Abstract
Surviving in a world with hidden rewards and dangers requires choosing the appropriate behaviours. Recent discoveries indicate that the habenula plays a prominent part in such behavioural choice through its effects on neuromodulator systems, in particular the dopamine and serotonin systems. By inhibiting dopamine-releasing neurons, habenula activation leads to the suppression of motor behaviour when an animal fails to obtain a reward or anticipates an aversive outcome. Moreover, the habenula is involved in behavioural responses to pain, stress, anxiety, sleep and reward, and its dysfunction is associated with depression, schizophrenia and drug-induced psychosis. As a highly conserved structure in the brain, the habenula provides a fundamental mechanism for both survival and decision-making.
The habenula is a phylogenetically old brain structure that is present in virtually all vertebrate species. In many of the vertebrates — fishes, amphibian, and reptiles — the habenula is larger on one side than the other, unlike most other brain structures1. The asymmetry of the habenula is often associated with asymmetries in neuronal organization2 and behaviour — for example, in social recognition, anti-predator response and locomotion3. In birds and mammals the habenula is a small area located at the posterior-dorsal-medial end of the thalamus and is divided into the medial habenula (MHb) and the lateral habenula (LHb).
It is thought that the habenula has evolved in close association with the pineal gland4, with which it has reciprocal connections5. The habenula also receives inputs from the limbic system and the basal ganglia, mainly through the stria medullaris6 (FIG. 1). The pineal gland, habenula and stria medullaris together form the epithalamus, a prominent structure that overlies the thalamus. The fasciculus retroflexus (also known as the habenula-interpeduncular tract) forms the output of the habenula. LHb neurons project to midbrain areas that are involved in the release of dopamine (the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA)) and serotonin (the median raphe nucleus (MRN) and dorsal raphe nucleus (DRN))7. It has recently been shown that LHb efferents are predominantly mediated by the rostromedial tegmental nucleus (RMTg)8. MHb neurons project to the interpeduncular nucleus (IPN), which projects to the MRN and DRN in addition to other areas9. It is plausible, therefore, that both the LHb and the MHb control the release of serotonin.
Figure 1. Anatomy of the habenula.
a | The habenula in the rhesus monkey. A coronal histological section (scale: 1 mm × 5) shows the habenula (indicated by the red circle). The medially located dark region corresponds to the medial habenula (MHb) and the lateral part corresponds to the lateral habenula (LHb). The violet line in the diagram of the monkey brain (viewed from the mesial side) corresponds to the vertical extent of the cross-section view of the habenula (scale: 5 mm × 2). The location of the habenula is also indicated in the diagram of the monkey brain (indicated by the orange circle). C, caudate nucleus; cc, corpus callosum; hc, habenular commissure; IC, inferior colliculus; MD, mediodorsal nucleus of the thalamus; N3, oculomotor nucleus; SC, superior colliculus; pc, posterior commissure; PT, pretectum; Pul, pulvinar; Th, thalamus. b | Afferent and efferent connections of the habenula. The MHb, LHb and pineal gland are collectively called the epithalamus. The MHb receives inputs mainly from the limbic system and sends outputs to the interpeduncular nucleus (IPN), which projects to the raphe nuclei. The LHb receives inputs mainly from the basal ganglia and sends outputs to the brain structures that contain dopaminergic neurons and serotonergic neurons, partly through the rostromedial tegmental nucleus (RMTg). Direct connections to dopaminergic and serotonergic neurons are not shown. The role of the RMTg for serotonergic neurons is less clear (indicated by the dashed lines). Light and dark grey lines indicate the axonal connections associated with the MHb and LHb, respectively. Many other connections are not shown, including reverse connections (for example, from the dorsal raphe nucleus and the ventral tegmental area (VTA) to the LHb). CPu, caudate and putamen; DBB, diagonal band of Broca; GPb, border region of the globus pallidus; LPO, lateral preoptic area; SNc, substantia nigra pars compacta.
The neural network that is outlined above indicates that the habenula may act as a node to link the forebrain to the midbrain regions that are involved in regulating emotional behaviours10. Indeed, experimental manipulations of this system — and in particular, lesions of the habenula — are followed by behavioural alterations in relation to pain, stress, anxiety, sleep, reward, and to cognitive and motor dysfunctions11. Although many studies have been performed since the 1950s, the precise function of the habenula has remained obscure.
Recently, however, the habenula has attracted a great deal of attention. One main reason for this is probably the part that it plays in the regulation of dopamine and serotonin systems. Experimental and clinical studies have shown that these neuromodulators are essential for normal motor and mental activities — as exemplified by the disorders that are associated with reduced levels of dopamine (for example, Parkinson’s disease) and serotonin (for example, major depression) — and the habenula is one of few regions that influence both the dopamine and serotonin systems. Another reason for the increased interest in the habenula may be the recent advances in human brain imaging studies. Until recently the resolution of the habenula in MRI and positron emission tomography (PET) studies was too low, but the habenula can now be visualized in imaging studies. The habenula has been shown to be hyperactive in patients with major depression12 and in healthy people when receiving negative feedback regarding a failed performance in a task13.
In this Review, I first discuss the various processes in which the habenula seems to be involved. Second, I show that the role of the habenula in these processes is characterized by a common theme, namely motor suppression (FIG. 2). Finally, I propose a common neural mechanism that may underlie the various functions of the habenula. I will not discuss in detail the comparative anatomy of the habenula, its development, its afferent and efferent connections and the neurotransmitters that are used by its neurons (and neurons associated with it), because excellent review articles have been published on these issues1,10,11,14.
Figure 2. Proposed common mechanisms for the diverse functions of the habenula.
The habenula is equipped with mechanisms by which body movements can be suppressed. How the motor suppression mechanisms are used depends on the information that is fed to the habenula. a | A phylogenetically old input to the habenula derives from the pineal gland, which encodes light–dark changes and regulates circadian rhythms. The habenula, which has reciprocal connections with the pineal glad, controls the level of motor activity according to sleep–wake states through its polysynaptic connections to neural circuits in the brainstem. b | A second input to the habenula derives from the basal ganglia, which encodes failure or punishment resulting from a motor action. Based on this input, the habenula inhibits dopamine neurons in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc). This results in suppression of the motor action. c | A third input to the habenula derives from the limbic system, which conveys information regarding aversive, painful or stressful events. In this case the habenula inhibits both serotonin and dopamine neurons and this results in a general suppression of body movement.
The habenula and sleep
Sleep is a fundamental phenomenon in which body and brain activities are suppressed. Several findings directly and indirectly suggest that the habenula has a role in the control of sleep, and this is probably related to its evolutional relationship with the pineal gland4 (see below).
First, the habenula seems to be crucial for rapid eye movement (REM) sleep. Removing the habenula output by lesioning the fasciculus retroflexus in rats reduces both the amount of time that rats spend in REM sleep and the atonia associated with REM sleep15,16. When fetal brain tissue that includes the habenula is transplanted into these rats the normal sleep pattern and atonia are restored15,17. Second, the firing of habenula neurons shows circadian rhythmicity18, even in slice preparations19. Third, neural activity of the LHb or the MHb increases markedly during drug-induced general anesthaesia20–22 and emerging evidence suggests that similar neural mechanisms underlie sleep and anesthaesia23, indicating that habenula activity may also increase during sleep. Fourth, in hibernating squirrels LHb neurons show increased activity24 and MHb neurons show elevated levels of the rate-limiting enzyme for the synthesis of melatonin25. This suggests that the habenula (like the pineal gland) synthesizes melatonin and may have a role in the generation and maintenance of hibernation. Finally, MHb neurons produce the cytokine interleukin (IL)-18 (REF. 26), which is known to promote sleep when it is injected intracerebroventricularly27.
The proposed role of the habenula in the regulation of sleep may be achieved through the influence of the habenula on serotonin neurons28, whereas its control of sleep-associated atonia may be mediated by descending circuits in the brainstem, possibly via the RMTg, without the involvement of serotonin or dopamine neurons29,30 (FIG. 2a).
The co-evolution of the habenula and the pineal gland4 may explain how the habenula acquired a sleep-regulatory function. The pineal gland regulates circadian and seasonal rhythmicity by releasing melatonin (this occurs at night in humans)31 and has mutual connections with the habenula32. In many non-mammalian vertebrates the pineal gland contains photosensitive cells, but in mammals photodetection takes place in the retina. Melanopsinmediated retinal ganglion cells send input to the LHb (and to the suprachiasmatic nucleus)33, which is therefore sensitive to photic stimulation19. It is possible that the habenula may use this photic information to regulate sleep.
Normal sleep and sleep-like states are considered to be beneficial for survival under stress because they minimize energy expenditure34,35. If the habenula regulates normal sleep, non-physiological sleep and sleep-like behaviour, as proposed above, it may have an instrumental role in minimizing energy expenditure by suppressing body movements during sleep-like states. However, this hypothesis remains to be examined in future studies.
Reward-based decision-making
Several studies suggest that the habenula also suppresses motor behaviours in awake animals. Animals with habenula lesions become hyperactive, distractible and make motor responses prematurely in a reaction-time task36,37. These effects may be mediated by the indirect connection of the LHb to dopamine neurons in the SNc or the VTA (FIG. 2b). Indeed, without dopamine neurons, humans and most animals are unable to initiate voluntary movements, as seen in patients with, and in animal models of, Parkinson’s disease38.
Several lines of evidence indicate that LHb neurons inhibit dopamine neurons: electrical stimulation of the LHb inhibits activity of dopamine neurons in the SNc and VTA39–41, and habenula lesions increase dopamine release in the cerebral cortex and the striatum42. This suggests that the motor hyperactivity that is induced by habenula lesions in rats may be due to the removal of the inhibition of dopamine neurons.
In apparent contradiction to the association between dopamine neuron activity and movement, dopamine neurons usually do not change their activity before or during a body movement. Instead, they respond to sensory events that predict changes in the animal’s motivational state (which in turn induce the animal to act and therefore to move)43. Typically, dopamine neurons are excited by larger-than-expected rewards (and their predictors) and are inhibited by smaller-than-expected rewards (and their predictors)43. It has been proposed that these changes in dopamine neuron activity drive reinforcement learning44. In this scheme, the excitation of dopamine neurons in response to receiving a larger-than-expected reward facilitates the action that leads to the larger reward, whereas the inhibition of dopamine neurons in response to receiving a smaller-than-expected reward suppresses the action that leads to the smaller reward (FIG. 3).
Figure 3. Role of the habenula in value-based decision-making.
This circuit is an extended form of the dopamine-mediated circuit shown in FIG. 2b. Input from the basal ganglia to the habenula, specifically the lateral habenula (LHb), derives from the border region of the internal segment of the globus pallidus (GPb), which receives inputs from the striatum — presumably the ‘striosome’ subterritory of the striatum (Striatum-S). Based on the basal ganglia input, the habenula influences dopamine neurons via inhibitory neurons in the rostromedial tegmental nucleus (RMTg). Negative reward prediction errors are encoded by an excitation of LHb neurons and consequently an inhibition of dopamine neurons. Positive reward prediction errors are encoded by an inhibition of LHb neurons and an excitation of dopamine neurons. Such bidirectional modulation of dopamine neurons contributes to the suppression of the to-be-less-rewarded motor action and the facilitation of the to-be-more-rewarded motor action. These effects on motor action are mediated by the innervation of the ‘matrix’ subterritory of the striatum (Striatum-M) by dopamine neurons in the ventral tegmental area (VTA) and substatia nigra pars compacta (SNc), and subsequently the innervation of the output region of the basal ganglia, that is, the substantia nigra pars reticulata (SNr) and the internal segment of the globus pallidus (GPi).
Recent studies have revealed that dopamine neurons may receive these reward-related signals from the LHb. A functional MRI (fMRI) study in which human subjects performed a motion-prediction task13 showed that the habenula (in addition to the anterior cingulate cortex and insula) was activated when a subject received feedback indicating that his or her response in the task was wrong. This finding was corroborated at the single-cell level by an experiment using macaque monkeys that had been trained to perform a visual saccade task with positionally-biased reward outcomes41 (FIG. 4). LHb neurons were excited by the appearance of a stimulus indicating that the monkey would receive a small reward and were inhibited by a stimulus indicating that a large reward would be received. LHb neurons also responded when the monkey received the actual reward (they were excited by a small reward and inhibited by a large reward), but only if the reward was not expected41. This response pattern is the opposite of that observed in dopamine neurons41,43.
Figure 4. The lateral habenula–dopamine system for modulation of saccadic eye movements.
a | Visual saccade task with position-biased reward outcomes. In one block of 20–30 trials, saccades in one direction (to the right) were rewarded, whereas saccades in the other direction (to the left) were not rewarded. The position–reward contingency was reversed in the next block without warning (not shown). In both blocks, after each correct trial a tone indicated that a correct response (that is, a saccade in the correct direction) had been made and a juice reward was delivered simultaneously with the tone. b | In each block, saccades to the rewarded position (shown by the red circles) became quicker (shorter latencies) whereas saccades to the unrewarded position (shown by the blue squares) became slower (longer latencies). c | The reward-indicating saccade target induced an inhibition in lateral habenula (LHb) neurons (top) and an excitation in dopamine (DA) neurons in the substantia nigra pars compacta (SNc) (bottom; shown by the red traces); the no-reward-indicating target induced an excitation in LHb neurons and an inhibition in dopamine neurons (shown by the blue traces). The LHb and dopamine neurons also responded strongly to the outcomes in the first trials of a block when the outcomes were unexpected (dashed red and blue traces). Figure is modified, with permission, from Nature REF. 41 © (2007) Macmillan Publishers Ltd. All rights reserved.
The results described above suggest that the LHb contributes to reinforcement learning through inhibitory action on dopamine neurons. However, subsequent studies have shown that the neural circuits that support LHb-mediated reinforcement learning are more complex (FIG. 3). First, negative reward prediction error signals are supplied to the LHb, at least partly, by neurons in the border region of the globus pallidus (GPb), more specifically, by neurons located between the internal and external segments of the globus pallidus45. These GPb neurons in turn receive input from the striatum, presumably from the subterritory known as the ‘striosome’46. Second, the inhibitory effect of LHb neurons on dopamine neurons seems to be mediated by GABA (γ-aminobutyric acid)-ergic inhibitory neurons40 in the RMTg8,47,48. Taken together, the data described above indicate that the GPb–LHb–RMTg–dopamine pathway has an important role in reward-based decision-making.
Avoidance of punishment
Failing to obtain a reward is disappointing and disheartening, but to be punished may be worse. LHb neurons have been shown to be excited49,50 or sometimes inhibited49 by painful — or otherwise aversive — stimuli. Furthermore, electrical stimulation of, or morphine injections into, the habenula induces analgesia51,52. These results suggest that the habenula may have a role in the response to aversive stimuli, including pain.
One method to investigate whether the habenula neurons indeed process motivational information rather than sensory information is to measure the response of habenula neurons to sensory stimuli that initially have no motivational value for the animal but that are subsequently conditioned using rewarding or aversive stimuli. In Pavlovian conditioning, such sensory stimuli are called conditioned stimuli (CSs) and the rewarding or aversive stimuli are called unconditioned stimuli (USs). If habenula neurons process negative motivational information, they should respond differently to the CS depending on the stimulus’ conditioned (or learned) values.
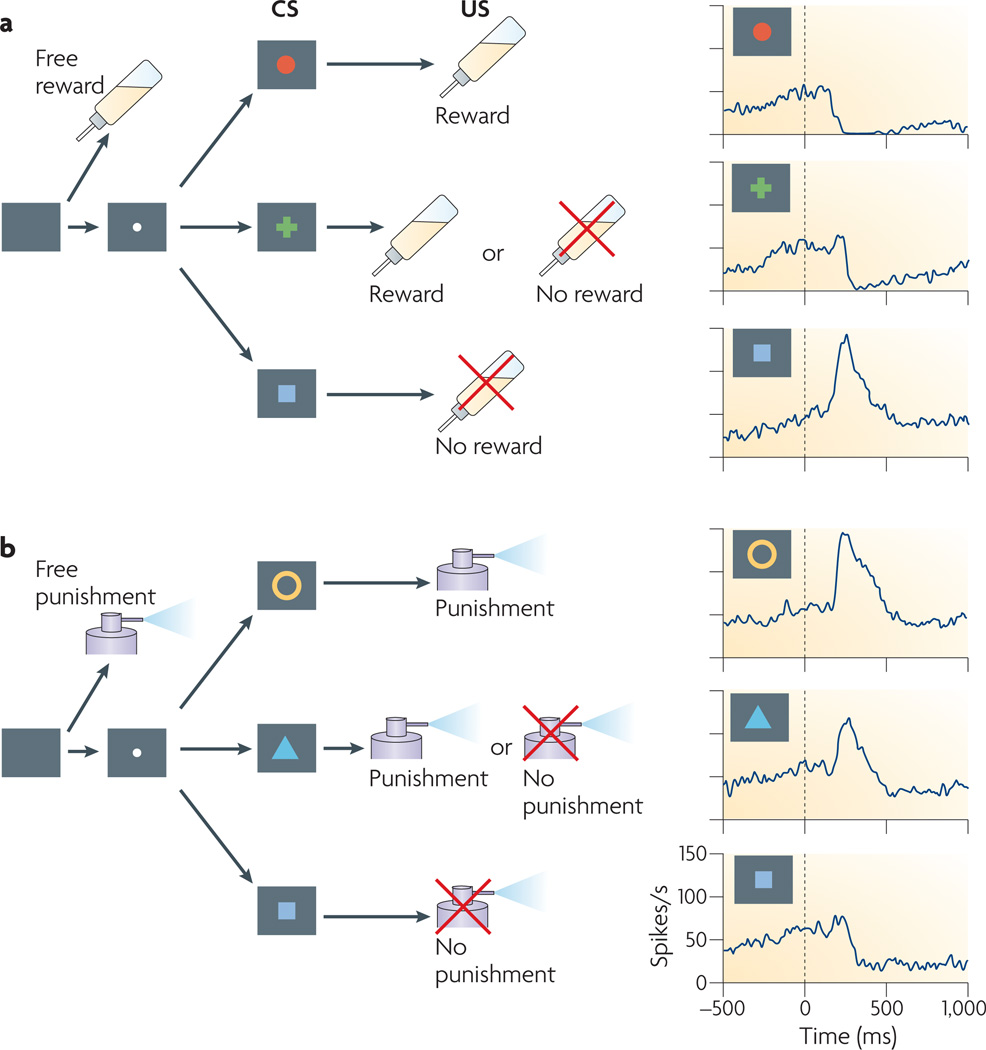
This idea was tested in an experiment using macaque monkeys53 (FIG. 5). A Pavlovian task was divided into two blocks: an ‘appetitive’ block in which juice was used as the US (FIG. 5a) and an ‘aversive’ block in which an air puff was used as the US (FIG. 5b). Three CSs (symbols, which appeared on a computer screen) indicated the probability (100%, 50% or 0%) of receiving the upcoming US. LHb neurons were strongly excited in the appetitive block by the CS that indicated a 0% probability of receiving the juice and in the aversive block by the CS that indicated a 100% probability of receiving the air puff (FIG. 5). In other words, LHb neurons were excited by the ‘worst outcome’ CS in each block. Conversely, the LHb neurons were inhibited by the CS indicating a 100% probability of receiving juice and (although only sometimes) by the CS indicating a 0% probability of receiving an air puff — that is, by the ‘best outcome’ CS in each block. Overall, the LHb neuron response was negatively correlated with the value of the outcome (US) as predicted by the CS, although the response was adjusted relative to the mean outcome value in each block.
Figure 5. The lateral habenula encodes motivational values.
A Pavlovian procedure with two distinct contexts (a and b). a | An appetitive block in which juice served as a reward. Three conditioned stimuli (CSs) were associated with the reward and indicated 100%, 50% and 0% probability of receiving the reward, respectively. b | An aversive block in which an air puff was delivered as a punishment. Three CSs were associated with the punishment and indicated 100%, 50% and 0% probability of receiving the punishment, respectively. Each trial of each block started after the presentation of a timing cue (a small central spot) on the screen. After 1 s, 1 of the 3 CSs was presented pseudo-randomly. After 1.5 s, the CS disappeared and the unconditioned stimulus (US; juice or air puff) was delivered. In addition to the cued trials, uncued trials were included in which a juice reward alone (free reward) was delivered during the appetitive block and an air puff alone (free punishment) was delivered during the aversive block. The activity of a lateral habenula neuron in response to the CSs is shown by the blue traces in the right hand column. Spike density functions are aligned by the onset of each CS (shown by the dashed black lines). Figure is modified, with permission, from Nature Neuroscience REF. 53 © (2009) Macmillan Publishers Ltd. All rights reserved.
These results indicate that the LHb responds to the negative value of a stimulus. By signalling this value — via the RMTg54 — to dopamine neurons in the substantia nigra and VTA, the habenula may contribute to the suppression of body movements that lead to an aversive outcome (FIG. 2b). Indeed, rats with habenula lesions show impairments in avoidance learning55–57 and furthermore, RMTg neurons are excited by various kinds of aversive stimuli54.
Behavioural responses to stress
Prolonged exposure to an environment in which aversive stimuli can occur at any time is stressful and can cause anxiety as well as depression58. Stress-inducing stimuli — including the repetition of aversive stimuli, physical constraint, open field exposure and social defeat — activate LHb neurons59 (FIG. 2c).
The stress-induced activation of the LHb (typically indicated by an increase in Fos-like immunoreactivity) is relatively confined to its medial part60, which receives input from the limbic system (the septum, diagonal band of Broca and medial frontal cortex)6 and from dopamine neurons in the VTA61. It is not known whether MHb neurons respond to stress by changing their activity. However, several studies suggest that in the MHb, stress induces various immune responses, such as increases in the level of the pro-inflammatory cytokine IL-18 (in response to restraint stress)62 and in the number of mast cells (in response to fighting-induced stress)63.
A typical behavioural response to stress is a general suppression of motor activity64. Repeated exposure to aversive stimuli is particularly stressful and in monkeys such aversive stimuli excite LHb neurons53 but inhibit a group of midbrain dopamine neurons65. The dopamine neurons that are inhibited by aversive stimuli tend to be inhibited strongly by LHb stimulation and are mainly located in the medial part of the SNc–VTA65. Congenitally stress-vulnerable rats that were rendered ‘helpless’ through repeated exposure to aversive stimuli have higher metabolic activity in the LHb and lower activity in the VTA66 compared with non-vulnerable, non-helpless rats. Thus, the stress-induced activation of LHb neurons in these rats would lead to a strong inhibition of dopamine neurons, which subsequently leads to the suppression of motor activity (FIG. 2c).
It is important to note that in monkeys that are performing behavioural tasks, the aversive stimulus-induced changes in LHb and dopamine neuron activity are transient and the background activity of LHb neurons and dopamine neurons is similar in appetitive and aversive blocks of trials53,65 (FIG. 5). Indeed, these monkeys do not show any signs of major depression. However, if the aversive stimuli are delivered repeatedly and for a long time, sensitization may occur in LHb neurons so that their background activity is continuously elevated and, consequently, the background activity of dopamine neurons is continuously reduced. This condition may account for the motor suppression that is associated with ‘learned helplessness’. Stress-induced activation of the LHb may also cause behavioural changes through alterations in the activity of serotonin neurons (FIG. 2c). For example, sustained uncontrollable stress can cause a lack of motivation to perform goal-directed actions (learned helplessness)64 and changes in serotonin transmission are thought to contribute to this behavioural change because stress activates serotonin neurons in the DRN and MRN67 and increases serotonin release in different brain regions68. The LHb has robust projections to the DRN and MRN7,69, which are the main source of serotonin neurons. Electrical stimulation of the LHb has been found to inhibit neurons in the DRN and MRN70 but both inhibitory71 and facilitatory effects on serotonin release have been reported72. Nevertheless, it is possible that stress-induced activation of the LHb stimulates serotonin neurons in the DRN and MRN, resulting in serotonin release.
The stress-induced elevation of serotonergic activity67 raises the possibility that serotonin neurons are excited by aversive stimuli. However, some DRN neurons encode reward values positively (that is, they are excited by reward), whereas other DRN neurons encode reward values negatively (that is, they are excited by the absence of reward)73. Unlike dopamine and LHb neuron activity (both of which represent reward prediction errors41), DRN neuron activity seems to represent the value of the current reward. Indeed, the change in activity in these neurons tended to be greatest after a trial with a reward or no reward74. Thus, it seems that DRN neurons monitor how good or bad the current condition is and this information can be useful for choosing an appropriate behaviour (for example, fight, flee or relax). The results described above suggest that DRN neurons may monitor the stressfulness of a situation.
Other functions of the habenula
Electromagnetic detection
Many animals detect changes in electric or magnetic fields and use this information to perform various kinds of behaviour, such as navigation and prey-capture75. In the lamprey this electromagnetic-induced behaviour seems to be controlled by the habenula–IPN system76. As the electric field intensity increases, the lamprey’s behaviour switches from a locomotive to a non-locomotive mode, perhaps mimicking the transition from migratory to preying states76. This presumed function of the habenula is particularly significant for elucidating the basic function of the habenula because the sea lamprey is thought to be the most primitive of the vertebrate species.
Navigation
Neurons that encode head direction have been found in several subcortical areas in the rat brain77. It has been suggested that the habenula–IPN system may provide idiothetic cues for head direction through its connection to the dorsal tegmental nucleus78. This hypothesis may be supported by the finding that some neurons in the LHb change their activity in relation to angular head velocity. Neurons that show activity that is related to running speed are also present in the LHb and the IPN79.
Maternal behaviour
Female rats with lesions of the LHb have severe disruptions of all components of maternal behaviour, including pup-retrieving, nursing, and nest-building80. The inputs from the medial and lateral preoptic area to the LHb may be crucial for its role in the regulation of maternal behaviour81. Dopamine release in the nucleus accumbens has also been shown to be crucial for maternal behaviour82 and this suggests that the LHb may contribute to maternal behaviour through its effect on dopamine neurons in the VTA, which project to the nucleus accumbens.
The habenula in psychiatric disorders
Major depression
Prolonged exposure to pain and stress in humans has been associated with major depression64. Several lines of evidence suggest that LHb neurons are hyperactive in individuals with depression. In rat models of depression (induced by stress, injections of α-methylpara-tyrosine or withdrawal from chronic amphetamine) neural activity (assessed with 2-deoxyglucose or cytochrome oxidase staining) has been found to be lower in most brain areas compared with control rats, but to be increased in the LHb83, MHb and IPN66. In accordance with these results, lesions of the habenula in rat models of depression have been found to cause a reduction in depression-like behaviour84. In human patients with depression, depleting plasma tryptophan to temporarily reduce brain serotonin synthesis was found to activate the habenula and DRN12,85. There was a strong correlation between activity in these two areas12 and in both areas activity also correlated negatively with mood ratings.
A major behavioural expression of depression is reduced motor activity83. In fact, motor suppression is used to assess depression-like behaviour in animals. It is possible that reduced neural activity in the basal ganglia contributes to reduced motor suppression in individuals with depression. Indeed, blood flow or metabolism is reduced in the basal ganglia nuclei in patients with major depression86 and this reduction may be related to reduced dopaminergic transmission87. Dopamine hypoactivity reduces motivation88, which is another hallmark of depression. Considering the inhibitory effect of the LHb on dopamine neurons, it is possible that the reduced motor activity in major depression is caused by hyperactivity of the LHb leading to hypoactivity of dopamine neurons (FIG. 2b), although this has not been investigated.
In addition, evidence suggests that major depression is mediated by serotonin neurons. A major theory of depression implicates altered serotonin levels in the brain as a cause of depression and this theory is supported by the successful treatment of depression with serotonin reuptake inhibitors89. It is possible that changes in serotonin release may be caused by an enhanced inhibitory signal from the LHb. In rat models of depression, lesioning of the habenula alleviates depression-like behaviour and eliminates the increased extracellular serotonin levels in the DRN90 and the increased serotonin turnover in the DRN84.
Depression is also associated with abnormalities in the circadian rhythm and with sleep disturbances34. These symptoms are often explained by dysfunctions of the serotonin system as serotonin is essential for normal sleep regulation34. However, based on the proposed role of the habenula in sleep that is discussed above it is possible that dysfunctions of the habenula underlie these sleep-related symptoms.
Hyperactivity of the habenula may also contribute to the symptoms of depression by modulating the neuro–immune system. Cytokines have been implicated as a contributing factor in mood disorders including depression91. In the brain, IL-18, a pro-inflammatory cytokine, is highly and uniquely expressed in the MHb62. Moreover, IL-18 levels in the MHb are elevated in response to bacterial infections and emotional stress, which cause sleep disturbances and possibly fatigue (aspects of ‘sickness behaviour’)26.
These data suggest, but do not provide direct evidence for, a link between habenula activity and major depression. They have nevertheless inspired proposals for the treatment of major depression using deep brain stimulation (DBS) to manipulate the activity of the habenula92,93. A first successful treatment of a patient with severe treatment-resistant depression using DBS of the habenula has been reported94. This result is consistent with the hypothesis that DBS suppresses the abnormally elevated activity of the habenula, as postulated for other DBS treatments (for example, DBS of the subgenual cingulate cortex for major depression95 and DBS of the subthalamic nucleus for Parkinson’s disease96).
Other psychiatric disorders
The habenula may also be involved in other psychiatric disorders, such as drug-induced psychosis and schizophrenia. Excessive intake of amphetamine or cocaine is associated with changes in central dopamine transmission and can cause paranoia, mania, delusions, hallucinations and thought disorder, all of which resemble symptoms of paranoid schizophrenia97. Interestingly, both amphetamine and cocaine, when continuously administered in rats, cause a strong and highly localized degeneration of the fasciculus retroflexus, the main efferent path from the habenula97. Chronic administration of cocaine also leads to long-term activity changes in GABA neurons in the VTA or the RMTg, which are the main targets of the LHb98,99. These findings raise the possibility that human drug-induced psychosis may be caused partly by damage to the habenula and that schizophrenia may be associated with habenula dysfunction97.
This hypothesis is supported by the finding that calcification of the epithalamus (including the habenula) — which presumably impairs the function of the habenula — is found much more frequently in patients with chronic schizophrenia than in control subjects100. Moreover, an fMRI study has shown altered activation of the habenula in patients with schizophrenia.101 Following an error in a difficult matching-to-sample task, the habenula was activated in control subjects, but not in patients with schizophrenia. Based on these findings it is possible to speculate that dopamine neurons in the VTA and SNc of patients with schizophrenia may not receive appropriate inhibitory signals from the habenula and that this may underlie the patients’ observed deficit in learning from the errors101.
A learning deficit such as this may coexist with (and possibly underlie) the impairments in working memory, attention and executive functions that have been observed in patients with schizophrenia102. Notably, information processing in cortico-hippocampal areas is influenced by various neuromodulators including dopamine, serotonin, noradrenaline and acetyl choline103, and the release of all of these neuromodulators is under the control of the habenula11. Indeed, schizophrenia-related cognitive impairments, including those that relate to spatial memory and attention, can be simulated in rats by lesioning the habenula104. Experiments have also suggested that these cognitive deficits are partly mediated by enhanced dopamine activity in the nucleus accumbens and that this enhanced activity could be caused by removal of the LHb-mediated inhibition of dopamine neurons105.
The habenula may also be involved in nicotine addiction. Nicotine withdrawal causes unpleasant physical and psychological symptoms106. Although nicotinic acetylcholine receptors are widely distributed in the brain, the subtypes that mediate nicotine withdrawal responses are highly concentrated in the MHb and IPN107. Nicotinic receptor activation in the habenula may be important for memory because chronic blockade of nicotinic acetylcholine receptors causes an increase in errors during spatial memory tasks108. In addition, blocking nicotinic receptors in the MHb or IPN in animals that have been chronically treated with nicotine enhances withdrawal responses107. Interestingly, a high dose of nicotine causes massive degeneration almost exclusively in the MHb and its output tract, the fasciculus retroflexus109. Thus, symptoms associated with nicotine withdrawal may be caused by dysfunctions of the MHb and its output.
However, the extent to which the habenula may contribute to bringing about drug-induced psychosis and schizophrenia is unclear as the evidence to date has often been based on a small number of clinical cases. More systematic studies are necessary to address this question.
Towards a unified theory of habenula function
The fact that the habenula is present in virtually all vertebrate species, including those that seemingly lack cognitive functions1, suggests that it has an important role in survival through a relatively simple mechanism that is shared by all vertebrates. As described above, the habenula seems to be involved in various behaviours. Nonetheless, it is probably useful to seek a core function or mechanism for the habenula that is common to these behaviours. One prominent feature of the habenula (the LHb and the MHb) is that it is involved in the processing of aversive information, such as pain, stress and failure. Animals may fight or try to escape from aversive events, but in many cases they simply stop moving (‘freeze’) to evade the aversive events110. I propose that the primary function of the habenula is to suppress motor activity under such adverse conditions.
This hypothesis consists of two propositions: first, the role of the habenula is to suppress motor activity; and second, the habenula is activated under adverse conditions. Evidence for the first proposition comes from the finding that the LHb exerts a powerful inhibition on dopamine neurons. As dopamine neurons are thought to be a key modulator of body movement and motivation, their LHb-induced inhibition could contribute to the suppression of body movements and to reduced motivation. Considering the close association of the habenula with the pineal gland, the retinal input to the habenula and the circadian variations in habenula activity, it is tempting to speculate that from an early stage of vertebrate evolution the habenula has played a part in controlling general motor activity based on circadian rhythmicity111. How this motor control mechanism was used later in evolution was probably dependent on the type of information that habenula neurons came to receive.
The second proposition — that the habenula becomes active under adverse conditions — needs to be discussed in more detail because from this proposition arise seemingly diverse functions of the habenula. LHb neurons are excited by aversive stimuli, including stimuli that were initially neutral but that have been conditioned to predict aversive events. In the MHb, aversive stimuli or stress induces enzymatic and immunological responses. It is therefore possible that the LHb and MHb both respond to aversive stimuli, but in different ways; that is, aversive signals would be mediated by neural processes and conveyed to the LHb in addition to being mediated by immunological and enzymatic processes and conveyed to the MHb. However, it is important to note that evidence in support of this hypothesis is still sparse, in particular for the purported role of the MHb, and remains a topic for further study.
In response to an aversive event an animal may act to remove itself from the source of that event (for example, escape), and this requires quick motor responses. Conversely, cues that predict aversive outcomes often produce marked inhibitions of motor behaviour110. The motor suppression that is associated with aversion prediction may be mediated by the LHb-dopamine circuit — because the majority of LHb neurons are excited by aversive events — leading to an inhibition of dopamine neurons that consequently leads to a general suppression of body movements. In addition, the aversion-predicting activation of the LHb (and possibly the MHb) activates serotonin neurons in the raphe nuclei and therefore influences neural processing in a large part of the brain, although the specific behavioural outcomes of LHb-mediated serotonin modulations are still unclear.
Under repeated or continuous stress, animals and humans may show depression-like behaviour, as proposed by the ‘learned helplessness theory’64 and the ‘rank theory’112 of depression. In both schemes, depression is considered to be a form of behavioural adaptation to adverse conditions. More importantly, in the state of depression sensitization of the LHb-dopamine and LHb-serotonin circuits seems to occur (FIG. 2c). Indeed, in humans with depression and in animal models of depression the LHb becomes hyperactive12,83. This may cause the general motor suppression (through inhibition of dopamine neurons) as well as the mood changes (through changes in serotonin transmission) that are associated with depression.
Thus far, I have proposed that the habenula evolved as a general motor controller that was originally devoted to circadian control of behaviour. According to my hypothesis, at some point in evolution the brain areas that encode aversive signals acquired connections to the habenula. The habenula then became a suppressor of motor activities in response to, or in anticipation of, aversive events. Why did these particular connections occur and why were these connections maintained throughout evolution? A conceivable explanation is that the habenula and its efferent circuit enabled the aversive signal-carrying areas of the brain to gain an evolutionary value. In this scheme, the habenula circuit could be more actively exploited; it could be used for the learning of voluntary behaviour.
Learning of voluntary behaviour starts when an animal acts on the external world spontaneously. If the outcome of the action is favourable, the action is likely to be repeated, but if the outcome is unfavourable, the action is likely to be suppressed113. In this case the aversive event occurs as a result of the animal’s own action (which can therefore be considered a ‘failure’). As the LHb circuit was already used for stress-induced motor suppression, it was easily implemented for failure-induced motor suppression. This means that the habenula circuit must have improved the quality of information that it carries in at least two ways. First, the value of the outcome (rewarding or aversive) must be judged with respect to the expected outcome value (better or worse than expected) for the animal to establish which action results in the best outcome114. That is, the neurons should encode a reward prediction error signal, a feature that is represented in LHb and dopamine neurons, but not in serotonin neurons41,73. Second, the motor suppression should be specific to the action that caused the aversive outcome; the action that caused a favourable outcome (reward) should not be suppressed. This algorithm may be achieved by specific interactions between dopamine neurons (which carry value signals) and cortical and striatal neurons (which carry action signals)115 (FIG. 3).
This hypothesis illustrates how a simple motor controller may have evolved to become part of an important learning mechanism. As more brain areas (which may have evolved after the habenula) formed connections to the habenula, the functions of the habenula seem to have become more diverse. However, the habenula seems to have retained its core mechanism, namely to suppress body movements. This is probably what has happened to many other brain areas: a core mechanism remains, but the functions that the brain area performs vary depending on the connections that have been added to it over time.
Acknowledgements
I would like to thank my colleagues Masayuki Matsumoto, Simon Hong and Ethan Bromberg-Martin, who have discovered various properties of the primate habenula and have provided me with excellent ideas on the function of the habenula. I also thank Ilya Monosov for helping me improve the manuscript. This research was supported by the Intramural Research Program at the National Institutes of Health, National Eye Institute.
Glossary
- Sleep-associated atonia
Depression of skeletal muscle tone that occurs during rapid eye movement sleep. The brain remains active with fast eye movements, but both sensory input and motor output are suppressed.
- Suprachiasmatic nucleus
The master circadian pacemaker in the mammalian brain. Its circadian rhythm is generated by a gene expression cycle in individual neurons in this nucleus, but it also receives light intensity signals directly from the retina.
- Reinforcement learning
A sub-area of machine learning concerned with how an agent learns from the consequences of its actions, rather than from being explicitly taught. It is essentially trial-and-error learning. The agent seeks to learn to select actions that maximize the accumulated reward over time.
- Avoidance learning
A type of learning in which a certain behaviour results in the cessation of an aversive stimulus — for example, a rat is placed in a box where a warning signal, such as a tone, is followed by an electric shock. As the sequence is repeated, the rat learns to jump over to the adjacent box before the shock is delivered.
Footnotes
Competing interests statement
The author declares no competing financial interests.
FURTHER INFORMATION
Author’s homepage: http://www.nei.nih.gov/intramural/lsr\hikosaka\hikosaka.asp
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Concha ML, Wilson SW. Asymmetry in the epithalamus of vertebrates. J. Anat. 2001;199:63–84. doi: 10.1046/j.1469-7580.2001.19910063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aizawa H, et al. Laterotopic representation of left-right information onto the dorso-ventral axis of a zebrafish midbrain target nucleus. Curr. Biol. 2005;15:238–243. doi: 10.1016/j.cub.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dadda M, Domenichini A, Piffer L, Argenton F, Bisazza A. Early differences in epithalamic left–right asymmetry influence lateralization and personality of adult zebrafish. Behav. Brain Res. 2010;206:208–215. doi: 10.1016/j.bbr.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Guglielmotti V, Cristino L. The interplay between the pineal complex and the habenular nuclei in lower vertebrates in the context of the evolution of cerebral asymmetry. Brain Res. Bull. 2006;69:475–488. doi: 10.1016/j.brainresbull.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Ronnekleiv OK, Moller M. Brain-pineal nervous connections in the rat: an ultrastructure study following habenular lesion. Exp. Brain Res. 1979;37:551–562. doi: 10.1007/BF00236823. [DOI] [PubMed] [Google Scholar]
- 6.Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J. Comp. Neurol. 1977;173:123–146. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- 7. Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J. Comp. Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. Together with a companion paper (reference 6) this comprehensive anatomical study on the rat habenula provides detailed axonal projection patterns to and from the lateral and medial habenula.
- 8.Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J. Comp. Neurol. 2009;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groenewegen HJ, Ahlenius S, Haber SN, Kowall NW, Nauta WJ. Cytoarchitecture, fiber connections, and some histochemical aspects of the interpeduncular nucleus in the rat. J. Comp. Neurol. 1986;249:65–102. doi: 10.1002/cne.902490107. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci. Biobehav. Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- 11.Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci. Biobehav. Rev. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 12. Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage. 1999;10:163–172. doi: 10.1006/nimg.1999.0455. During transient depressive relapses that were induced by tryptophan depletion in volunteer patients, neural activity assessed by positron emission tomography increased in the habenula and the dorsal raphe. A linear correlation between habenula and raphe activity was observed in patients who experienced strong depressive mood.
- 13. Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J. Neurosci. 2003;23:4308–4314. doi: 10.1523/JNEUROSCI.23-10-04308.2003. Functional MRI carried out on human subjects while they performed a dynamically adaptive motion prediction task showed that negative feedback activated the rostral cingulate motor area, inferior anterior insula and habenula.
- 14.Geisler S, Trimble M. The lateral habenula: no longer neglected. CNS Spectr. 2008;13:484–489. doi: 10.1017/s1092852900016710. [DOI] [PubMed] [Google Scholar]
- 15. Haun F, Eckenrode TC, Murray M. Habenula and thalamus cell transplants restore normal sleep behaviors disrupted by denervation of the interpeduncular nucleus. J. Neurosci. 1992;12:3282–3290. doi: 10.1523/JNEUROSCI.12-08-03282.1992. Lesions of the fasciculus retroflexus (habenula output) in rats markedly decreased the muscle atonia component of REM sleep and reduced the duration of sleep episodes. Transplants of fetal habenula cells in the lesioned rats restored the normal frequency of REM atonia.
- 16.Valjakka A, et al. The fasciculus retroflexus controls the integrity of REM sleep by supporting the generation of hippocampal theta rhythm and rapid eye movements in rats. Brain Res. Bull. 1998;47:171–184. doi: 10.1016/s0361-9230(98)00006-9. [DOI] [PubMed] [Google Scholar]
- 17.Eckenrode TC, Murray M, Haun F. Habenula and thalamus cell transplants mediate different specific patterns of innervation in the interpeduncular nucleus. J. Neurosci. 1992;12:3272–3281. doi: 10.1523/JNEUROSCI.12-08-03272.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur. J. Neurosci. 2007;25:3195–3216. doi: 10.1111/j.1460-9568.2007.05581.x. [DOI] [PubMed] [Google Scholar]
- 19. Zhao H, Rusak B. Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience. 2005;132:519–528. doi: 10.1016/j.neuroscience.2005.01.012. Cells in the lateral and medial habenula were activated or suppressed by retinal illumination and showed higher activity during the day than during the night. Cells in the lateral habenula, but not the medial habenula, maintained the circadian rhythmicity even in slice preparation.
- 20.Herkenham M. Anesthetics and the habenulo-interpeduncular system: selective sparing of metabolic activity. Brain Res. 1981;210:461–466. doi: 10.1016/0006-8993(81)90927-6. [DOI] [PubMed] [Google Scholar]
- 21.van Nieuwenhuijzen PS, McGregor IS, Hunt GE. The distribution of γ-hydroxybutyrate-induced Fos expression in rat brain: comparison with baclofen. Neuroscience. 2009;158:441–445. doi: 10.1016/j.neuroscience.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Abulafia R, Zalkind V, Devor M. Cerebral activity during the anesthesia-like state induced by mesopontine microinjection of pentobarbital. J. Neurosci. 2009;29:7053–7064. doi: 10.1523/JNEUROSCI.1357-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allada R. An emerging link between general anesthesia and sleep. Proc. Natl Acad. Sci. USA. 2008;105:2257–2258. doi: 10.1073/pnas.0711532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilduff TS, Sharp FR, Heller HC. [14C]2-deoxyglucose uptake in ground squirrel brain during hibernation. J. Neurosci. 1982;2:143–157. doi: 10.1523/JNEUROSCI.02-02-00143.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu EZ, Hallenbeck JM, Cai D, McCarron RM. Elevated arylalkylamine-N.-acetyltransferase (AA-NAT) gene expression in medial habenular and suprachiasmatic nuclei of hibernating ground squirrels. Brain Res. Mol. Brain Res. 2002;102:9–17. doi: 10.1016/s0169-328x(02)00138-9. [DOI] [PubMed] [Google Scholar]
- 26.Sugama S, Conti B. Interleukin-18 and stress. Brain Res. Rev. 2008;58:85–95. doi: 10.1016/j.brainresrev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Kubota T, Fang J, Brown RA, Krueger JM. Interleukin-18 promotes sleep in rabbits and rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R828–R838. doi: 10.1152/ajpregu.2001.281.3.R828. [DOI] [PubMed] [Google Scholar]
- 28.Pavel S, Eisner C. A GABAergic habenulo–raphe pathway mediates both serotoninergic and hypnogenic effects of vasotocin in cats. Brain Res. Bull. 1984;13:623–627. doi: 10.1016/0361-9230(84)90193-x. [DOI] [PubMed] [Google Scholar]
- 29.Vetrivelan R, Fuller PM, Tong Q, Lu J. Medullary circuitry regulating rapid eye movement sleep and motor atonia. J. Neurosci. 2009;29:9361–9369. doi: 10.1523/JNEUROSCI.0737-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holstege G. The mesopontine rostromedial tegmental nucleus and the emotional motor system: role in basic survival behavior. J. Comp. Neurol. 2009;513:559–565. doi: 10.1002/cne.21990. [DOI] [PubMed] [Google Scholar]
- 31.Brainard GC, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J. Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semm P, Schneider T, Vollrath L. Morphological and electrophysiological evidence for habenular influence on the guinea-pig pineal gland. J. Neural Transm. 1981;50:247–266. doi: 10.1007/BF01249146. [DOI] [PubMed] [Google Scholar]
- 33.Hattar S, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nature Rev. Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel JM. Sleep viewed as a state of adaptive inactivity. Nature Rev. Neurosci. 2009;10:747–753. doi: 10.1038/nrn2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee EH, Huang SL. Role of lateral habenula in the regulation of exploratory behavior and its relationship to stress in rats. Behav. Brain Res. 1988;30:265–271. doi: 10.1016/0166-4328(88)90169-6. [DOI] [PubMed] [Google Scholar]
- 37.Lecourtier L, Kelly PH. Bilateral lesions of the habenula induce attentional disturbances in rats. Neuropsychopharmacology. 2005;30:484–496. doi: 10.1038/sj.npp.1300595. [DOI] [PubMed] [Google Scholar]
- 38.Selby G. In: Handbook of Clinical Neurology. Vinken PJ, Bruyn GW, editors. Vol. 6. Amsterdam: North Holland Publishing Company; 1968. pp. 173–211. [Google Scholar]
- 39.Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J. Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J. Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 42.Nishikawa T, Fage D, Scatton B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373:324–336. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- 43.Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Hikosaka O. Basal ganglia mechanisms of reward-oriented eye movement. Ann. N. Y. Acad. Sci. 2007;1104:229–249. doi: 10.1196/annals.1390.012. [DOI] [PubMed] [Google Scholar]
- 45. Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron. 2008;60:720–729. doi: 10.1016/j.neuron.2008.09.035. In the monkey, during the performance of a saccade task with positionally biased reward outcomes, neurons that project to the lateral habenula were found mainly in the dorsal and ventral borders of the internal segment of the globus pallidus. A majority of them were excited by the no-reward-predicting target and inhibited by the reward-predicting target, similarly to lateral habenula neurons.
- 46.Rajakumar N, Elisevich K, Flumerfelt BA. Compartmental origin of the striato-entopeduncular projection in the rat. J. Comp. Neurol. 1993;331:286–296. doi: 10.1002/cne.903310210. [DOI] [PubMed] [Google Scholar]
- 47.Kim U. Topographic commissural and descending projections of the habenula in the rat. J. Comp. Neurol. 2009;513:173–187. doi: 10.1002/cne.21951. [DOI] [PubMed] [Google Scholar]
- 48.Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J. Comp. Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- 49.Dafny N, Qiao JT. Habenular neuron responses to noxious input are modified by dorsal raphe stimulation. Neurol. Res. 1990;12:117–121. doi: 10.1080/01616412.1990.11739929. [DOI] [PubMed] [Google Scholar]
- 50.Matsumoto N, Yahata F, Kawarada K, Kamata K, Suzuki TA. Tooth pulp stimulation induces c-fos expression in the lateral habenular nucleus of the cat. Neuroreport. 1994;5:2397–2400. doi: 10.1097/00001756-199411000-00046. [DOI] [PubMed] [Google Scholar]
- 51.Mahieux G, Benabid AL. Naloxone-reversible analgesia induced by electrical stimulation of the habenula in the rat. Brain Res. 1987;406:118–129. doi: 10.1016/0006-8993(87)90776-1. [DOI] [PubMed] [Google Scholar]
- 52.Cohen SR, Melzack R. The habenula and pain: repeated electrical stimulation produces prolonged analgesia but lesions have no effect on formalin pain or morphine analgesia. Behav. Brain Res. 1993;54:171–178. doi: 10.1016/0166-4328(93)90076-3. [DOI] [PubMed] [Google Scholar]
- 53.Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nature Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. GABAergic neurons in the rostromedial tegmental nucleus, which receive inputs from the lateral habenula and project to midbrain dopamine neurons, showed phasic activation and/or Fos induction after aversive stimuli and inhibitions after rewards or reward-predicting stimuli. Lesions of this nucleus markedly reduced passive fear behaviours.
- 55.Nielson HC, McIver AH. Cold stress and habenular lesion effects on rat behaviors. J. Appl. Physiol. 1966;21:655–660. doi: 10.1152/jappl.1966.21.2.655. [DOI] [PubMed] [Google Scholar]
- 56.Rausch LJ, Long CJ. Habenular Lesions and avoidance learning deficits in allbins rats. Physiol. Behav. 1974;2:352–356. [Google Scholar]
- 57.Thornton EW, Bradbury GE, Evans JA, Wickens A. A failure to support cross-sensitization between effects of apomorphine and lesions of the habenula nucleus. Pharmacol. Biochem. Behav. 1989;32:77–81. doi: 10.1016/0091-3057(89)90213-x. [DOI] [PubMed] [Google Scholar]
- 58.Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci. Biobehav. Rev. 1981;5:247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- 59.Shumake J, Gonzalez-Lima F. Brain systems underlying susceptibility to helplessness and depression. Behav. Cogn. Neurosci. Rev. 2003;2:198–221. doi: 10.1177/1534582303259057. [DOI] [PubMed] [Google Scholar]
- 60.Wirtshafter D, Asin KE, Pitzer MR. Dopamine agonists and stress produce different patterns of Fos-like immunoreactivity in the lateral habenula. Brain Res. 1994;633:21–26. doi: 10.1016/0006-8993(94)91517-2. [DOI] [PubMed] [Google Scholar]
- 61.Phillipson OT, Pycock CJ. Dopamine neurones of the ventral tegmentum project to both medial and lateral habenula. Some implications for habenular function. Exp. Brain Res. 1982;45:89–94. doi: 10.1007/BF00235766. [DOI] [PubMed] [Google Scholar]
- 62.Sugama S, et al. Neurons of the superior nucleus of the medial habenula and ependymal cells express IL-18 in rat CNS. Brain Res. 2002;958:1–9. doi: 10.1016/s0006-8993(02)03363-2. [DOI] [PubMed] [Google Scholar]
- 63.Cirulli F, Pistillo L, de Acetis L, Alleva E, Aloe L. Increased number of mast cells in the central nervous system of adult male mice following chronic subordination stress. Brain Behav. Immun. 1998;12:123–133. doi: 10.1006/brbi.1998.0505. [DOI] [PubMed] [Google Scholar]
- 64.Seligman ME. Learned helplessness. Annu. Rev. Med. 1972;23:407–412. doi: 10.1146/annurev.me.23.020172.002203. [DOI] [PubMed] [Google Scholar]
- 65.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003;963:274–281. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- 67.Takase LF, et al. Inescapable shock activates serotonergic neurons in all raphe nuclei of rat. Behav. Brain Res. 2004;153:233–239. doi: 10.1016/j.bbr.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 68.Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 69.Aghajanian GK, Wang RY. Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res. 1977;122:229–242. doi: 10.1016/0006-8993(77)90291-8. [DOI] [PubMed] [Google Scholar]
- 70.Stern WC, Johnson A, Bronzino JD, Morgane PJ. Effects of electrical stimulation of the lateral habenula on single-unit activity of raphe neurons. Exp. Neurol. 1979;65:326–342. doi: 10.1016/0014-4886(79)90102-x. [DOI] [PubMed] [Google Scholar]
- 71.Nishikawa T, Scatton B. Inhibitory influence of GABA on central serotonergic transmission. Involvement of the habenulo-raphe pathways in the GABAergic inhibition of ascending cerebral serotonergic neurons. Brain Res. 1985;331:81–90. doi: 10.1016/0006-8993(85)90717-6. [DOI] [PubMed] [Google Scholar]
- 72.Kalen P, Lindvall O, Bjorklund A. Electrical stimulation of the lateral habenula increases hippocampal noradrenaline release as monitored by in vivo microdialysis. Exp. Brain Res. 1989;76:239–245. doi: 10.1007/BF00253642. [DOI] [PubMed] [Google Scholar]
- 73.Nakamura K, Matsumoto M, Hikosaka O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J. Neurosci. 2008;28:5331–5343. doi: 10.1523/JNEUROSCI.0021-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bromberg-Martin ES, Hikosaka O, Nakamura K. Coding of task reward value in the dorsal raphe nucleus. J. Neurosci. 2010;30:6262–6272. doi: 10.1523/JNEUROSCI.0015-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnsen S, Lohmann KJ. The physics and neurobiology of magnetoreception. Nature Rev. Neurosci. 2005;6:703–712. doi: 10.1038/nrn1745. [DOI] [PubMed] [Google Scholar]
- 76.Chung-Davidson YW, Yun SS, Teeter J, Li W. Brain pathways and behavioral responses to weak electric fields in parasitic sea lampreys (Petromyzon marinus) Behav. Neurosci. 2004;118:611–619. doi: 10.1037/0735-7044.118.3.611. [DOI] [PubMed] [Google Scholar]
- 77.Taube JS. The head direction signal: origins and sensory-motor integration. Annu. Rev. Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- 78.Clark BJ, Sarma A, Taube JS. Head direction cell instability in the anterior dorsal thalamus after lesions of the interpeduncular nucleus. J. Neurosci. 2009;29:493–507. doi: 10.1523/JNEUROSCI.2811-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharp PE, Turner-Williams S, Tuttle S. Movement-related correlates of single cell activity in the interpeduncular nucleus and habenula of the rat during a pellet-chasing task. Behav. Brain Res. 2006;166:55–70. doi: 10.1016/j.bbr.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 80.Matthews-Felton T, Corodimas KP, Rosenblatt JS, Morrell JI. Lateral habenula neurons are necessary for the hormonal onset of maternal behavior and for the display of postpartum estrus in naturally parturient female rats. Behav. Neurosci. 1995;109:1172–1188. doi: 10.1037//0735-7044.109.6.1172. [DOI] [PubMed] [Google Scholar]
- 81.Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82:267–281. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- 82.Champagne FA, et al. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J. Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Caldecott-Hazard S, Mazziotta J, Phelps M. Cerebral correlates of depressed behavior in rats, visualized using 14C-2-deoxyglucose autoradiography. J. Neurosci. 1988;8:1951–1961. doi: 10.1523/JNEUROSCI.08-06-01951.1988. Regional glucose metabolism was measured using 2-deoxyglucose in three rat models of depressed behaviour (induced by injections of α-methyl-paratyrosine, withdrawal from chronic amphetamine, or stress). Glucose metabolism was elevated in the lateral habenula in each of the three models.
- 84.Yang LM, Hu B, Xia YH, Zhang BL, Zhao H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav. Brain Res. 2008;188:84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 85.Roiser JP, et al. The effects of tryptophan depletion on neural responses to emotional words in remitted depression. Biol. Psychiatry. 2009;66:441–450. doi: 10.1016/j.biopsych.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Soares JC, Mann JJ. The functional neuroanatomy of mood disorders. J. Psychiatr. Res. 1997;31:393–432. doi: 10.1016/s0022-3956(97)00016-2. [DOI] [PubMed] [Google Scholar]
- 87.Kapur S, Mann JJ. Role of the dopaminergic system in depression. Biol. Psychiatry. 1992;32:1–17. doi: 10.1016/0006-3223(92)90137-o. [DOI] [PubMed] [Google Scholar]
- 88.Wise RA. Dopamine, learning and motivation. Nature Rev. Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 89.Middlemiss DN, Price GW, Watson JM. Serotonergic targets in depression. Curr. Opin. Pharmacol. 2002;2:18–22. doi: 10.1016/s1471-4892(01)00116-3. [DOI] [PubMed] [Google Scholar]
- 90.Amat J, et al. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917:118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- 91.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sartorius A, Henn FA. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med. Hypotheses. 2007;69:1305–1308. doi: 10.1016/j.mehy.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 93.Hauptman JS, DeSalles AA, Espinoza R, Sedrak M, Ishida W. Potential surgical targets for deep brain stimulation in treatment-resistant depression. Neurosurg. Focus. 2008;25:e3. doi: 10.3171/FOC/2008/25/7/E3. [DOI] [PubMed] [Google Scholar]
- 94.Sartorius A, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol. Psychiatry. 2009;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 95.Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 96.Benabid AL. Deep brain stimulation for Parkinson’s disease. Curr. Opin. Neurobiol. 2003;13:696–706. doi: 10.1016/j.conb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 97.Ellison G. Stimulant-induced psychosis, the dopamine theory of schizophrenia, and the habenula. Brain Res. Brain Res. Rev. 1994;19:223–239. doi: 10.1016/0165-0173(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 98.Perrotti LI, et al. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur. J. Neurosci. 2005;21:2817–2824. doi: 10.1111/j.1460-9568.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- 99.Geisler S, et al. Prominent activation of brainstem and pallidal afferents of the ventral tegmental area by cocaine. Neuropsychopharmacology. 2008;33:2688–2700. doi: 10.1038/sj.npp.1301650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sandyk R. Pineal and habenula calcification in schizophrenia. Int. J. Neurosci. 1992;67:19–30. doi: 10.3109/00207459208994773. [DOI] [PubMed] [Google Scholar]
- 101.Shepard PD, Holcomb HH, Gold JM. Schizophrenia in translation: the presence of absence: habenular regulation of dopamine neurons and the encoding of negative outcomes. Schizophr. Bull. 2006;32:417–421. doi: 10.1093/schbul/sbj083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gold JM, Weinberger DR. Cognitive deficits and the neurobiology of schizophrenia. Curr. Opin. Neurobiol. 1995;5:225–230. doi: 10.1016/0959-4388(95)80030-1. [DOI] [PubMed] [Google Scholar]
- 103.Joyce JN. The dopamine hypothesis of schizophrenia: limbic interactions with serotonin and norepinephrine. Psychopharmacology (Berl.) 1993;112:S16–S34. doi: 10.1007/BF02245004. [DOI] [PubMed] [Google Scholar]
- 104.Lecourtier L, Neijt HC, Kelly PH. Habenula lesions cause impaired cognitive performance in rats: implications for schizophrenia. Eur. J. Neurosci. 2004;19:2551–2560. doi: 10.1111/j.0953-816X.2004.03356.x. [DOI] [PubMed] [Google Scholar]
- 105.Lecourtier L, et al. Habenula lesions alter synaptic plasticity within the fimbria-accumbens pathway in the rat. Neuroscience. 2006;141:1025–1032. doi: 10.1016/j.neuroscience.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 106.De Biasi M, Salas R. Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal. Exp. Biol. Med. (Maywood) 2008;233:917–929. doi: 10.3181/0712-MR-355. [DOI] [PubMed] [Google Scholar]
- 107.Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J. Neurosci. 2009;29:3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sanders D, et al. Nicotinic receptors in the habenula: importance for memory. Neuroscience. 2010;166:386–390. doi: 10.1016/j.neuroscience.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 109.Carlson J, Noguchi K, Ellison G. Nicotine produces selective degeneration in the medial habenula and fasciculus retroflexus. Brain Res. 2001;906:127–134. doi: 10.1016/s0006-8993(01)02570-7. [DOI] [PubMed] [Google Scholar]
- 110.Bracha HS. Freeze, flight, fight, fright, faint: adaptationist perspectives on the acute stress response spectrum. CNS Spectr. 2004;9:679–685. doi: 10.1017/s1092852900001954. [DOI] [PubMed] [Google Scholar]
- 111.Tavakoli-Nezhad M, Schwartz WJ. Hamsters running on time: is the lateral habenula a part of the clock? Chronobiol. Int. 2006;23:217–224. doi: 10.1080/07420520500521947. [DOI] [PubMed] [Google Scholar]
- 112.Price J, Sloman L, Gardner R, Jr, Gilbert P, Rohde P. The social competition hypothesis of depression. Br. J. Psychiatry. 1994;164:309–315. doi: 10.1192/bjp.164.3.309. [DOI] [PubMed] [Google Scholar]
- 113.Thorndike EL. Animal Intelligence: Experimental Studies. New York: Macmillan; 1911. [Google Scholar]
- 114.Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu. Rev. Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- 115.Wickens JR, Horvitz JC, Costa RM, Killcross S. Dopaminergic mechanisms in actions and habits. J. Neurosci. 2007;27:8181–8183. doi: 10.1523/JNEUROSCI.1671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]