Abstract
Background. Retinoblastoma (RB) and transforming growth factor-β1 (TGF-β1) are important tumor-related factors. Methods. A series of 30 EBV-associated gastric carcinoma (EBVaGC) and 38 matched EBV-negative gastric carcinoma (EBVnGC) tissues were examined for the promoter methylation of RB by methylation-specific PCR (MSP) method. The expression of RB and TGF-β1 in gastric carcinoma tissues was detected by immunohistochemistry. Results. The methylation rate of RB gene in EBVaGC and EBVnGC was 80.0% (24/30) and 50.0% (19/38), respectively. The difference of RB methylation rate between EBVaGC and EBVnGC was significant (χ 2 = 6.490, P = 0.011). There was no significant difference for RB expression between EBVaGC (43.3%, 13/30) and EBVnGC (63.2%, 24/38), and also for TGF-β1 between EBVaGC (56.7%, 17/30) and EBVnGC (63.2%, 24/38). RB methylation was not reversely correlated with RB expression in gastric carcinoma tissues (χ 2 = 2.943, P = 0.086, r = 0.208). RB methylation, loss expression of RB, and TGF-β1 expression were significantly associated with tumor invasion and lymph node metastasis (P < 0.05), but was not associated with sex, age, histological subtype (differentiation status) and tumor location. Conclusions. Methylation of RB is a common event in gastric carcinomas and EBV induces methylation of RB in EBVaGC, which may contribute to the development of gastric carcinomas. EBV has no significant effect on induction of TGF-β1 expression. Detection of RB methylation, RB expression, and TGF-β1 expression may be helpful to judge the status of tumor invasion and lymph node metastasis in gastric carcinomas.
1. Introduction
Gastric carcinoma is the second leading cause of cancer-related death worldwide [1]. Epstein-Barr virus (EBV) is a tumor-related herpes virus associated with the transformation of various types of cells, such as lymphoid, dendritic, smooth muscle, and epithelial cells [2]. EBV-associated gastric carcinoma (EBVaGC) is characterized by the monoclonal growth of EBV-infected epithelial cells, and the entity was recognized by Imai in 1994 [3]. EBVaGC is distributed worldwide with an annual incidence of more than 90,000 patients (10% of total gastric carcinoma (GC)) [4]. Following infection, EBV remains in a latent state in EBVaGC, which is classified as latency I. Compared with EBV-negative gastric carcinoma (EBVnGC), EBVaGC has unique clinical and pathological features, such as a younger age of incidence, high incidence in men than in women, and more diffuse than intestinal types [5–7], suggestive of a particular oncogenic mechanism of EBVaGC.
Epigenetic alterations, including methylation of CpG dinucletides in promoters and changes in chromatin structure, can affect gene expression without modifying the underlying in genetic sequences. Aberrant methylation of promoters in tumor-related genes is now regarded as one of the major mechanisms in the development of gastric carcinoma [8]. Tumor-related genes p16, p14, E-cadherin, PTEN (phosphatase and tensin homolog deleted on chromosome ten), RASSF1A (Ras association domain family 1A), GSTP1 (Glutathione S-transferase pi 1), MGMT (O (6)-methylguanine-DNA-methyltransferase), and MINT2 (Munc18-1-interacting protein 2) are hypermethylated in EBVaGC [9–11], suggesting that EBV-related aberrant methylation may play an important role in development of EBVaGC.
Retinoblastoma (RB) and transforming growth factor-β1 (TGF-β1) are important regulatory factors in cell growth and differentiation, whose abnormal transcription or expression are closely associated with tumor occurrence and development. RB was the first successfully cloned human tumor suppressor gene (TSG). Its inactivation may result in cell proliferation leading to tumorigenesis [12]. TGF-β1 is a multifunctional cytokine and triggers an intracellular signal transduction protein to regulate numerous developmental and homoeostatic processes via regulation of gene induction. It plays a dual regulatory role in cell proliferation and differentiation. In the early stage of cancer, TGF-β1 can inhibit cell proliferation through arrest in the G1 phase and be regarded as a tumor suppressor; in the late stages, TGF-β1 becomes a tumor promoting factor by stimulating angiogenesis, cell spread, immune suppression, and synthesis of extracellular matrix [13–16]. It has been reported that EBV latent membrane protein 1 (LMP1) has a resistant to the TGF-β1-mediated growth inhibition in EBV-positive gastric carcinoma cell lines (GT38 and GT39) and indicated that TGF-β1 may be a key factor for EBV reactivation and selective growth of EBV-infected epithelial cells in vivo [17, 18]. However, the LMP1 expression is absent in EBVaGC tissues [19]. The identified role of TGF-β1 in EBVaGC has not been understood well and needs further research.
The absence of RB expression and overexpression of TGF-β1 have been found in gastric carcinomas [20, 21], and the mutations and methylation of RB gene in gastric carcinomas were also reported in the literature [22, 23]. Mukherjee et al. [24] found TGF-β1 treatment in late G(1) acutely blocks S-phase entry, this acute block by requiring the function of RB and loss of RB abrogates late-G(1) arrest by TGF-β1, suggesting a novel role for RB in mediating this effect of TGF-β1 late-G(1) arrest through direct interaction with and control of the MCM helicase. However, there is no report about the expression and promoter methylation status of RB and TGF-β1 in EBVaGC and EBVnGC to our knowledge. In this study, we examined RB methylation status, RB and TGF-β1 protein expression in EBVaGC and matched EBVnGC. The aim of the study is to understand the relationship among EBV, RB and TGF-β1 and their role in gastric carcinoma tumorgenesis.
2. Materials and Methods
2.1. Patients and Tissue Samples
Fresh and paraffin-embedded gastric carcinoma tissues were obtained from 1678 gastric carcinoma patients in Shangdong Province, China from 2001 to 2009. The positivity of EBV in GC tissues was determined by EBV-encoded small RNA 1 in situ hybridization, as described previously [25]. The clinical features (gender, age, pathologic grade, location, invasion and lymph node metastasis) matched 30 EBVaGC and 38 EBVnGC samples were chosen for study. The study was approved by the Medical Ethics Committee at the Medical College of Qingdao University, China, and informed consent was received from all patients.
2.2. DNA Extraction
DNA was extracted from fresh tumor tissues using the standard method with proteinase K digestion and phenol-chloroform purification. The QIAamp DNAFFPE Tissue kit (QIAGEN GmbH, Hilden, Germany) was used to extract the DNA from paraffin-embedded tumor tissues.
2.3. Immunohistochemistry (IHC)
Paraffin sections were deparaffinized and hydrated as per routine. Rabbit antihuman polyclonal antibody TGF-β1 and mouse antihuman monoclonal antibody RB (ZSGB-Bio) were diluted to 1 : 50. The reagents (PV9000 and DAB) were obtained from ZSGB-Bio and staining was performed as per protocol. PBS (phosphate buffer saline) was used in replacement of primary antibody as a blank control. The section was considered as expressing the protein if cellular staining ≥5%, following the methods described previously [26, 27].
2.4. Bisulfite Treatment of Genomic DNA and Methylation-Specific PCR (MSP)
5 μg DNA was denatured in 33.3 μL of 0.3 mol/L NaOH at 37°C for 15 minutes. Denatured DNA was mixed directly with 333 μL of bisulfite solution and treated in darkness. The bisulfite solution was prepared as either 2.4 mol/L sodium metabisulfite (pH 5.0–5.2) (Sigma S-1516, St. Louis, MO, USA)/0.5 mmol/L hydroquinone (Sigma H-7148) for a 4-hour treatment [28]. DNA was desalted and purified using the QIAEX Gel Extraction system (QIAGEN, Cat. no.20021). DNA was then treated with 0.3 mol/L NaOH at 37°C for 15 minutes and precipitated with 3 mol/L ammonium acetate (pH 7.0) and 1 mol/L sodium acetate (pH 5.2). Recovered DNA was dissolved in 100 μL of TE buffer (pH 8.0) and stored at −20°C.
RB promoter methylation status was determined using MSP. In this method, bisulfite treatment converts unmethylated cytosine to uracil, but does not affect the methylated cytosine. Thus, PCR primers can be designed that anneal selectively to methylated or unmethylated DNA after bisulfite conversion. The sequences of the unmethylated DNA and methylated DNA-specific primers are listed in Table 1. The primer UF/UR pair was designed specifically for amplification of the bisulfite-converted unmethylated promoter, while the MF/MR primer pair was designed specifically for the amplification of the bisulfite-converted methylated promoter. MSP results determined whether the samples are methylated or unmethylated. If there is M primers amplified band, the sample was considered to be in the methylation status. One microliter of bisulfite-treated DNA (around 25 ng) was amplified with 1.5 mmol/L MgCl2 and 0.2 mmol/L dNTP in a 25 μL reaction volume. Primers were used at a final concentration of 0.4 mmol/L each. The PCR involved an initial denaturation at 94°C for 10 minutes, followed by 40 cycles consisting of 94°C for 30 seconds, predetermined optimal annealing temperature for 30 seconds, 72°C for 30 seconds, and a final extension at 72°C for 5 minutes. Eight microliters of PCR product were analyzed on a 2.0% agarose gel. Water was used as a negative control.
Table 1.
List of primers used in MSP.
| Primers | Sequence | Product size (bp) | Annealing temp (0°C) | Genomic position |
|---|---|---|---|---|
| RBMF | 5′GGGAGTTTCGCGGACGTGAC3′ | 163 | 60 | −61 to 102 |
| RBMR | 5′ACGTCGAAACACGCCCCG3′ | |||
| RBUF | 5′GGGAGTTTTGTGGATGTGAT3′ | 163 | 58 | −61 to 102 |
| RBUR | 5′ACATCAAAACACACCCCA3′ |
2.5. Statistical Analysis
RB promoter methylation status, RB expression and TGF-β1 expression between EBVaGC and EBVnGC was compared using the Chi-square test. The correlation between promoter methylation and the protein expression was analyzed by Paired fourfold table Chi-square test. The association of clinical features with RB promoter methylation, RB expression, and TGF-β1 expression was compared by chi-square test. P < 0.05 was considered to be statistically significant.
3. Results
3.1. Comparison of Clinicopathological Data between EBVaGC and EBVnGC Patients
102 of 1678 (6.1%) cases of gastric carcinoma were EBV positive. 30 EBVaGC and 38 EBVnGC tumor tissues with matching clinical parameters were chosen for methylation detection. The clinical and pathological data are listed in Table 2. The two kinds of gastric carcinomas were similar in gender, age, pathologic grade, location, invasion, and lymph node metastasis.
Table 2.
Comparison of clinicopathological data between EBVaGC and EBVnGC patients.
| EBVaGC (n = 30) | EBVnGC (n = 38) | χ 2 | P | |
|---|---|---|---|---|
| Age (yr) | ||||
| <50 | 18 | 19 | 0.676 | 0.411 |
| ≥50 | 12 | 19 | ||
| Gender | ||||
| Male | 27 | 31 | — | 0.494 |
| Female | 3 | 7 | ||
| Pathologic grade | ||||
| Poorly differentiated | 28 | 32 | — | 0.288 |
| Well-moderately differentiated | 2 | 6 | ||
| Location | ||||
| Gastric cardia | 7 | 7 | — | 0.738 |
| Gastric body | 13 | 15 | ||
| Antrum | 10 | 16 | ||
| Depth of invasion | ||||
| Invasion to serosa and invasion through serosa | 22 | 24 | 0.793 | 0.373 |
| Not invading serosa | 8 | 14 | ||
| Lymph node metastasis | ||||
| Positive | 17 | 21 | 0.134 | 0.908 |
| Negative | 13 | 17 |
3.2. The Promoter Methylation Status of RB Gene in EBVaGC and EBVnGC
Promoter methylation of the RB gene was detected by MSP (Figure 1(a)). In total, 43/68 (63.2%) cases of gastric carcinomas demonstrated RB gene promoter methylation. The difference in the percent of positive methylation bands detected by MSP for RB was statistically different between EBVaGC (24/30, 80%) and EBVnGC (19/38, 50.0%) (P = 0.011).
Figure 1.
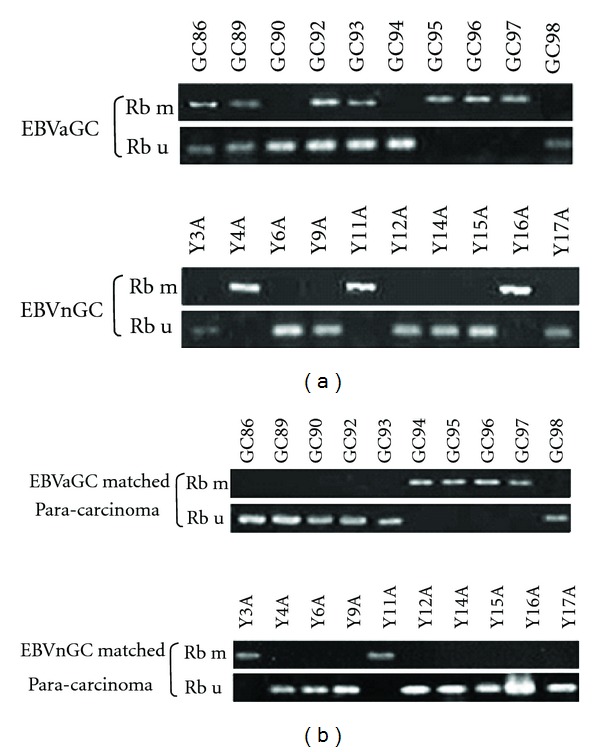
RB promoter methylation in EBVaGC, EBVnGC, and matched paracarcinoma tissues. (a) Representative RB promoter methylation in EBVaGC and EBVnGC by MSP. U, PCR product from the MSP assay using primers specific for the unmethylated allele; M, PCR product from the MSP assay using primers specific for the methylated allele. (b) Representative RB MSP assay results for matched paracarcinoma tissues.
The association of clinicopathological parameters of 68 cases with RB gene methylation status was studied. There was no relationship of RB gene methylation status with patient age, gender, pathologic types, and tumor location. However, the methylation status was associated with the depth of tumor invasion (P = 0.036) and lymph node metastasis (P = 0.012), (Table 3).
Table 3.
Correlation of methylation status of RB gene with clinicopathological data of gastric carcinoma patients.
| n | Methylated (n) | Unmethylated (n) | χ 2 | P | |
|---|---|---|---|---|---|
| EBV infection | |||||
| EBVaGC | 30 | 24 | 6 | 6.490 | 0.011 |
| EBVnGC | 38 | 19 | 19 | ||
| Age (yr) | |||||
| <50 | 37 | 22 | 15 | 0.498 | 0.481 |
| ≥50 | 31 | 21 | 10 | ||
| Gender | |||||
| Male | 58 | 36 | 22 | — | 0.835 |
| Female | 10 | 7 | 3 | ||
| Pathologic grade | |||||
| Poorly differentiated | 60 | 40 | 20 | — | 0.216 |
| Well-moderately differentiated | 8 | 3 | 5 | ||
| Location | |||||
| Gastric cardia | 14 | 10 | 4 | 3.172 | 0.205 |
| Gastric body | 28 | 20 | 8 | ||
| Antrum | 26 | 13 | 13 | ||
| Depth of invasion | |||||
| Invasion to serosa and invasion through serosa | 46 | 33 | 13 | 4.423 | 0.036 |
| Not invading serosa | 22 | 10 | 12 | ||
| Lymph node metastasis | |||||
| Positive | 38 | 29 | 9 | 6.339 | 0.012 |
| Negative | 30 | 14 | 16 |
3.3. The Promoter Methylation Status of RB Gene in GC and Corresponding Adjacent Normal Gastric Tissues
The promoter methylation of RB gene was detected in EBVaGC and EBVnGC corresponding adjacent normal gastric tissues (Figure 1(b)). The percent of positive methylation bands by MSP for RB in gastric carcinoma and corresponding adjacent normal gastric tissues was 63.2% (43/68) and 39.7% (27/68); the difference was significant (P = 0.006).
3.4. The Protein Expression of RB and TGF-β1
RB and TGF-β1 protein expression was detected by IHC, shown in Figures 2(a) and 2(b). The percent of EBVaGC that were positive by IHC for RB was 43.3% (13/30), lower than in EBVnGC (63.2%, 24/38), but not significantly different (P = 0.103). There was not obvious difference of TGF-β1 protein expression between EBVaGC (56.7%, 17/30) and EBVnGC (63.2%, 24/38) (P = 0.587) (Table 4).
Figure 2.
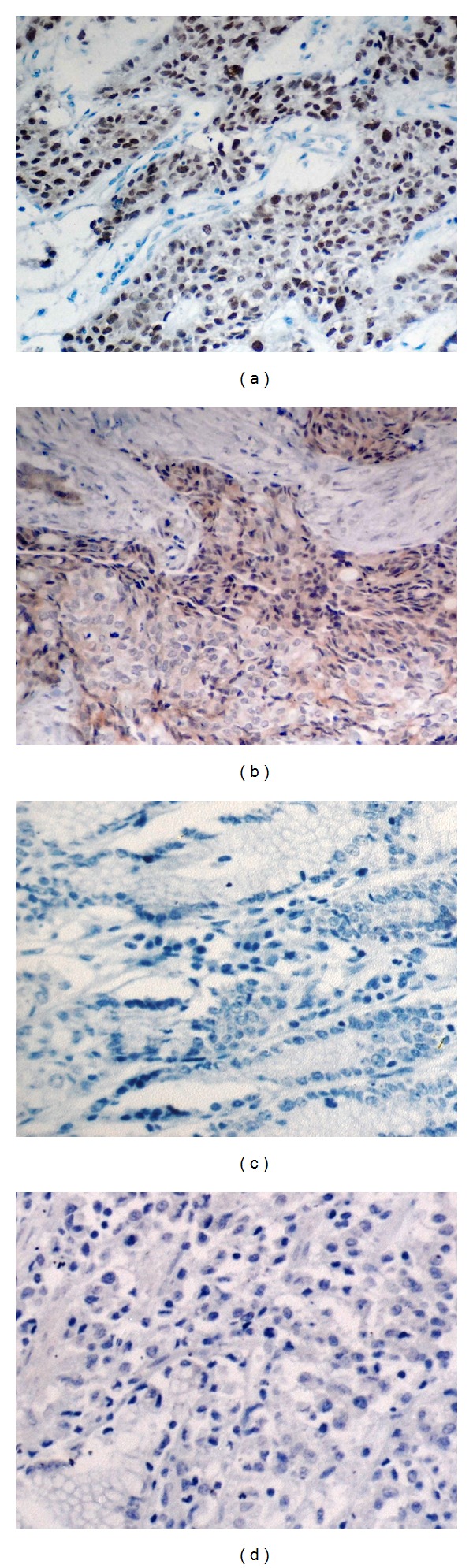
The protein expression of RB and TGF-β1 by immunohistochemistry (magnification ×100). (a) Positive immunohistochemistry result of RB in paraffin section. Expression of RB was found in nuclei of gastric carcinoma cells. (b) Positive immunohistochemistry result of TGF-β1 in paraffin section. Expression of TGF-β1 was found in cytoplasm of gastric carcinoma cells. (c) Negative immunohistochemistry result of RB in paraffin section. (d) Negative immunohistochemistry result of TGF-β1 in paraffin section.
Table 4.
Comparisons of the expression of RB and TGF-β1 between EBVaGC and EBVnGC.
| n | RB expression | TGF-β1 expression | |||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| EBVaGC | 30 | 13 | 17 | 17 | 13 |
| EBVnGC | 38 | 24 | 14 | 24 | 14 |
| χ 2 | 2.656 | 0.295 | |||
| P | 0.103 | 0.587 | |||
The correlation of RB protein expression with RB promoter methylation was studied. There were 23 negative RB protein expression cases in 43 RB gene methylated gastric carcinoma (53.5%), which was higher than in RB gene unmethylated gastric carcinoma (32.0%, 8/25), but without reverse correlation (P = 0.09, r = 0.21), (Table 5).
Table 5.
Correlation of methylation status of RB gene with its protein expression.
| Methylation status | Protein expression | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Methylated | 20 | 23 | 43 |
| Unmethylated | 17 | 8 | 25 |
|
| |||
| Total | 37 | 31 | 68 |
The association between RB expression and clinicopathological parameters is shown in Table 6. There was no relationship between RB protein expression and patients' age, gender, pathological grade, and tumor location, but it was related with the depth of tumor invasion (P = 0.04) and lymph node metastasis (P = 0.02).
Table 6.
Correlation of expression of RB and TGF-β1 protein with clinicopathological data of gastric carcinoma patients.
| n | RB | TGF-β1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Expression (n) | Absent (n) | χ 2 | P | Expression (n) | Absent (n) | χ 2 | P | ||
| Age (yr) | |||||||||
| <50 | 37 | 21 | 16 | 0.180 | 0.671 | 20 | 17 | 1.320 | 0.251 |
| ≥50 | 31 | 16 | 15 | 21 | 10 | ||||
| Gender | |||||||||
| Male | 58 | 31 | 27 | — | 0.972 | 35 | 23 | — | 0.742 |
| Female | 10 | 6 | 4 | 6 | 4 | ||||
| Pathologic grade | |||||||||
| Poorly differentiated | 60 | 31 | 29 | — | 0.428 | 38 | 22 | — | 0.295 |
| Well-moderately differentiated | 8 | 6 | 2 | 3 | 5 | ||||
| Location | |||||||||
| Gastric cardia | 14 | 7 | 7 | 2.091 | 0.352 | 8 | 6 | 0.318 | 0.853 |
| Gastric body | 28 | 13 | 15 | 18 | 10 | ||||
| Antrum | 26 | 17 | 9 | 15 | 11 | ||||
| Depth of invasion | |||||||||
| Invasion to serosa and invasion through serosa | 46 | 21 | 25 | 4.398 | 0.036 | 32 | 14 | 5.105 | 0.024 |
| Not invading serosa | 22 | 16 | 6 | 9 | 13 | ||||
| Lymph node metastasis | |||||||||
| Positive | 38 | 16 | 22 | 5.259 | 0.022 | 29 | 9 | 9.235 | 0.002 |
| Negative | 30 | 21 | 9 | 12 | 18 | ||||
The association between TGF-β1 expression and clinicopathological parameters is shown in Table 6. There was no relationship between TGF-β1 protein expression and patients' age, gender, pathological types, and tumor location, but there was positive association between TGF-β1 protein expression and the depth of tumor invasion (P = 0.02) and lymph node metastasis (P = 0.002).
4. Discussion
In this study, the percent of EBVaGC with positive methylation bands by MSP for RB was significantly higher than that of EBVnGC, indicating that EBV may induce RB promoter methylation during infection. Previous studies showed that EBVaGC had higher methylation frequency and promoter CpGI methylation density than EBVnGC in some TSGs, such as p16, E-cadherin and p73, and the methylation status was reverse correlated with protein expression [4, 10, 29]. These results indicate that methylation and silence of TSGs induced by EBV may be an important oncogenic mechanism for the development of EBVaGC. Only a few studies detected the methylation of RB in gastric carcinomas. Zhao et al. [30] found that the percent of positive methylation bands for RB gene was 44.6% (45/101), similar to our study of 38 EBVnGC (50%), but less than that of 30 EBVaGC cases (80%), which provides further support that EBV induces RB gene methylation in EBVaGC.
Promoter CpG island methylation is considered an important mechanism of TSG inactivation. In the present study, 23 of 43 (53.5%) methylated gastric carcinoma tissues lost RB protein expression, which was higher than that in unmethylated gastric carcinoma tissues (32.0%, 8/25), but RB promoter methylation was not reversely correlated with RB protein expression (P = 0.086). This phenomenon was also found between p16INK4 gene methylation status and expression in meningiomas by Tse et al. [31]. The possible explanations include: (1) gene methylation in gastric carcinoma tissue is heterogeneous; (2) gene methylation may occur in only one allele of cancer cells, while the other allele remains unmethylated. The above reasons may also explain the existence of methylated and unmethylated gene bands by MSP. Increasing of CpG island methylation density is a dynamic process, and only the methylation density increases to a certain extent, it results in the complete loss of the expression. The RB promotor methylation could result the decrease or loss of protein expression. Thus, lacking reverse correlation between RB promotor methylation and protein expression was not contradictory. Because of the limitation of major disadvantage of MSP, the methylation status of single CpG site in primer binding sequences is not be detected [32]. The correlation between RB promoter methylation dynamic change and protein expression need further study. These reasons above can also be used to explain why there wasn't a significant difference in RB protein expression between EBVaGC and EBVnGC, even though RB promoter methylation of EBVaGC was significantly higher than EBVnGC. If the RB gene promoter methylation and its protein expression were negatively correlated, RB protein expression in EBVaGC should have been significantly lower than that in EBVnGC. In this study, the percent of EBVaGC and EBVnGC that were positive by IHC for RB were 43.3% and 63.2%, respectively. Although no significant difference was found of the positive rate of RB protein expression between EBVaGC and EBVnGC, the relatively lower expression rate in EBVaGC also suggests that EBV-induced RB promoter methylation could lead to inhibition of RB protein expression to some extent. Moreover, the promoter methylation of RB gene was also detected in GC and corresponding adjacent normal gastric tissues and the difference was significant, which confirmed that RB promoter methylation is involved with the development of GC.
Similar to the result of RB protein expression, the positive rate of TGF-β1 protein expression was 56.7% (17/30) and 63.2% (24/38) in EBVaGC and EBVnGC, respectively, without significant difference (P = 0.404), suggesting that EBV is not related to the TGF-β1 expression in EBVaGC. Kim et al. [33] examined the association of EBV with RB and p53 protein expression in classic Hodgkin lymphoma and found that EBV wasn't associated with RB and p53 protein expression. Xu et al. [34] found TGF-β1 level in the serum of nasopharyngeal cancer patients was significantly higher than that in normal persons, and also the advanced stage was higher than the early stage, and recurrent tumors was higher than primary tumors, which indicating that serum TGF-β1 can be used for diagnosis and judgment for prognosis of NPC, and EBV infection can induce the synthesis and release of TGF-β1. This result was different from our result of TGF-β1 in EBVaGC.
Previous studies showed that TGF-β1 expression rate and expression level were higher in gastric carcinoma tissues than that in normal tissues, and the TGF-β1 expression were associated with gastric invasion, metastases, and prognosis [35–37]. In the advanced cancer, TGF-β1 can provide the microenvironment suitable for tumor growth, invasion, and metastases by stimulating angiogenesis, cell spread, immune suppression, and synthesis of extracellular matrix. In gastric carcinoma, RB protein loss was also found to be associated with metastases and prognosis [38–41]. The RB protein expression rate was 40% ~ 90% [38–40, 42, 43] and TGF-β1 protein expression rate was 22.8% ~ 71% [35–37, 44] in previous studies. In the present study, RB protein loss and TGF-β1 protein wasn't associated with patient age, gender, pathologic types, and tumor location, but associated with the depth of tumor invasion and lymph node metastasis. At the same time, we also confirmed that RB promoter methylation was associated with tumor invasion and lymph node metastasis, indicating that RB promoter methylation, RB and TGF-β1 protein expression can be as clinical reference index for judgement of gastric carcinoma invasion and metastasis.
5. Conclusion
Our study showed that Aberrant RB promoter methylation was common in gastric carcinoma. EBV could induce RB gene methylation and affect the gene expression in EBVaGC development. EBV has no significant effect on TGF-β1 expression.
Conflict of Interests
None of the authors has any conflicts of interests.
Acknowledgments
This research was supported by the Grant from National Natural Science Foundation of China (NSFC 30970157). The authors thank Stacey Barron (University of Pittsburgh, Pittsburgh, PA, USA) for her helpful review of the paper.
Abbreviations
- GC:
Gastric carcinoma
- EBV:
Epstein-Barr virus
- EBVaGC:
Epstein-Barr virus-associated gastric carcinoma
- EBVnGC:
EBV-negative gastric carcinoma
- TSG:
Tumor suppressor gene
- RB:
Retinoblastoma
- TGF-β1:
Transforming growth factor-β1.
References
- 1.Correa P, Piazuelo MB, Camargo MC. The future of gastric cancer prevention. Gastric Cancer. 2004;7(1):9–16. doi: 10.1007/s10120-003-0265-0. [DOI] [PubMed] [Google Scholar]
- 2.Uozaki H, Fukayama M. Epstein-Barr virus and gastric carcinoma—viral carcinogenesis through epigenetic mechanisms. International Journal of Clinical and Experimental Pathology. 2008;1(3):198–216. [PMC free article] [PubMed] [Google Scholar]
- 3.Imai S, Koizumi S, Sugiura M, et al. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(19):9131–9135. doi: 10.1073/pnas.91.19.9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ushiku T, Chong JM, Uozaki H, et al. p73 gene promoter methylation in Epstein-Barr virus-associated gastric carcinoma. International Journal of Cancer. 2007;120(1):60–66. doi: 10.1002/ijc.22275. [DOI] [PubMed] [Google Scholar]
- 5.Akiba S, Koriyama C, Herrera-Goepfert R, Eizuru Y. Epstein-Barr virus associated gastric carcinoma: epidemiological and clinicopathological features. Cancer Science. 2008;99(2):195–201. doi: 10.1111/j.1349-7006.2007.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JH, Kim SH, Han SH, An JS, Lee ES, Kim YS. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: a meta-analysis. Journal of Gastroenterology and Hepatology. 2009;24(3):354–365. doi: 10.1111/j.1440-1746.2009.05775.x. [DOI] [PubMed] [Google Scholar]
- 7.Fukayama M, Hino R, Uozaki H. Epstein-Barr virus and gastric carcinoma: virus-host interactions leading to carcinoma. Cancer Science. 2008;99(9):1726–1733. doi: 10.1111/j.1349-7006.2008.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taby R, Issa JP. Cancer epigenetics. Cancer Journal for Clinicians. 2010;60(6):376–392. doi: 10.3322/caac.20085. [DOI] [PubMed] [Google Scholar]
- 9.Sakuma K, Chong JM, Sudo M, et al. High-density methylation of p14ARF and P16INK4A in Epstein-Barr virus-associated gastric carcinoma. International Journal of Cancer. 2004;112(2):273–278. doi: 10.1002/ijc.20420. [DOI] [PubMed] [Google Scholar]
- 10.Sudo M, Chong JM, Sakuma K, et al. Promoter hypermethylation of E-cadherin and its abnormal expression in Epstein-Barr virus-associated gastric carcinoma. International Journal of Cancer. 2004;109(2):194–199. doi: 10.1002/ijc.11701. [DOI] [PubMed] [Google Scholar]
- 11.Kang GH, Lee S, Kim WH, et al. Epstein-Barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. American Journal of Pathology. 2002;160(3):787–794. doi: 10.1016/S0002-9440(10)64901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiman KG. The retinoblastoma gene: role in cell cycle control and cell differentiation. FASEB Journal. 1993;7(10):841–845. doi: 10.1096/fasebj.7.10.8393817. [DOI] [PubMed] [Google Scholar]
- 13.Akhurst RJ, Derynck R. TGF-β signaling in cancer—a double-edged sword. Trends in Cell Biology. 2001;11(11):S44–S51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 14.Attisano L, Wrana JL. Signal transduction by the TGF-β superfamily. Science. 2002;296(5573):1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 15.Lutz M, Knaus P. Integration of the TGF-β pathway into the cellular signalling network. Cellular Signalling. 2002;14(12):977–988. doi: 10.1016/s0898-6568(02)00058-x. [DOI] [PubMed] [Google Scholar]
- 16.Huang SS, Leal SM, Chen CL, Liu IH, Huang JS. Cellular growth inhibition by TGF-beta1 involves IRS proteins. FEBS Letters. 2004;565(1–3):117–121. doi: 10.1016/j.febslet.2004.03.082. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda M, Kurosaki W, Yanagihara K, Kuratsune H, Sairenji T. A mechanism in epstein-barr virus oncogenesis: inhibition of transforming growth factor-β1-mediated induction of MAPK/p21 by LMP1. Virology. 2002;302(2):310–320. doi: 10.1006/viro.2002.1619. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda M, Ikuta K, Yanagihara K, et al. Effect of transforming growth factor-β1 on the cell growth and Epstein-Barr virus reactivation in EBV-infected epithelial cell lines. Virology. 2001;288(1):109–118. doi: 10.1006/viro.2001.1071. [DOI] [PubMed] [Google Scholar]
- 19.Imai S, Koizumi S, Sugiura M, et al. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(19):9131–9135. doi: 10.1073/pnas.91.19.9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cito L, Pentimalli F, Forte I, Mattioli E, Giordano A. Rb family proteins in gastric cancer. Oncology Reports. 2010;24(6):1411–1418. doi: 10.3892/or_00001000. [DOI] [PubMed] [Google Scholar]
- 21.Ananiev J, Gulubova M, Tchernev G, et al. Relation between transforming growth factor-beta1 expression, its receptor and clinicopathological factors and survival in HER2-negative gastric cancers. Wiener Klinische Wochenschrift. 2011;123(21-22):668–673. doi: 10.1007/s00508-011-0078-9. [DOI] [PubMed] [Google Scholar]
- 22.Galetsky SA, Tsvetnov VV, Land CE, et al. Epstein-Barr-Virus-associated gastric cancer in Russia. International Journal of Cancer. 1997;73(6):786–789. doi: 10.1002/(sici)1097-0215(19971210)73:6<786::aid-ijc2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 23.Chang MS, Lee HS, Kim CW, Kim YI, Kim WH. Clinicopathologic characteristics of Epstein-Barr virus-incorporated gastric cancers in Korea. Pathology Research and Practice. 2001;197(6):395–400. doi: 10.1078/0344-0338-00052. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee P, Winter SL, Alexandrow MG. Cell cycle arrest by transforming growth factor β1 near G 1/S is mediated by acute abrogation of prereplication complex activation involving an Rb-MCM interaction. Molecular and Cellular Biology. 2010;30(3):845–856. doi: 10.1128/MCB.01152-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Kanai K, Satoh Y, Luo B, Sairenji T. Carboxyl-terminal sequence variation of latent membrane protein 1 gene in Epstein-Barr virus-associated gastric carcinomas from eastern China and Japan. Intervirology. 2007;50(3):229–236. doi: 10.1159/000100566. [DOI] [PubMed] [Google Scholar]
- 26.Khaled HM, Bahnassy AA, Raafat AA, Zekri ARN, Madboul MS, Mokhtar NM. Clinical significance of altered nm23-H1, EGFR, RB and p53 expression in bilharzial bladder cancer. BMC Cancer. 2009;9:p. 32. doi: 10.1186/1471-2407-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valkov A, Sorbye SW, Kilvaer TK, et al. The prognostic impact of TGF-β1, fascin, NF-κb and PKC-ζ expression in soft tissue sarcomas. PLoS ONE. 2011;6(3) doi: 10.1371/journal.pone.0017507.e17507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao Q, Huang H, Geiman TM, et al. Defective de novo methylation of viral and cellular DNA sequences in ICF syndrome cells. Human Molecular Genetics. 2002;11(18):2091–2102. doi: 10.1093/hmg/11.18.2091. [DOI] [PubMed] [Google Scholar]
- 29.Osawa T, Chong JM, Sudo M, et al. Reduced expression and promoter methylation of p16 gene in Epstein-Barr virus-associated gastric carcinoma. Japanese Journal of Cancer Research. 2002;93(11):1195–1200. doi: 10.1111/j.1349-7006.2002.tb01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao YF, Zhang YG, Tian XX, Du J, Zheng J. Aberrant methylation of multiple genes in gastric carcinomas. International Journal of Surgical Pathology. 2007;15(3):242–251. doi: 10.1177/1066896907302117. [DOI] [PubMed] [Google Scholar]
- 31.Tse JYM, Ng HK, Lo KW, et al. Analysis of cell cycle regulators: p161NK4A, pRb, and CDK4 in low- and high-grade meningiomas. Human Pathology. 1998;29(11):1200–1207. doi: 10.1016/s0046-8177(98)90246-5. [DOI] [PubMed] [Google Scholar]
- 32.Dahl C, Guldberg P. DNA methylation analysis techniques. Biogerontology. 2003;4(4):233–250. doi: 10.1023/a:1025103319328. [DOI] [PubMed] [Google Scholar]
- 33.Kim LH, Peh SC, Poppema S. Expression of retinoblastoma protein and P16 proteins in classic Hodgkin lymphoma: relationship with expression of p53 and presence of Epstein-Barr virus in the regulation of cell growth and death. Human Pathology. 2006;37(1):92–100. doi: 10.1016/j.humpath.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Menezes J, Prasad U, Ahmad A. Elevated serum levels of transforming growth factor beta1 in Epstein-Barr virus-associated nasopharyngeal carcinoma patients. International Journal of Cancer. 1999;8484(4):396–399. doi: 10.1002/(sici)1097-0215(19990820)84:4<396::aid-ijc11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 35.Vagenas K, Spyropoulos C, Gavala V, Tsamandas AC. TGFbeta1, TGFbeta2, and TGFbeta3 protein expression in gastric carcinomas: correlation with prognostics factors and patient survival. Journal of Surgical Research. 2007;139(2):182–188. doi: 10.1016/j.jss.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Saito H, Tsujitani S, Oka S, et al. The expression of transforming growth factor-beta1 is significantly correlated with the expression of vascular endothelial growth factor and poor prognosis of patients with advanced gastric carcinoma. Cancer. 1999;86(8):1455–1462. doi: 10.1002/(sici)1097-0142(19991015)86:8<1455::aid-cncr11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 37.Maehara Y, Kakeji Y, Kabashima A, et al. Role of transforming growth factor-β1 in invasion and metastasis in gastric carcinoma. Journal of Clinical Oncology. 1999;17(2):607–614. doi: 10.1200/JCO.1999.17.2.607. [DOI] [PubMed] [Google Scholar]
- 38.Kouraklis G, Katsoulis IE, Theocharis S, et al. Does the expression of cyclin E, pRb, and p21 correlate with prognosis in gastric adenocarcinoma? Digestive Diseases and Sciences. 2009;54(5):1015–1020. doi: 10.1007/s10620-008-0464-y. [DOI] [PubMed] [Google Scholar]
- 39.Song HS, Kim IH, Sohn SS, Kwon KY, Lee WS. Prognostic significance of immunohistochemical expression of p53 and retinoblastoma gene protein (pRB) in curatively resected gastric cancer. Korean Journal of Internal Medicine. 2005;20(1):1–7. doi: 10.3904/kjim.2005.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feakins RM, Nickols CD, Bidd H, Walton SJ. Abnormal expression of pRb, p16, and Cyclin D1 in Gastric adenocarcinoma and its lymph node metastases: relationship with pathological features and survival. Human Pathology. 2003;34(12):1276–1282. doi: 10.1016/j.humpath.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Chou NH, Chen HC, Chou NS, Hsu PI, Tseng HH. Expression of altered retinoblastoma protein inversely correlates with tumor invasion in gastric carcinoma. World Journal of Gastroenterology. 2006;12(44):7188–7191. doi: 10.3748/wjg.v12.i44.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kishimoto I, Mitomi H, Ohkura Y, Kanazawa H, Fukui N, Watanabe M. Abnormal expression of p16INK4a, cyclin D1, cyclin-dependent kinase 4 and retinoblastoma protein in gastric carcinomas. Journal of Surgical Oncology. 2008;98(1):60–66. doi: 10.1002/jso.21087. [DOI] [PubMed] [Google Scholar]
- 43.He XS, Rong YH, Su Q, et al. Expression of p16 gene and Rb protein in gastric carcinoma and their chinicopathological significance. World Journal of Gastroenterology. 2005;11(15):2218–2223. doi: 10.3748/wjg.v11.i15.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zolota V, Batistatou A, Tsamandas AC, Iliopoulos G, Scopa CD, Bonikos DS. Immunohistochemical expression of TGF-β1, p21WAF1, p53, Ki67, and angiogenesis in gastric carcinomas: a clinicopathologic study. International Journal of Gastrointestinal Cancer. 2002;32(2-3):83–89. doi: 10.1385/IJGC:32:2-3:83. [DOI] [PubMed] [Google Scholar]


