrueFISP (a balanced gradient echo sequence) and VIBE (an ultrafast gradient echo sequence) series, acquired on a 1.5T Siemens Symphony scanner (Siemens Canada, Burlington, Ontario). The TRs ranged from 5.08 to 5.41 ms, and TE from 2.54 to 2.72 ms. Slice thicknesses were 6.6 and 7.8 mm, and 2.5 mm and 1.5 to 2.5 mm for dorsal and transverse TrueFISP and VIBE images, respectively. Field of view varied from 247 × 330 mm up to 400 × 400 mm. Images were collected prior to contrast injection, and at 5, 10, 15, and 20 min post-injection (3 dogs) and 5, 10, and 15 min post-injection (2 dogs) for the gadoversetamide studies, and at 15, 30, and 60 min post-injection for the Gadoflourine M studies. The timing of post-contrast image acquisition was based on previous indirect MR lymphography studies in dogs, which reported a maximum enhancement of lymph nodes within 15 min using a conventional contrast agent, and between 60 to 90 min for Gadoflourine M (1,3). The 20 min time point was added for the gadoversetamide arm of the study after it was noticed that the first 2 dogs did not exhibit a noticeable decline in enhancement by 15 min. The dogs were monitored for adverse reactions for 1 h after contrast administration, and the injection sites were examined every 24 h for the first 7 d after each procedure.
All images were analyzed using the segmentation editor in the Amira Visualization Software version 5.2.2 (Visage Imaging, Richmond, Australia). VIBE sequences were used for all analyses because of better and more consistent image quality and visualization of the lymph nodes. Lymphatic structures are usually embedded in fat, and the VIBE sequence was chosen for its fat suppression T1 signal characteristic. The mandibular, retropharyngeal, and superficial cervical lymph nodes were segmented using a combination of threshold segmentation and manual selection. Threshold was used to highlight all soft tissue intensity, followed by manual selection of the node in each slice. For each node, the whole node was included, not just those areas within the node that were enhanced with contrast. Lymphatic vessels were not included as part of the lymph node analysis. Each set of lymph nodes was separated into left- and right-sided groups for analysis. For each scan, the analysis was first performed on the latest time point post-contrast, which generally provided the best visualization of the lymphatic structures. That analysis was then used as a template for the other scans, with adjustments made for variation in patient position. This allowed for a highly reproducible segmentation between analyses. Regions of interest (ROIs) were also drawn symmetrically in the left and right epaxial musculature of the first cervical vertebra on each scan. These circular ROIs were standardized between all scans in a given plane, and were large enough to incorporate as much muscle tissue as possible without incorporating non-muscle. Background ROIs were then placed symmetrically on both the left and right side on this same slice. The size of these background ROIs was approximately twice that of the musculature ROIs. The volume and signal intensity (SI) were calculated for all measured tissues. In 1 case, an artifact occurred that interfered with signal intensity measurement in the pre-contrast imaging for 1 dog injected with Gadofluorine M. The data from this dog were omitted from analysis. The lymph node enhancement ratio (ER), signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were calculated using the following formulae (9):
Results
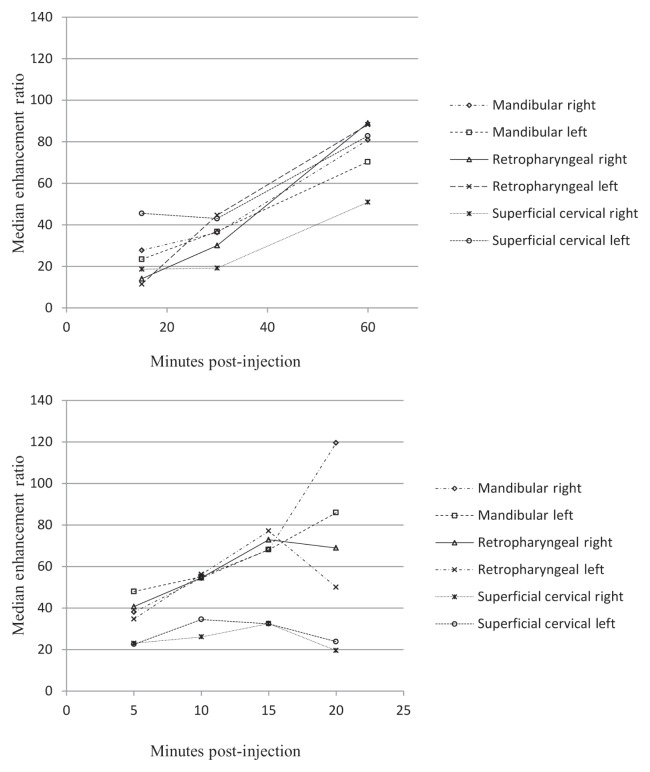
Intradermal administration of both gadoversetamide and Gadofluorine M resulted in a consistently increased enhancement of the featured lymph nodes in the expected lymphatic drainage region from the injection site with time to the point of maximum enhancement (60 min for Gadofluorine M, 15 min for gadoversetamide) (Figure 1). Examples of these contrast-enhanced nodes (retropharyngeal and superficial cervical nodes) are presented in Figure 2. The mandibular lymph nodes showed a similar increase in enhancement with time, with the maximum enhancement occurring 20 min after injection of gadoversetamide. The maximum median ER was higher for Gadofluorine M than for gadoversetamide for the retropharyngeal lymph nodes (18% higher), and for the superficial cervical lymph nodes (79% higher). The maximum median ER was 27% higher for gadoversetamide than for Gadofluorine M for the mandibular lymph nodes. The maximum median ER for gadoversetamide was approximately 2 to 3 times greater for the mandibular and retropharyngeal lymph nodes than for the superficial cervical lymph nodes. The parotid lymph nodes were not adequately delineated on pre- and post-contrast images for selection of the node and signal intensity measurement. The CNR and SNR showed a similar pattern as the ER for both contrast agents for most nodes, with maximum median CNR and SNR at 15 to 20 min for gadoversetamide and 60 min for Gadofluorine M.
Figure 1.
Median enhancement ratio [ER = (Postcontrast SI — Precontrast SI)/(Precontrast SI)] of lymph nodes in the head and neck after intracutaneous injection of 5 μmol/kg body weight (BW) Gadofluorine M (top) and 1 mL of gadoversetamide (bottom) in the right and left mandibular, temporal, and lateral neck regions (n = 5 dogs).
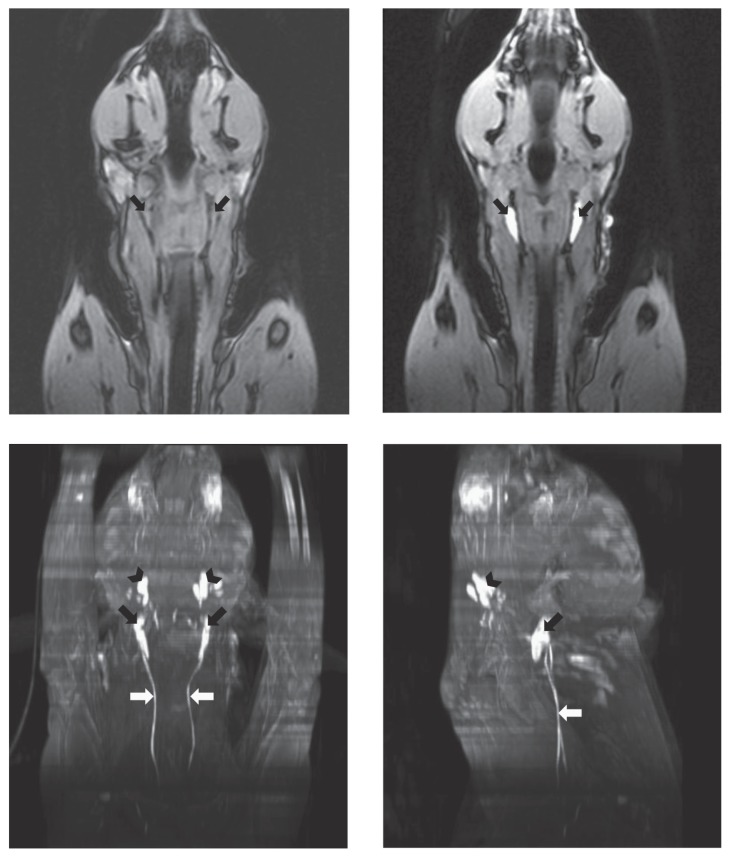
Figure 2.
VIBE dorsal plane images of the head and neck before (top left) and 60 min after intracutaneous injection of Gadofluorine M (top right). The medial retropharyngeal lymph nodes show intense, uniform enhancement (arrows). Ventral (bottom left) and left lateral (bottom right) maximum intensity projections of the same dog 60 min after Gadofluorine M. The mandibular (black arrowhead) and medial retropharyngeal (black arrow) lymph nodes show intense enhancement, and the right and left tracheal trunks are enhanced (white arrows).
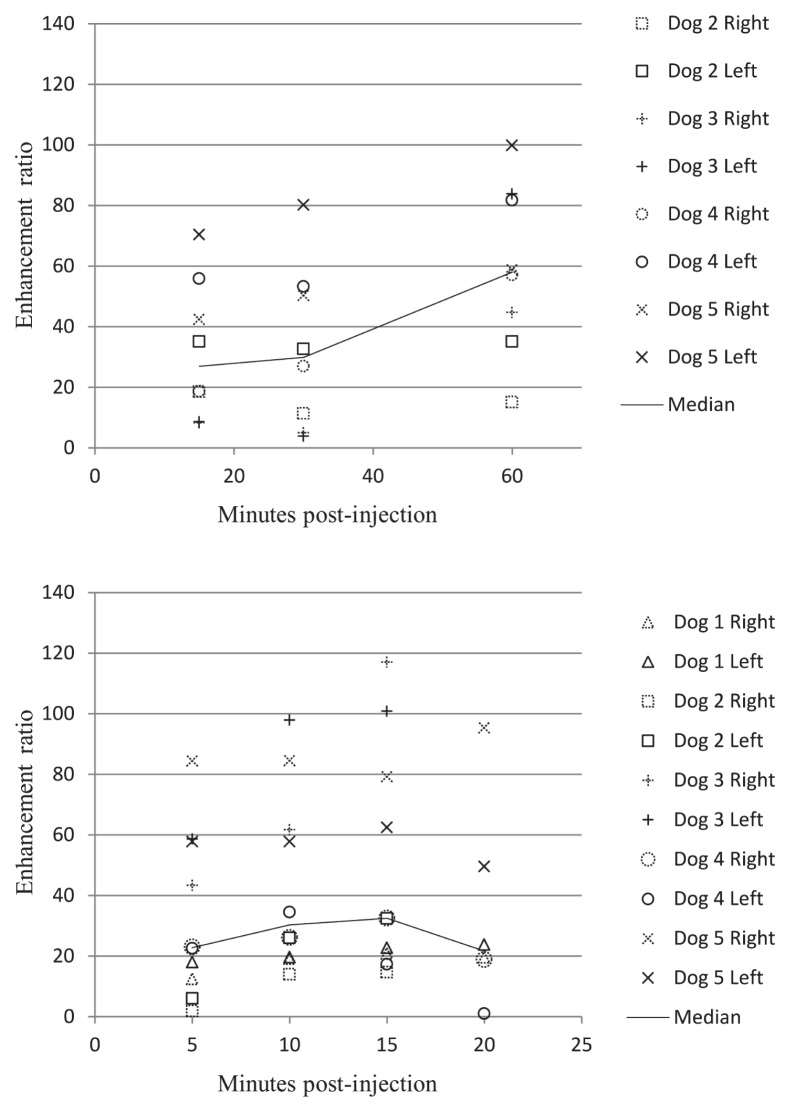
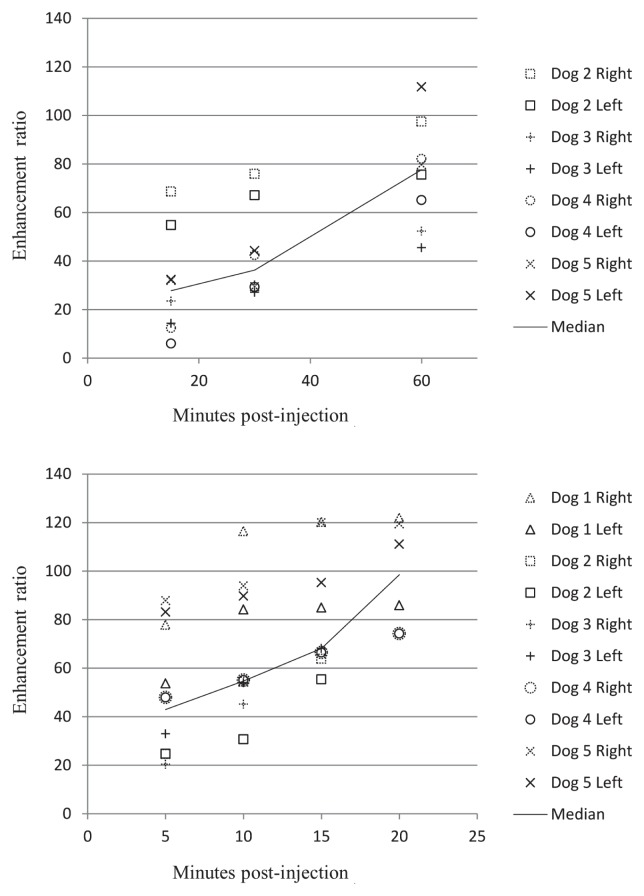
The most variation in enhancement ratios between dogs was found in the superficial cervical lymph nodes for both contrast agents (Figure 3). The least variation between dogs was found in the mandibular lymph nodes for both agents (Figure 4).
Figure 3.
The maximum variation in enhancement ratio [ER = (Postcontrast SI — Precontrast SI)/(Precontrast SI)] between dogs was found with the superficial cervical lymph nodes for both gadofluorine M (top) and gadoversetamide (bottom) (n = 5 dogs).
Figure 4.
The least variation in enhancement ratio between dogs was found with the mandibular lymph nodes for both gadofluorine M (top) and gadoversetamide (bottom) (n = 5 dogs).
Three dogs injected with gadoversetamide and 2 dogs injected with Gadofluorine M had mild swelling and erythema at injection sites which resolved within 12 h with no treatment. Within 3 to 5 d after injection, dry crusting developed at 1 to 4 injection sites in 3 dogs injected with gadoversetamide and 3 dogs injected with Gadofluorine M. The crusting resolved after 4 to 5 d with no treatment.
Discussion
Regional lymph nodes are the first site of metastasis for some common tumors in dogs, and accurate identification of the first nodes to receive lymph from a tumor guides the choice of which nodes to biopsy during staging of the patient, as well as which lymph nodes to excise or irradiate during treatment. Lymphatic drainage patterns may be altered from normal patterns when a tumor is present; therefore, assessment of the drainage pattern in individual patients prior to treatment is ideal (10). Various methods for determining the pattern of lymphatic drainage from a specific anatomical location have been described in dogs, including lymphoscintigraphy, contrast-enhanced ultrasonography, intradermal dye injection, and computed tomographic (CT) and MR lymphography (1,3,11–14). Lymphoscintigraphy provides limited information on anatomical landmarks and geometry, and requires a nuclear medicine service, and intradermal dye injection involves surgical dissection to identify the stained nodes. Advantages of CT and MR lymphography over other techniques include the high resolution three-dimensional images combined with functional information about lymphatic drainage, as well as the potential to perform these procedures under the same anesthetic episode as the primary tumor imaging.
Two contrast agents used for indirect MR lymphography were investigated in this study, gadoversetamide, a commercially available conventional gadolinium-containing contrast agent, and Gadofluorine M, a lymphotropic contrast agent that is not yet commercially available. Gadoversetamide most likely enters lymphatic vessels from the interstitial space due to a combination of pressure, volume, and osmotic effects, while Gadofluorine M is a micelle-forming compound that has been shown to accumulate in lymph nodes after interstitial administration (4,15). These different mechanisms lead to a difference in time to maximum enhancement after interstitial injection, as well as in length of time that enhancement persists (1,4). A wider window of time would exist for imaging with Gadofluorine M due to the prolonged enhancement. A disadvantage of Gadofluorine M, at least for dogs, would be the need for prolonged anesthesia with the longer uptake times, assuming the dogs would need to be under general anesthesia for intradermal injection as in this study. Although we did not attempt intradermal injection other than under general anesthesia, it may be possible to perform after sedation, avoiding the need for general anesthesia until the time of imaging.
In this study, intradermal injection of both the conventional contrast agent gadoversetamide and the lymphotropic agent Gadofluorine M effectively identified lymph nodes draining a specific site in the head and neck region in healthy dogs. Application of this technique in dogs with tumors in the head and neck region should enhance nodes most likely to contain metastatic cells and therefore guide selection of nodes for biopsy as well as for treatment. The safety profile for gadoversetamide in dogs and human patients has been established, while relatively limited data are available for Gadofluorine (16,17). Our findings of reliable enhancement of draining nodes after intradermal injection are consistent with canine studies using Gadofluorine M in the pelvic limb, and conventional MR contrast agents in the pelvic limb and mammary regions (1,3,4).
The canine parotid lymph node is not identified consistently on MR images due to its close association with the parotid salivary gland and lack of signal contrast between the 2 structures (18). We had hoped that enhancement of the node with a contrast agent would assist in its identification; however, indirect MR lymphography with either contrast agent did not enhance the parotid lymph node. The injection site used is drained by the parotid lymph node, and the physical distance from injection site to node was comparable to the other sites used in the study (5). It is possible that the parotid lymph node did take up contrast but was not distinguishable from the surrounding parotid salivary gland.
Although consistent landmarks were used to locate injection sites for each dog, there may have been small differences in location of injections between dogs leading to some of the variability in lymph node enhancement between dogs. The number and size of lymph nodes is variable between individual dogs, which may also have resulted in variability in enhancement between dogs (5).
Mild, temporary swelling has been reported with interstitial injection of gadopentetate dimeglumine and gadoteridol in human and canine subjects (2,3). Subcutaneous administration of gadolinium-containing contrast agents, including gadoversetamide, caused inflammation, edema, and necrosis at sites of injection in a mouse model (19). This study reported a substantial variability in the degree of tissue damage from 1 animal to another. The tissue damage associated with interstitial injection of gadolinium-containing contrast agents accounts for the dermal crusting seen in the week after imaging in 3 of the dogs after gadoversetamide injection. The dermal lesions in 3 dogs injected with Gadofluorine M use were identical to those seen after gadoversetamide injection. With the small volumes of contrast agents used for interstitial MR lymphography, this toxicity is not likely to be clinically significant.
The limitations of this pilot study include not being able to carry out statistical comparisons between the 2 contrast agents that a study using a larger number of dogs would allow. As well, we expected maximum enhancement for the gadoversetamide to occur within 15 min based on previous studies, and it would have been ideal to follow the enhancement pattern for a longer period of time in order to identify the time of maximum enhancement for the mandibular lymph nodes. The post-contrast imaging time for the Gadofluorine M was limited due to our use of a human MR facility, but it would have been desirable to follow these dogs to 120 min to confirm previous study findings of maximum enhancement between 60 and 90 min (1).
Indirect MR lymphography using both gadoversetamide and Gadofluorine M provided anatomical information regarding lymphatic drainage combined with high resolution anatomical imaging for the mandibular, retropharyngeal, and superficial cervical lymph nodes. Gadoversetamide and Gadofluorine M were comparable in the degree of enhancement of the lymph nodes but at different post-injection time points. If image acquisition over a longer period of time is desirable, use of Gadofluorine M may be preferable. This technique could be applied to tumor-bearing dogs at the time of primary tumor imaging, to identify those lymph nodes at highest risk of metastatic disease, and to guide staging and treatment. CVJ
Footnotes
The Gadofluorine M used in this study was provided by Bayer Schering Pharma AG.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Misselwitz B, Platzek J, Radüchel B, Oellinger JJ, Weinmann HJ. Gadofluorine 8: Initial experience with a new contrast medium for interstitial MR lymphography. MAGMA. 1999;8:190–195. doi: 10.1007/BF02594598. [DOI] [PubMed] [Google Scholar]
- 2.Loo BW, Jr, Draney MT, Sivanandan R, et al. Indirect MR lymphangiography of the head and neck using conventional gadolinium contrast: A pilot study in humans. Int J Radiat Oncol Biol Phys. 2006;66:462–468. doi: 10.1016/j.ijrobp.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 3.Suga K, Yuan Y, Ogasawara N, Okada M, Matsunaga N. Localization of breast sentinel lymph nodes by MR lymphography with a conventional gadolinium contrast agent. Preliminary observations in dogs and humans. Acta Radiol. 2003;44:35–42. [PubMed] [Google Scholar]
- 4.Suga K, Yuan Y, Ogasawara N, Okada M, Matsunaga N. Visualization of normal and interrupted lymphatic drainage in dog legs with interstitial MR lymphography using an extracellular MR contrast agent, gadopentetate dimeglumine. Invest Radiol. 2003;38:349–357. [PubMed] [Google Scholar]
- 5.Bezuidenhout AJ. The lymphatic system. In: Evans HE, editor. Miller’s Anatomy of the Dog. 3rd ed. Philadelphia, Pennsylvania: WB Saunders; 1993. pp. 717–757. [Google Scholar]
- 6.Martini FH, Timmons MJ, McKinley MP. Human Anatomy. 3rd ed. Upper Saddle River, New Jersey: Prentice Hall; 2000. The lymphatic system; pp. 600–619. [Google Scholar]
- 7.Suami H, Shin D, Uygur F, Chang DW. Comparative anatomical study of the lymphatic system of the upper extremity in canine and human. Clin Anat. 2010;23:1036. [Google Scholar]
- 8.Covidien Pharmaceuticals [homepage on the Internet] Hazelwood: c 2012. [Last accessed August 1, 2012]. Optimark package insert. Available from http://imaging.covidien.com/imageServer.aspx/doc133680.pdf?contentID=19019&contenttype=application/pdf. [Google Scholar]
- 9.Tsuda N, Tsuji T, Kato N. Interstitial magnetic resonance lymphography using gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid in rabbits with lymph node metastasis. Invest Radiol. 2005;40:306–312. doi: 10.1097/01.rli.0000160606.54347.15. [DOI] [PubMed] [Google Scholar]
- 10.Patsikas MN, Karayannopoulou M, Kaldrymidoy E, et al. The lymph drainage of the neoplastic mammary glands in the bitch: A lymphographic study. Anat Histol Embryol. 2006;35:228–234. doi: 10.1111/j.1439-0264.2005.00664.x. [DOI] [PubMed] [Google Scholar]
- 11.Pereira CT, Luiz Navarro MF, Williams J, Wlademir De MB, Primo BP. 99mTc-labeled dextran for mammary lymphoscintigraphy in dogs. Vet Radiol Ultrasound. 2008;49:487–491. doi: 10.1111/j.1740-8261.2008.00414.x. [DOI] [PubMed] [Google Scholar]
- 12.Gelb HR, Freeman LJ, Rohleder JJ, Snyder PW. Feasibility of contrast-enhanced ultrasound-guided biopsy of sentinel lymph nodes in dogs. Vet Radiol Ultrasound. 2010;51:628–633. doi: 10.1111/j.1740-8261.2010.01712.x. [DOI] [PubMed] [Google Scholar]
- 13.Wells S, Bennett A, Walsh P, Owens S, Peauroi J. Clinical usefulness of intradermal fluorescein and patent blue violet dyes for sentinel lymph node identification in dogs. Vet Comp Oncol. 2006;4:114–122. doi: 10.1111/j.1476-5810.2006.00099.x. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi H, Tangoku A, Suga K, et al. CT lymphography-navigated sentinel lymph node biopsy in patients with superficial esophageal cancer. Surgery. 2006;139:224–235. doi: 10.1016/j.surg.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Misselwitz B. MR contrast agents in lymph node imaging. Eur J Radiol. 2006;58:375–382. doi: 10.1016/j.ejrad.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 16.Wible JH, Jr, Troup CM, Hynes MR, et al. Toxicological assessment of gadoversetamide injection (OptiMARK), a new contrast-enhancement agent for use in magnetic resonance imaging. Invest Radiol. 2001;36:401–412. doi: 10.1097/00004424-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Brown JJ, Kristy RM, Stevens GR, Pierro JA. The OptiMARK clinical development program: Summary of safety data. J Magn Reson Imaging. 2002;15:446–455. doi: 10.1002/jmri.10091. [DOI] [PubMed] [Google Scholar]
- 18.Kneissl S, Probst A. Magnetic resonance imaging features of presumed normal head and neck lymph nodes in dogs. Vet Radiol Ultrasound. 2006;47:538–541. doi: 10.1111/j.1740-8261.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- 19.Runge VM, Dickey KM, Williams NM, Peng X. Local tissue toxicity in response to extravascular extravasation of magnetic resonance contrast media. Invest Radiol. 2002;37:393–398. doi: 10.1097/00004424-200207000-00006. [DOI] [PubMed] [Google Scholar]