Abstract
An atypical case of Mycoplasma pneumonia with an unusual radiographic and computed tomographic pattern was diagnosed in a Siamese kitten. The cat showed no response to broad-spectrum antibiotic therapy including enrofloxacin. The administration of doxycycline led to a dramatic clinical and radiographic improvement.
Résumé
Pneumonie atypique associée à un isolat de Mycoplasma chez un chaton. Un cas atypique de pneumonie à Mycoplasma avec image radiographique et tomodensitométrie inusitée a été diagnostiqué chez un chaton Siamois. Le chat n’a manifesté aucune réaction à un traitement antibiotique à large spectre d’efficacité incluant l’enrofloxacine. L’administration de doxycycline a provoqué une amélioration clinique et radiographique spectaculaire.
(Traduit par les auteurs)
A 3-month-old male Siamese kitten was presented to the emergency service of the Centre Hospitalier Universitaire Vétérinaire of the Faculty of Veterinary Medicine at the University of Montreal, for evaluation of acute respiratory distress. The cat had been adopted from a pet shop 5 d previously. It was initially alert and had a good appetite. No evidence of respiratory disease was observed by the owners. The cat started showing lethargy and inappetence 4 d after its adoption. It developed difficulty breathing and coughed without any associated nasal discharge or sneezing. The following day, the kitten was presented to its regular veterinarian who noticed that it was tachypneic and dyspneic. The cat was immediately referred to the Faculty of Veterinary Medicine after the application of dextrose on its buccal mucous membranes.
Case description
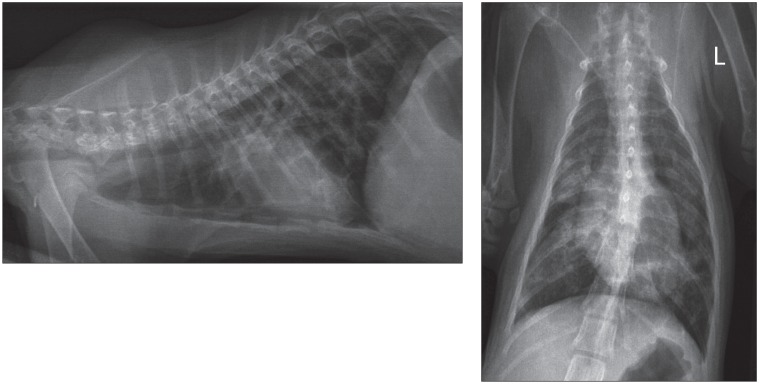
On presentation (day 1), the cat was lethargic and slightly hypothermic (37.6°C). Tachypnea and mixed dyspnea with a preponderant expiratory component were present. Respiratory auscultation showed a diffuse and symmetric increase in bronchovesicular sounds. Cardiac auscultation and the rest of the physical examination did not identify any other abnormalities. The cat received an injection of butorphanol (Torbugesic; Pfizer Santé Animale, Pfizer Canada, Kirkland, Quebec), 0.1 mg/kg body weight (BW), IM, and was placed in an oxygen cage. A complete blood (cell) count (CBC) revealed a slight neutrophilia [14.61 × 109/L; reference range (RR): 3.0 to 13.4 × 109/L]. The biochemistry profile showed a slight increase in alkaline phosphatase (75 U/L; RR: 0 to 50 U/L) and alanine aminotransferase (98 U/L; RR: 16 to 63 U/L) activities, hyper-phosphatemia (3.43 mmol/L; RR: 0.96 to 1.96 mmol/L) and hyperglobulinemia (50.1 g/L; RR: 29 to 47 g/L). Thoracic radiographs revealed a diffuse and severe unstructured interstitial pulmonary pattern with multifocal alveolar foci, predominantly in the right middle, right caudal, and left caudal lung lobes (Figure 1). Multiple linear soft-tissue opacity trabeculations were observed and gave an impression of septation of the pulmonary parenchyma; these were interpreted as areas of thickening or fibrosis of the visceral pleura, but the hypothesis of congenital pulmonary bullae was also considered. The size of the heart and pulmonary vessels was difficult to assess given the severe pulmonary abnormalities, but a slight generalized cardiomegaly and congestion of pulmonary vessels were suspected. The cat received 2 injections of furosemide (Salix™ injection; Merck Santé Animale, Intervet Canada Corp., Kirkland, Quebec), 0.5 mg/kg BW, IM, 1 h apart, but there was no improvement of its respiratory distress. Echocardiography and abdominal ultrasound showed no abnormalities.
Figure 1.
Initial (day 1) right latero-lateral and ventro-dorsal radiographic projections of the thorax of a 3-month-old Siamese kitten showing a diffuse unstructured interstitial pulmonary pattern with multifocal alveolar foci and multiple linear soft tissue opacity trabeculations.
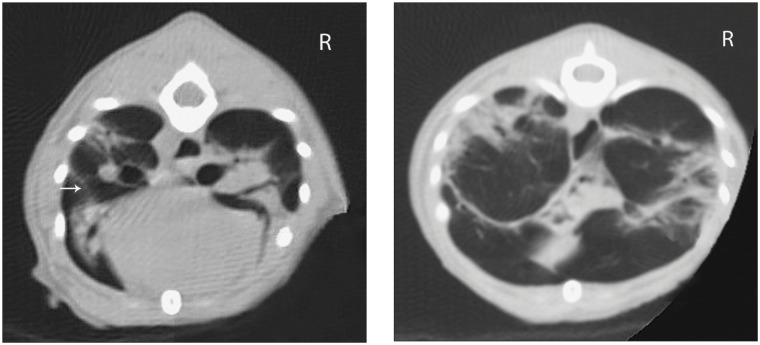
On day 2, computed tomographic examination of the thorax was performed under general anesthesia to characterize the unusual and severe pulmonary changes. Butorphanol (Torbugesic; Pfizer Santé Animale), 0.05 mg/kg BW, IV, was administered for premedication. Anesthesia was induced with ketamine (Vetalar, Bioniche Animal Health Canada, Belleville, Ontario), 5 mg/kg BW, IV and midazolam (Sandoz Canada, Boucherville, Quebec), 0.25 mg/kg BW, IV, and was maintained with isoflurane. Computed tomography (CT) was performed with a single slice helical scanner using the following parameters: 120 kV, 250 mA, 2 mm slice thickness, 1 mm slice interval, 1.0 pitch, matrix: 512 × 512 pixels, scan field of view: 250 mm, reconstruction (display) field of view: 120 mm, reconstruction algorithm: chest (high frequency). The cat was positioned in sternal recumbency and breath hold was performed during the acquisition of the images. The CT revealed a diffuse “ground glass” unstructured interstitial pulmonary pattern with multifocal alveolar consolidation of the lung parenchyma (Figure 2). Multiple linear soft tissue attenuation foci were observed on the surface of pulmonary lobes and inside the parenchyma, leading to an impression of septation of the lung field. A blind bronchoalveolar lavage was performed before recovery from anesthesia. Cytologic examination showed suppurative to mixed inflammation (50% degenerative neutrophils, 40% vacuolated macrophages, 10% mature lymphocytes) without evidence of microorganisms. Bronchoalveolar lavage was submitted for aerobic and Mycoplasma culture.
Figure 2.
Transverse 2-mm conventional computed tomography (CT) image of the thorax showing a diffuse unstructured interstitial pulmonary pattern with multifocal alveolar consolidation of lung parenchyma. Multiple linear soft tissue attenuation foci can be observed on the surface of pulmonary lobes and inside the parenchyma (arrow).
Initial treatment consisted of inhalation therapy with albuterol sulfate (Ventolin; GlaxoSmithKline, Mississauga, Ontario), 108 μg as needed, delivered through a 1-way valve spacer device, and injections of ticarcillin-clavulanic acid (Timentin, GlaxoSmithKline), 40 mg/kg BW, IV, q8h, while culture results were pending. The cat also received 1 injection of dexamethasone (Dexamethasone 2; Vétoquinol Canada, Lavaltrie, Quebec) at an anti-inflammatory dose, 0.08 mg/kg IV. The following day (day 3), enrofloxacin (Baytril; Bayer Healthcare, Santé Animale Division, Bayer, Toronto, Ontario), 5 mg/kg BW, IV, q24h, was added because the dyspnea became more severe. On day 4, routine culture of the bronchoalveolar lavage was negative. Inhalation of fluticasone propionate (Flovent; GlaxoSmithKline) was started (220 μg q12h delivered through a 1-way valve spacer device). On day 5, a second injection of dexamethasone (Dexamethasone 2; Vétoquinol Canada) was given, 0.15 mg/kg BW, IV, because of the lack of response to treatment.
Culture of the bronchoalveolar lavage resulted in isolation of Mycoplasma sp. Administration of doxycycline (Novo-doxylin; Novopharm Canada, Toronto, Ontario) was started, 7.5 mg/kg BW, PO, q24h. The cat experienced a dramatic improvement in its respiratory pattern and activity level during the first 24 h and continued to improve during the following days. Administration of enrofloxacin and ticarcillin-clavulanic acid was discontinued on day 8. On day 9, the cat was weaned from oxygen. Thoracic radiographs were repeated; a persistent diffuse unstructured interstitial pattern with multifocal alveolar consolidation was observed, but a redistribution of the pulmonary lesions was evident. On day 10, the cat was discharged with a prescription for doxycycline (7.5 mg/kg BW, PO, q24h) and inhalation of albuterol sulfate (108 μg q12h), based on the hypothesis that bronchospasms could contribute to the expiratory dyspnea.
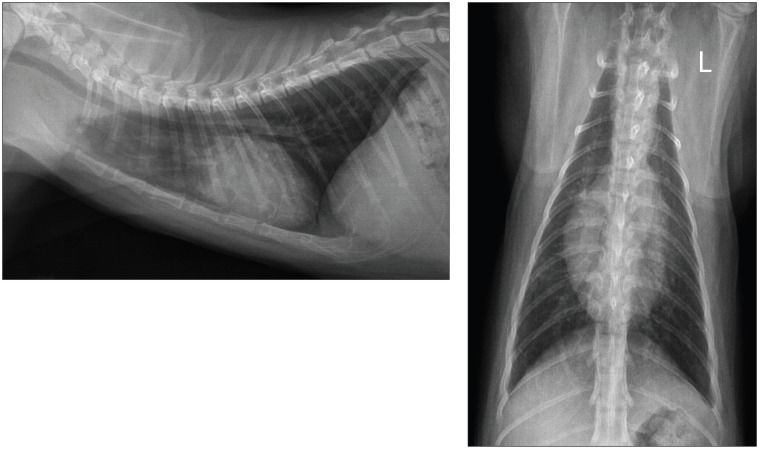
On day 24, the cat was revaluated. The owners reported the cat had experienced an excellent response to treatment and was completely normal at home. The respiratory pattern and auscultation were unremarkable. Thoracic radiographs revealed a marked decrease in severity of the interstitial and alveolar pulmonary lesions. The administration of albuterol sulfate was discontinued. On day 56, the multifocal alveolar pulmonary pattern was no longer observed on thoracic radiographs. The owners were advised to continue the administration of doxycycline for an additional 2 wk. The total duration of doxycycline administration was consequently 65 d. On day 106, the kitten was bright and alert, had a normal respiration, and had normal weight gain and growth since the last evaluation. New thoracic radiographs revealed a mild diffuse unstructured interstitial pulmonary pattern and few linear opacities superimposed over the lung field (Figure 3). Overall, the pulmonary lesions remained unchanged from the previous evaluation, but were considered mild and possibly due to residual pulmonary fibrosis.
Figure 3.
Post-treatment (day 106) left latero-lateral and ventro-dorsal radiographic projections of the thorax showing a complete resolution of the alveolar pulmonary pattern. A residual mild diffuse unstructured interstitial pulmonary pattern and few linear opacities can be observed.
Discussion
Mycoplasma species are small bacteria that lack a peptidoglycan cell wall. They belong to the normal commensal flora of the conjunctiva and upper airways (pharynx, larynx, oral cavity, nasal cavity) in cats and are a well-recognized cause of conjunctivitis and upper respiratory infection in this species (1–3). However, their role as a primary cause of lower respiratory disease has been debated for several years. Mycoplasma spp. have never been isolated from the trachea, bronchi, or lung of healthy cats (4,5). In most reported canine or feline cases with isolation of Mycoplasma spp. in the lower airways, a co-infection with other bacteria or an underlying disease leading to aspiration of gastric content, impairment of local defense mechanisms, or systemic immunosuppression were identified (5–9). Thus, Mycoplasma colonization and proliferation were considered a secondary event.
Few reports argue for a primary pathogenic role of Mycoplasma spp. in lower respiratory disease in cats. A case series identified Mycoplasma spp. as the sole bacterial isolate cultured from airway washings in 3 cats with clinical and radiographic signs of bronchitis (9). However, the role of Mycoplasma spp. as primary pathogens was unclear because of the suspicion of concomitant feline bronchial disease (feline asthma/chronic bronchitis) in these 3 cats. A recent retrospective study on lower respiratory tract infections in cats reported a pure airway growth of Mycoplasma spp. in 11/21 cats (10). A case of primary severe Mycoplasma pneumonia with reversible respiratory failure was recently reported in a cat (11). In the present report, the isolation of Mycoplasma spp. in pure culture from the lower airways, the absence of response to broad-spectrum antibiotic therapy followed by a complete and rapid response to a specific antibiotic (doxycycline) known to be active against Mycoplasma, and the absence of recurrence of clinical or radiographic respiratory signs after completion of treatment suggest a primary etiologic role for Mycoplasma spp. The hypothesis of a viral infection leading to a secondary Mycoplasma pneumonia cannot be definitively excluded but appears unlikely because the cat did not show any signs of upper airway or ocular involvement, which are the typical manifestations of respiratory viral infection in cats. In other animal species (cattle, goats, swine, and poultry), Mycoplasma spp. are well-recognized primary pathogens of the lower respiratory tract (12–14). In humans, Mycoplasma pneumoniae is a primary cause of pneumonia in otherwise healthy patients and is the main etiologic agent of community-acquired atypical pneumonia of older children and teenagers (15,16).
The cat in this report had unusual radiographic features consisting of a diffuse unstructured interstitial pulmonary pattern associated with a multifocal patchy alveolar opacification and linear opacities superimposed on the lungs. Radiographic changes associated with Mycoplasma lower respiratory infection are not well-described in the veterinary literature. Thoracic radiographs can be normal or feature a bronchial pattern without parenchymal involvement in some cases (8–10). Other pulmonary changes consist of interstitial and focal or lobar alveolar consolidation (8,10), or multifocal patchy alveolar pattern (10,11), similar to the case herein, and no case of linear pulmonary markings has been described.
In the present case, CT examination of the thoracic cavity was performed to better characterize the pulmonary changes. To our knowledge, only 1 previous report describes the use of CT in a case of feline Mycoplasma pneumonia (11). Interestingly, CT depicted a number of similarities with the case of this report, describing a multifocal patchy air space consolidation associated with a diffuse ground glass attenuation and nodular and reticular markings. In humans, CT findings associated with Mycoplasma are well-described (17,18). Adult human patients with M. pneumoniae pneumonia often present diffuse or multifocal areas of ground glass attenuation and consolidation with a lobular distribution, with frequent thickening of interlobular septa. This pattern of CT features appears distinct from the pattern observed in pediatric patients (lobar or segmental consolidation with frequent pleural effusion and regional lymphadenopathy) (18). The cat in this report seems to share some CT features with adult human patients with M. pneumoniae pneumonia. To our knowledge, this report is the first to document the long-term radiographic evolution after the successful treatment of Mycoplasma pneumonia in a cat. Despite the severe and diffuse radiographic parenchymal changes, a marked improvement of pulmonary lesions was evident with resolution of the multifocal alveolar pattern. The residual diffuse interstitial pattern and linear opacities were interpreted as parenchymal fibrosis and pleural or interlobular fibrosis, respectively. However, histopathologic analysis was not available for confirmation.
Similar to the results of a recent study (19), the mycoplasmal organisms were not observed cytologically. Moreover, the specific Mycoplasma species was not identified in this cat. Most studies focusing on feline respiratory mycoplasmosis did not identify the particular species. Consequently, little is known about the relation between clinical presentation, radiographic changes, or zoonotic potential of feline respiratory Mycoplasma infection and the Mycoplasma species implicated. Traditionally, the identification of mycoplasmas depends on serological methods, using a panel of species-specific diagnostic antisera. Recently, molecular methods, such as polymerase chain reaction (PCR) with or without a restriction enzyme digestion step, were developed for identification of canine and feline mycoplasmas. Advantages of PCR testing include the elimination of the need for complex and expensive Mycoplasma-specific culture medium and antisera, the shortened delay before diagnosis of Mycoplasma infection and identification of species, and possibly the identification of non-cultivable species (20,21).
The cat of this report did not show clinical improvement after the administration of enrofloxacin. Fluoroquinolones are generally considered active against mycoplasmas, just as macrolides, azalides, lincosamides, tetracyclines, or chloramphenicol (22–24). Mycoplasmas are naturally resistant to all beta-lactam antibiotics because they lack a cell wall. In the present case, resistance of the Mycoplasma isolate to fluoroquinolones is likely but could not be confirmed because an in vitro antibiotic susceptibility profile was not performed. Defective pulmonary diffusion appears less probable considering the pharmacokinetic properties of fluoroquinolones.
This case report highlights the importance of considering Mycoplasma sp. as a possible etiologic agent when an atypical pneumonia unresponsive to first-line antibiotics (particularly beta-lactams) is diagnosed in a cat. The presence of Mycoplasma sp. in the lower airways should be confirmed using specialized bacteriologic or molecular techniques. Susceptibility to fluoroquinolones should not be assumed and the choice of the antibiotic should ideally be based on an in vitro susceptibility profile. A dramatic clinical and radiographic improvement can be expected if appropriate treatment is implemented. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Bannasch MJ, Foley JE. Epidemiological evaluation of multiple respiratory pathogens in cats in animal shelters. J Feline Med Surg. 2005;7:109–119. doi: 10.1016/j.jfms.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson LR, Foley JE, DeCock HEV, Clarke HE, Maggs DJ. Assessment of infectious organisms associated with chronic rhinosinusitis in cats. J Am Vet Med Assoc. 2005;227:579–585. doi: 10.2460/javma.2005.227.579. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann AD, Hawley J, Werckenthin C, Lappin MR, Hartmann K. Detection of bacterial and viral organisms from the conjunctiva of cats with conjunctivitis and upper respiratory tract disease. J Feline Med Surg. 2010;12:775–782. doi: 10.1016/j.jfms.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padrid PA, Feldman BF, Funk K, Samitz EM, Reil D, Cross CE. Cytologic, microbiologic, and biochemical analysis of bronchoalveolar lavage fluid obtained from 24 healthy cats. Am J Vet Res. 1991;52:1300–1307. [PubMed] [Google Scholar]
- 5.Randolph JF, Moise NS, Scarlett JM, Shin SJ, Blue JT, Corbett JR. Prevalence of mycoplasmal and ureaplasmal recovery from tracheobronchial lavages and of mycoplasmal recovery from pharyngeal swab specimens in cats with or without pulmonary disease. Am J Vet Res. 1993;54:897–900. [PubMed] [Google Scholar]
- 6.Randolph JF, Moise NS, Scarlett JM, Shin SJ, Blue JT, Bookbinder PR. Prevalence of mycoplasmal and ureaplasmal recovery from tracheobronchial lavages and prevalence of mycoplasmal recovery from pharyngeal swab specimens in dogs with or without pulmonary disease. Am J Vet Res. 1993;54:387–391. [PubMed] [Google Scholar]
- 7.Jameson PH, King LA, Lappin MR, Jones RL. Comparison of clinical signs, diagnostic findings, organisms isolated, and clinical outcome in dogs with bacterial pneumonia: 93 cases (1986–1991) J Am Vet Med Assoc. 1995;206:206–209. [PubMed] [Google Scholar]
- 8.Foster SF, Barrs VR, Martin P, Malik R. Pneumonia associated with Mycoplasma spp. in three cats. Aust Vet J. 1998;76:460–464. doi: 10.1111/j.1751-0813.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 9.Chandler JC, Lappin MR. Mycoplasmal respiratory infections in small animals: 17 cases (1988–1999) J Am Anim Hosp Assoc. 2002;38:111–119. doi: 10.5326/0380111. [DOI] [PubMed] [Google Scholar]
- 10.Foster SF, Martin P, Allan GS, Barrs VR, Malik R. Lower respiratory tract infections in cats: 21 cases (1995–2000) J Feline Med Surg. 2004;6:167–180. doi: 10.1016/j.jfms.2003.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trow AV, Rozanski EA, Tidwell AS. Primary mycoplasma pneumonia associated with reversible respiratory failure in a cat. J Feline Med Surg. 2008;10:398–402. doi: 10.1016/j.jfms.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholas RA, Ayling RD. Mycoplasma bovis: Disease, diagnosis, and control. Res Vet Sci. 2003;74:105–112. doi: 10.1016/s0034-5288(02)00155-8. [DOI] [PubMed] [Google Scholar]
- 13.Sibila M, Pieters M, Molitor T, Maes D, Haesebrouck F, Segalés J. Current perspectives on the diagnosis and epidemiology of Mycoplasma hyopneumoniae infection. Vet J. 2009;181:221–231. doi: 10.1016/j.tvjl.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stipkovits L, Kempf I. Mycoplasmoses in poultry. Rev Sci Tech. 1996;15:1495–1525. doi: 10.20506/rst.15.4.986. [DOI] [PubMed] [Google Scholar]
- 15.Principi N, Esposito S, Blasi F, Allegra L. Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community- acquired lower respiratory tract infections. Clin Infect Dis. 2001;32:1281–1289. doi: 10.1086/319981. [DOI] [PubMed] [Google Scholar]
- 16.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697–728. doi: 10.1128/CMR.17.4.697-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reittner P, Ward S, Heyneman L, Johkoh T, Müller NL. Pneumonia: High-resolution CT findings in 114 patients. Eur Radiol. 2003;13:515–21. doi: 10.1007/s00330-002-1490-3. [DOI] [PubMed] [Google Scholar]
- 18.Lee I, Kim TS, Yoon HK. Mycoplasma pneumoniae pneumonia: CT features in 16 patients. Eur Radiol. 2006;16:719–725. doi: 10.1007/s00330-005-0026-z. [DOI] [PubMed] [Google Scholar]
- 19.Johnson LR, Vernau W. Bronchoscopic findings in 48 cats with spontaneous lower respiratory tract disease (2002–2009) J Vet Intern Med. 2011;25:236–243. doi: 10.1111/j.1939-1676.2011.00688.x. [DOI] [PubMed] [Google Scholar]
- 20.Chalker VJ, Owen WM, Paterson CJ, Brownlie J. Development of a polymerase chain reaction for the detection of Mycoplasma felis in domestic cats. Vet Microbiol. 2004;100:77–82. doi: 10.1016/j.vetmic.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Johnson LR, Drazenovich NL, Foley JE. A comparison of routine culture with polymerase chain reaction technology for the detection of Mycoplasma species in feline nasal samples. J Vet Diagn Invest. 2004;16:347–351. doi: 10.1177/104063870401600418. [DOI] [PubMed] [Google Scholar]
- 22.Brunner H, Weidner W. Chemotherapy of human Mycoplasma diseases. Isr J Med Sci. 1981;17:656–660. [PubMed] [Google Scholar]
- 23.Kato H, Murakami T, Takase S, Ono K. Sensitivities in vitro to antibiotics of Mycoplasma isolated from canine sources. Jap J Vet Sci. 1972;34:197–206. doi: 10.1292/jvms1939.34.197. [DOI] [PubMed] [Google Scholar]
- 24.Chalker VJ. Canine mycoplasmas. Res Vet Sci. 2005;79:1–8. doi: 10.1016/j.rvsc.2004.10.002. [DOI] [PubMed] [Google Scholar]