Abstract
Patients with severe COPD often exhale along the same flow-volume curve during quite breathing as during forced expiratory vital capacity manoeuvre, and this has been taken as indicating expiratory flow limitation at rest (EFLT). Therefore, EFLT, namely, attainment of maximal expiratory flow during tidal expiration, occurs when an increase in transpulmonary pressure causes no increase in expiratory flow. EFLT leads to small airway injury and promotes dynamic pulmonary hyperinflation with concurrent dyspnoea and exercise limitation. In fact, EFLT occurs commonly in COPD patients (mainly in GOLD III and IV stage) in whom the latter symptoms are common. The existing up-to-date physiological methods for assessing expiratory flow limitation (EFLT) are reviewed in the present work. Among the currently available techniques, the negative expiratory pressure (NEP) has been validated in a wide variety of settings and disorders. Consequently, it should be regarded as a simple, non invasive, most practical, and accurate new technique.
1. Introduction
Some experts use the term chronic airflow limitation as a synonym for chronic obstructive pulmonary disease (COPD) to indicate the reduction in maximum expiratory flow that occurs in this disease (and indeed in other pulmonary diseases). Patients with severe COPD often exhale along the same flow-volume curve during quite breathing as during forced expiratory vital capacity manoeuvre, and this has been taken as indicating flow limitation at rest (EFLT). Consequently, the term tidal expiratory flow limitation (EFLT) is used to indicate that maximal expiratory flow is achieved during tidal breathing at rest or during exercise. This is characteristic of intrathoracic flow obstruction. The former term does not imply that EFLT actually occurs during tidal breathing [1]. The location of expiratory flow limitation is considered to be in the central airways (4th–7th generation) and move to the periphery during forced expiratory manoeuvres. It is located beyond the 7th (i.e., from the 8th onwards) generation during tidal breathing [2–4].
Tidal expiratory flow limitation (EFLT) [5–8] plays a central role according to a recent hypothesis [5] on the transition from small airways disease (SAD) to overt COPD in smokers. EFLT implies inhomogeneity of ventilation distribution with concurrent impairment of gas exchange and unevenly distributed stress and strain within the lung, which is amplified by tissue interdependence [6, 7] and may lead to small airway injury [5–8]. Initially, the latter is histologically characterized by denuded epithelium, rupture of alveolar-airway attachments, and increased number of polymorphonuclear leucocytes [5–7]. Studies in which heliox (80% He/20% O2) was administered in COPD patients provided also corroborative evidence that EFLT was located in the peripheral airways [2–4]. EFLT promotes dynamic pulmonary hyperinflation and PEEPi with concurrent dyspnoea and exercise limitation [8]. In fact, EFLT occurs commonly in GOLD III and IV stage patients causing dynamic hyperinflation and severe dyspnoea [9]. It should be noted that the important role of expiratory flow limitation in COPD patients has been studied in a variety of clinical settings (during mechanical ventilation and exercise, correlation with dyspnoea, orthopnoea, and the other lung function indexes, before and after bronchodilatation, various postures).
2. Clinical Significance of EFL
The important role of EFLT in chronic dyspnoea and exercise impairment for a surprisingly wide range of clinical circumstances was enlightened by the techniques of detecting it, but mainly by the use of negative expiratory pressure (NEP) technique. EFLT measured with the NEP technique is a much better predictor of chronic dyspnoea than FEV1, and FEV1 is not a specific predictor of EFLT in COPD patients. These findings suggest that EFLT measured by the NEP technique may be more useful in the evaluation of dyspnoea in COPD patients than spirometric measurements [10].
The improvement of inspiratory capacity (IC) after bronchodilator administration [11], which is mainly limited to patients with EFL at rest and therefore usually exhibits a reduction of baseline IC, entails reduction in dyspnoea both at rest and during light exercise [12]. The fact that after bronchodilator administration there is a significant reduction of dynamic hyperinflation (DH) only in patients with EFL at rest further supports the usefulness of stratifying COPD patients in subgroups with and without EFL in order to predict an improvement in DH [11]. COPD patients with EFL may experience less breathlessness after a bronchodilator, at least during light exercise, than those without EFL. This beneficial effect, which is closely related to an increase in IC at rest, occurs even in the absence of a significant improvement of FEV1 [12]. Though, in the past, bronchodilator testing focused on changes of FEV1, the scrutiny of changes in IC in non-EFLT and EFLT COPD patients should provide useful information. In contrast, the detection of EFLT did not predict the changes of EELV or dyspnoea occurring after bronchodilation [13].
Díaz et al. [14] found that IC was the only spirometric parameter, in which there was almost no overlap between non-EFLT and EFLT COPD patients. The non-EFLT patients had almost all normal IC whilst the EFLT all had <80% pred in a group of 52 COPD patients. Linear regression analysis performed separately for these EFLT and non-EFLT patients showed that in the EFLT patients the sole predictor of exercise capacity was IC% pred, whilst in the non-EFLT the ratio FEV1/FVC% pred was the sole predictor. Díaz et al. [15] also reported that in EFLT COPD patients, the maximal tidal volume and hence maximal oxygen consumption are closely related to the reduced IC. The EFLT patients also exhibited a significant increase in PaCO2 and a decrease in PaO2 during peak exercise. O'Donnell et al. [16] extended the findings of Díaz et al. [14, 15] reporting that since the pathophysiological hallmark of COPD is EFL (occurring during exercise and in the advanced disease even at rest), the latter promoted DH which was correlated best with resting IC. DH curtailed VT response to exercise. The inability to expand VT in response to increasing ventilatory demand contributed to exercise intolerance in COPD.
The main finding of these studies was that detection of tidal EFL plays an important role in identifying the factors that limit exercise tolerance because resting EFL clearly separates two populations of patients with significant differences in exercise tolerance. More importantly, their detection provides useful information about the mechanisms limiting exercise tolerance. The detection of EFL during exercise should be carried out also using the NEP technique, as the conventional method for detecting flow limitation based on comparison of tidal with maximal flow-volume curves is not reliable [17]. In the presence of tidal EFL, DH appears to be the main determinant of exercise performance and the magnitude of resting IC, a well-recognized marker of DH, the best clinical predictor [14, 17].
EFL may be absent at rest but can be developed and hence detected during any exercise level by the use of NEP. That explains the fact that COPD patients, who are not hyperinflated at rest, develop DH during exercise [17]. It should be noted here that there are instances when DH (reflected by a reduced IC) can occur in the absence of tidal EFL [18, 19], and the presence of tidal EFL may not necessarily result in DH if the available expiratory flow is sufficient to sustain resting ventilation without the need to increase EELV. This is reflected by the fact that there are patients with EFLT and normal IC. Thus, measurement of IC and detection of EFL are complimentary ways for assessing bronchodilator and exercise responsiveness in COPD patients.
It was found that almost all COPD patients who require mechanical ventilation are flow-limited over the entire range of tidal expiration and that the supine posture promotes flow limitation [20].
Despite these potentially adverse consequences of EFL, its prevalence has not been extensively studied until recently, probably due to the lack of simple and noninvasive techniques. The aim of this work was to review the existing physiological techniques of assessing tidal expiratory flow limitation (EFLT).
3. Oesophageal Balloon Techniques
3.1. Fry Method
The definition of EFL implies that a further increase in transpulmonary pressure will cause no further increase in expiratory flow [21]. Therefore, direct assessment of expiratory flow limitation requires determination of iso-volume relationships between flow and transpulmonary pressure (F-P). Fry et al. [22] were the first who developed such curves in 1950s. The explanation of an isovolumic pressure flow curve lies in understanding its construction. Flow, volume and oesophageal pressure (Poes) are measured simultaneously during the performance of repeated expiratory vital capacity efforts by a subject seated in a volume body plethysmograph, in which gas compression artifact is corrected. The subject is instructed to exhale with varying amounts of effort that are reflected by changes in Poes. From a series of such efforts (~30), it is possible to plot flow against Poes at any given lung volume [21]. The flow reached a plateau at a low positive pleural pressure and that once maximum flow for that volume is reached, it remains constant despite increasing Poes by making expiratory efforts of increasing intensity.
3.2. Mead-Whittenberger's Method
The Mead-Whittenberger method [23] directly relates alveolar pressure to flow. Mead-Whittenberger's graphs can be obtained by plotting the flow measured at the airway opening versus the resistive pressure drop during a single breath. In such a way the phenomenon of flow limitation is documented.
These techniques are technically complex and time consuming. Furthermore, these techniques are invasive because they require the insertion of an oesophageal balloon [22, 23].
3.3. Conventional (Hyatt's) Method
Until recently, the “conventional” method used to detect expiratory flow-limitation during tidal breathing was the one proposed by Hyatt [24] in 1961. It consists in superimposing a flow volume loop of a tidal breath within a maximum flow-volume curve. This analysis and the “concept of EFL” have been the basics for understanding respiratory dynamics. Flow limitation is not present when the patient breaths tidally below the maximal expiratory flow-volume (MEFV) curve. According to this technique, normal subjects do not reach flow limitation even at maximum exercise [1, 25]. In contrast, flow limitation is present when a patient breathes tidally along or higher than the MEFV curve. Patients with severe chronic obstructive pulmonary disease (COPD) may exhibit flow limitation even at rest, as reflected by the fact that they breathe tidally along or above their maximal flow-volume curve [1, 21–25]. However, the conventional method to detect flow limitation based on comparison of maximal to tidal expiratory flow-volume curves suffers from several methodological deficiencies. These include the following.
(a) Thoracic Gas Compression Artefacts. Volume should be measured with a body-box, instead of using, as is common practice, a pneumotachograph or a spirometer in order to minimize such errors [26]. Consequently, in practice, flow limitation can be assessed only in seated subjects at rest.
(b) Incorrect Alignment of Tidal and Maximal Expiratory F-V Curves. Such alignment is usually made considering the total lung capacity (TLC) as a fixed reference point. This assumption may not always be valid [27, 28].
(c) Effect of Previous Volume and Time History. Comparison of tidal and maximal F-V curves is incorrect, since the previous volume and time history of a spontaneous tidal breath is necessarily different from that of an FVC manoeuvre. Therefore, it is axiomatic that comparison of tidal with maximal F-V curves is problematic. In fact, there is not a single maximal F-V curve but rather a family of different curves, which depend on the time course of the inspiration preceding the FVC manoeuvre [29–31].
(d) Respiratory Mechanics and Time Constant Inequalities. These are different during the tidal and maximal expiratory efforts again making comparisons of the two F-V curves problematic [32–34].
(e) Exercise. Exercise may result in bronchodilation or bronchoconstriction and other changes of lung mechanics, which may also affect correct comparisons of the two F-V curves [35].
(f) Patient's Cooperation. Another important limitation of the conventional method is that it requires patient's cooperation. This is not always feasible [27, 28].
In fact, it has been clearly demonstrated in several studies [11, 17, 36, 37] comparing the NEP with the conventional technique that the latter is not accurate. As a result, the use of the conventional method is no longer recommended.
4. The Negative Expiratory Pressure (NEP) Technique
In order to overcome these technical and conceptual difficulties, the negative expiratory pressure (NEP) technique has been introduced [10, 17, 27, 36]. The NEP technique has been first applied and validated in mechanically ventilated ICU patients by concomitant determination of isovolume flow-pressure relationships [38]. This method does not require performance of FVC manoeuvres, collaboration on the part of the patient, or use of a body-box. It can be used during spontaneous breathing in any body position [39], during exercise [17, 40], and ICU settings [20]. With this technique the volume and time history of the control and test expiration are axiomatically the same.
Briefly, a flanged plastic mouthpiece is connected in series to a pneumotachograph and a T-tube (Figure 1). One side of the T-tube is open to the atmosphere, whilst the other side is equipped with a one-way pneumatic valve, which allows for the subject to be rapidly switched to negative pressure generated by a vacuum cleaner or a Venturi device. The pneumatic valve consists of an inflatable balloon connected to a gas cylinder filled with pure helium and a manual pneumatic controller. The latter permits remote-control balloon deflation, which is accomplished quickly (30–60 ms) and quietly, allowing rapid exposure to negative pressure during expiration (NEP). Alternatively, a solenoid rapid valve can be used. The NEP (usually set at about −5 cm H2O) can be adjusted with a potentiometer on the vacuum cleaner or by controlling the Venturi device. Airflow () is measured with the heated pneumotachograph and pressure at the airway opening (Pao) is simultaneously measured through a side port on the mouthpiece (Figure 1). Volume (V) is obtained by digital integration of the flow signal, and correction of electrical drift is mandatory [36]. While performing the testing, the subjects should be watched closely for leaks at the mouthpiece. Only those tests, in which there is no leak, are valid [41]. Tidal EFL is assessed while seated upright in a comfortable chair or if needed lying supine on a comfortable couch, at least 2 h after eating or taking coffee. Patients are asked to breathe room air through the equipment assembly with the noseclip on (Figure 1). Each subject has an initial 10–15 min trial run, in order to become accustomed to the apparatus and procedure. The flow, volume, and pressure are continuously monitored on the computer screen. When regular breathing is resumed, a series of test breaths are performed with regular breaths in between the test breaths, in which NEP is applied at the beginning of expiration and maintained throughout the ensuing expiration [36].
Figure 1.
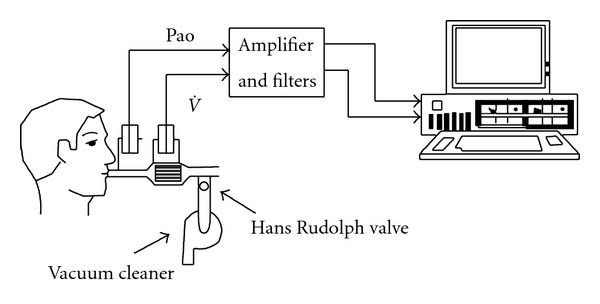
Schematic diagram of equipment setup. Pao: pressure at the airway opening; : gas flow (from [36]).
The NEP technique is based on the principle that in the absence of flow limitation, the increase in pressure gradient between the alveoli and the airway opening caused by NEP should result in increased expiratory flow. By contrast, in flow-limited subjects application of NEP should not change the expiratory flow. Our analysis essentially consists in comparing the expiratory -V curve obtained during a control breath with that obtained during the subsequent expiration in which NEP is applied [36].
Subjects in whom application of NEP does not elicit an increase of flow during part or all of the tidal expiration (Figures 2(b) and 2(c)) are considered flow-limited (EFL). By contrast, subjects in whom flow increases with NEP throughout the control tidal volume range (Figure 2(a)) are considered as non-flow-limited (non-EFLT). If tidal EFL is present when NEP is applied, there is a transient increase of flow (spike), which mainly reflects sudden reduction in volume of the compliant oral and neck structures. To a lesser extent a small artefact due to common-mode rejection ratio of the system of measuring flow may also contribute to the flow transients [10, 36]. Such spikes are useful markers of EFL.
Figure 2.
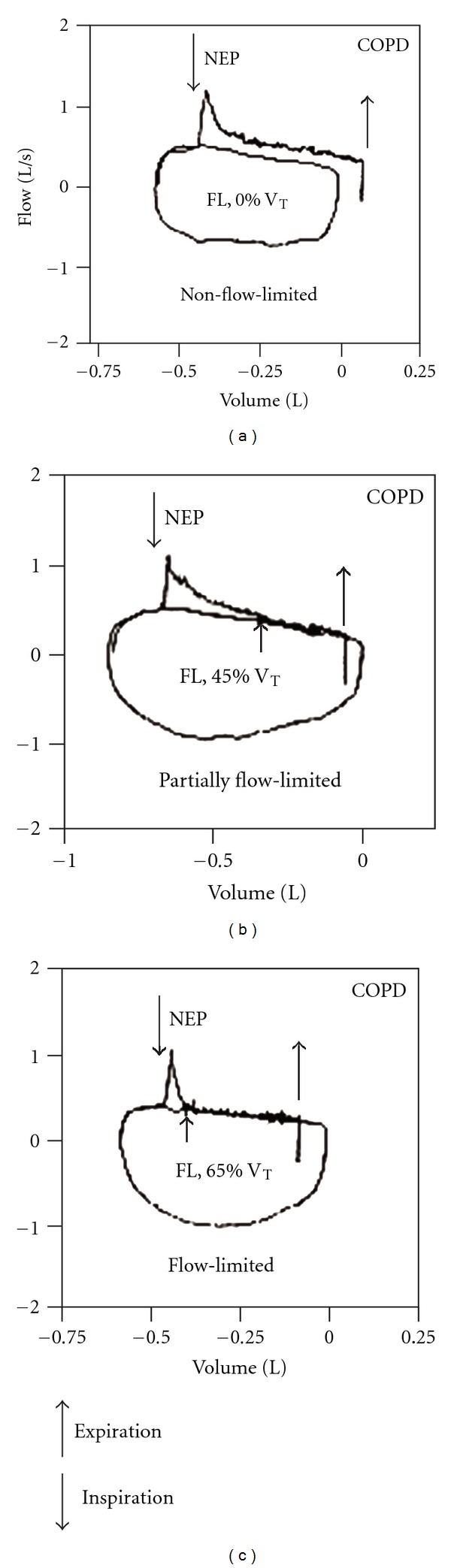
Flow-volume loops of test breaths and preceding control breaths of three representative COPD patients with different degrees of flow-limitation: not flow-limited (NFL) (a), flow-limited (EFL) over less than 50% VT (b), and flow-limited from peak expiratory flow (EFL) (c). Arrows indicate points at which NEP was applied and removed (modified from [10]).
The degree of flow limitation can be assessed using three different EFLT indices: (a) as a continuous variable expressed as % VT in both seated and supine positions (Figure 2) [36], (b) as a discrete variable in the form of three categories classification, that is, non-EFLT both seated and supine, EFLT supine but not seated, and EFLT both seated and supine [36], and (c) as discrete variable in the form of the five-category classification (5-point EFLT score) [10].
In all studies employing the NEP technique, the latter was not associated with any unpleasant sensation, cough, or other side effects [10, 17, 36]. The finding of O'Donnell et al. [42] that application of −9.7 cm H2O/L/s of expiratory assistance for 4 min during inspiration and expiration caused unpleasant respiratory sensation can be attributed to negative pressure application differences, that is, NEP, usually at −5 cm H2O level, is applied only during expiration every 5–10 breaths intervals.
The use of the NEP technique during tidal flow-volume analysis studies has led to realization of the important role of expiratory flow limitation in exertional dyspnoea and ventilatory impairment for a surprisingly wide range of clinical circumstances, for example, before and after bronchodilation, exercise, ICU, and heliox administration at rest and during exercise [8, 43, 44]. Up to date, no study has questioned reliability and accuracy of the NEP technique. Currently, therefore, the NEP technique can be regarded as the new gold standard to detect EFLT, if one takes into account the pros and cons of each available technique. It is a novel, simple, non-invasive, useful research and clinical lung function tool.
5. Submaximal Expiratory Manoeuvres
Pellegrino and Brusasco [45] proposed an alternative technique to detect expiratory flow limitation. EFLT was inferred from the impingement of the tidal flow-volume loop on the flow recorded during submaximally forced expiratory manoeuvres initiated from end-tidal inspiration in a body-box. After regular breathing with no volume drift, the subject performs a forced expiration from end-tidal inspiration without breath holding (partial expiratory manoeuvre). Care is taken to coach the subjects not to slow down the inspiration preceding the partial forced manoeuvre, thus minimizing the dependence of forced flows on the time of the preceding inspiration. A deep inspiration to TLC recorded soon after the gentle forced manoeuvre allowed the loops to be superimposed and compared at absolute lung volume. Flow limitation is defined as the condition of tidal expiratory flow impinging on the maximal flow generated during the gentle forced expiratory manoeuvre. Since this method requires a body box measurements cannot be made in different body postures, ICU, or during exercise testing.
6. Squeezing the Abdomen during Expiration
Workers in Brussels have shown that manual compression of the abdomen coinciding with the onset of expiration can be used as a simple way of detecting flow limitation at rest [46] and during exercise [47]. With one hand placed on the lower back of the patient and other applied with the palm at the level of the umbilicus perpendicular to the axis between the xiphoid process and the pubis, the operator first detects a respiratory rhythm by gentle palpation, and then after warning the subject applies a forceful pressure at the onset of expiration. As in the NEP technique, the resulting expiratory flow-volume loop recorded at the mouth is superimposed on the preceding tidal breath. Failure to increase expiratory flow indicates flow limitation. This technique produces clear differences between normal subjects and patients with COPD. The presence of flow limitation during exercise detected during exercise in COPD patients was associated with increases in the end-expiratory lung volume (EELV) [47]. Interestingly, not all subjects with COPD exhibited flow limitation when lung volume changed, a finding which requires confirmation. The method is appealingly simple, not influenced by the upper airway compliance, and like the NEP method, it avoids problems with the preceding volume history of the test breath. Despite initial concerns about the possibility that gas compression in the alveoli would produce false positive results, this does not seem to be a practical problem. However, unlike the NEP method, it is virtually impossible to squeeze at the precise of expiration. Thus far this technique has not been widely applied despite its relative simplicity.
7. Forced Oscillation Technique (FOT)
Another approach for detecting EFLT has been the forced oscillation technique (FOT) previously applied to look at the frequency dependence of resistance in a range of lung diseases and now available commercially in a modified form using impulse oscillometry [48, 49]. The principle here is that flow limitation will only be present in patients with obstructive pulmonary disease during expiration. Normally oscillatory pressures generated by a loud-speaker system at the mouth are transmitted throughout the respiratory system, and by studying the resulting pressures which are in and out of phase with the signal, both the respiratory system resistance and reactance (a measure of the elastic properties of the system) can be computed. When flow limitation occurs, wave speed theory predicts that a choke point will develop within the airway subtended by that “unit” of the lung. In these circumstances, the oscillatory pressure applied at the mouth will no longer reach the alveoli and the reactance will reflect the mechanical properties of the airway wall rather than those of the whole respiratory system. As a result, reactance becomes much more negative and there is a clear within breath difference between inspiration and expiration. Dellacá and colleagues [49] used this property to investigate the distribution of changes in intrabreath reactance in normal subjects and COPD patients who were instrumented with balloon catheters. In a recent study Dellacá et al. [50] found a good agreement between NEP and FOT despite the fact that the FOT method may detect regional as well as overall EFLT. NEP detects the condition in which all possible pathways between airway opening and the alveoli are choked. When this occurs, the total expiratory flow is independent of the expiratory pressure, a condition of “global” expiratory flow limitation. In contrast, FOT assesses the amount of the lung that is choked during expiration only. This measures “regional” flow limitation, and a threshold value may indicate when the regional flow limitation reaches the condition of “global” flow limitation. Therefore, when “global” expiratory flow limitation is reached, the two techniques should produce the same response [50].
It does appear to hold considerable promise, but to date, only a few studies to detect EFLT with this method have been reported. On the other hand, FOT is very complex, expensive as it requires the special FOT equipment, and time consuming.
8. Technegas Method
Technegas is an aerosol of 99mTc-labeled carbon molecules with small diameter (<0.01 μm) [19] capable of depositing even in the most peripheral regions of the lung. Pellegrino et al. [19] used the inhalation of Technegas to reveal sites (“hot spots”) of EFLT after induced bronchocontsriction in asthmatic patients. During forced expiration, the flow-limiting segment is known to be located first in the large intrathoracic airways and then to move peripherally. However, the present scintigraphic technique cannot precisely define the anatomical location of the flow-limiting segment during tidal breathing. Therefore, what the “hot spots” represent appears to be uncertain. The authors claim that this technique is useful to detect “regional” EFLT well before the NEP and submaximal expiratory manoeuvre techniques.
9. Breath-by-Breath Method
The most recent method to detect EFLT is the one using breath-by-breath quantification of progressive airflow limitation during exercise applied in stable COPD patients [51]. The authors have noted that during heavy exercise in COPD patients, dynamic airways compression leads to a progressive fall in intrabreath flow. This is manifested by an increasing concavity in the spontaneous expiratory flow-volume (SEFV) curve. The new method consists in quantifying the SEFV curve configuration breath-by-breath during incremental exercise utilizing a computerized analysis. For each breath's SEFV curve, points of highest flow and end-expiration were identified to define a rectangle's diagonal. Fractional area within the rectangle below the SEFV curve was defined as the “rectangular area ratio” (RAR). RAR < 0.5 signifies concavity of the SEFV curve. However, this method may be useful only during exercise because inspection of SEFV curve during resting breathing is not a reliable means in detecting EFLT [41]. Severe COPD patients often exhibit a mechanically active expiration, which is characterized by abdominal activity. This necessarily affects the shape of SEFV curve, making it concave with respect to the volume axis, even in the absence of EFLT [52].
10. Conclusions
The newer aforementioned techniques represent a substantial advance on traditional approaches which compared tidal and maximal flow-volume loops or even the more robust but time-consuming method of determining partial expiratory flow-volume loops. By freeing both parts, the doctor and the patient, from the limitations of the oesophageal balloon catheters and body plethysmograph, they have opened up a new era in understanding modern physiological principles like the tidal expiratory flow limitation [8, 43, 44]. Among the available physiological techniques to detect EFLT, the NEP should probably be regarded as the new gold standard. This view is supported by the data obtained from the NEP's application in a wide variety of settings [8, 43, 44]. However, extensive comparisons between these different methods are needed before the best “test” or combination of techniques can be unequivocally recommended to correctly assess EFLT.
References
- 1.Pride NB. Tests of forced expiration and inspiration. In: Hughes JMB, Pride NB, editors. Lung Function Tests: Physiological Principles and Clinical Applications. London, UK: WB Saunders; 1999. pp. 3–25. [Google Scholar]
- 2.Pecchiari M, Pelucchi A, D’Angelo E, Forest A, Milic-Emili J, D’Angelo E. Effect of heliox breathing on dynamic hyperinflation in COPD patients. Chest. 2004;125(6):2075–2082. doi: 10.1378/chest.125.6.2075. [DOI] [PubMed] [Google Scholar]
- 3.Brighenti C, Barbini P, Gnudi G, Cevenini G, Pecchiari M, D’Angelo E. Helium-oxygen ventilation in the presence of expiratory flow-limitation: a model study. Respiratory Physiology and Neurobiology. 2007;157(2-3):326–334. doi: 10.1016/j.resp.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 4.D’Angelo E, Santus P, Civitillo MF, Centanni S, Pecchiari M. Expiratory flow-limitation and heliox breathing in resting and exercising COPD patients. Respiratory Physiology and Neurobiology. 2009;169(3):291–296. doi: 10.1016/j.resp.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Milic-Emili J. Does mechanical injury of the peripheral airways play a role in the genesis of COPD in smokers? COPD. 2004;1(1):85–92. doi: 10.1081/COPD-120028700. [DOI] [PubMed] [Google Scholar]
- 6.Milic-Emili J, Torchio R, D’Angelo E. Closing volume: a reappraisal (1967–2007) European Journal of Applied Physiology. 2007;99(6):567–583. doi: 10.1007/s00421-006-0389-0. [DOI] [PubMed] [Google Scholar]
- 7.D’Angelo E, Koulouris NG, Della Valle P, Gentile G, Pecchiari M. The fall in exhaled nitric oxide with ventilation at low lung volumes in rabbits: an index of small airway injury. Respiratory Physiology and Neurobiology. 2008;160(2):215–223. doi: 10.1016/j.resp.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Calverley PMA, Koulouris NG. Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology. European Respiratory Journal. 2005;25(1):186–199. doi: 10.1183/09031936.04.00113204. [DOI] [PubMed] [Google Scholar]
- 9.Gennimata SA, Palamidas A, Karakontaki F, et al. Pathophysiology of evolution of small airways disease to overt COPD. COPD. 2010;7(4):269–275. doi: 10.3109/15412555.2010.497515. [DOI] [PubMed] [Google Scholar]
- 10.Eltayara L, Becklake MR, Volta CA, Milic-Emili J. Relationship between chronic dyspnea and expiratory flow limitation in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1996;154(6):1726–1734. doi: 10.1164/ajrccm.154.6.8970362. [DOI] [PubMed] [Google Scholar]
- 11.Tantucci C, Duguet A, Similowski T, Zelter M, Derenne JP, Milic-Emili J. Effect of salbutamol on dynamic hyperinflation in chronic obstructive pulmonary disease patients. European Respiratory Journal. 1998;12(4):799–804. doi: 10.1183/09031936.98.12040799. [DOI] [PubMed] [Google Scholar]
- 12.Boni E, Corda L, Franchini D, et al. Volume effect and exertional dyspnoea after bronchodilator in patients with COPD with and without expiratory flow limitation at rest. Thorax. 2002;57(6):528–532. doi: 10.1136/thorax.57.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadcroft J, Calverley PMA. Alternative methods for assessing bronchodilator reversibility in chronic obstructive pulmonary disease. Thorax. 2001;56(9):713–720. doi: 10.1136/thorax.56.9.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Díaz O, Villafranca C, Ghezzo H, et al. Role of inspiratory capacity on exercise tolerance in COPD patients with and without tidal expiratory flow limitation at rest. European Respiratory Journal. 2000;16(2):269–275. doi: 10.1034/j.1399-3003.2000.16b14.x. [DOI] [PubMed] [Google Scholar]
- 15.Díaz O, Villafranca C, Ghezzo H, et al. Breathing pattern and gas exchange at peak exercise in COPD patients with and without tidal flow limitation at rest. European Respiratory Journal. 2001;17(6):1120–1127. doi: 10.1183/09031936.01.00057801. [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2001;164(5):770–777. doi: 10.1164/ajrccm.164.5.2012122. [DOI] [PubMed] [Google Scholar]
- 17.Koulouris NG, Dimopoulou I, Valta P, Finkelstein R, Cosio MG, Milic-Emili J. Detection of expiratory flow limitation during exercise in COPD patients. Journal of Applied Physiology. 1997;82(3):723–731. doi: 10.1152/jappl.1997.82.3.723. [DOI] [PubMed] [Google Scholar]
- 18.Tantucci C, Ellaffi M, Duguet A, et al. Dynamic hyperinflation and flow limitation during methacholine-induced bronchoconstriction in asthma. European Respiratory Journal. 1999;14(2):295–301. doi: 10.1183/09031936.99.142. [DOI] [PubMed] [Google Scholar]
- 19.Pellegrino R, Biggi A, Papaleo A, Camuzzini G, Rodarte JR, Brusasco V. Regional expiratory flow limitation studied with technegas in asthma. Journal of Applied Physiology. 2001;91(5):2190–2198. doi: 10.1152/jappl.2001.91.5.2190. [DOI] [PubMed] [Google Scholar]
- 20.Alvisi V, Romanello A, Badet M, Gaillard S, Philit F, Guérin C. Time course of expiratory flow limitation in COPD patients during acute respiratory failure requiring mechanical ventilation. Chest. 2003;123(5):1625–1632. doi: 10.1378/chest.123.5.1625. [DOI] [PubMed] [Google Scholar]
- 21.Murray JF. Ventilation. In: Murray JF, editor. The Normal Lung: The Basis for Diagnosis and Treatment of Pulmonary Disease. 2nd edition. London, UK: WB Saunders; 1986. pp. 83–119. [Google Scholar]
- 22.Fry DL, Ebert RV, Stead WW, Brown CC. The mechanics of pulmonary ventilation in normal subjects and in patients with emphysema. American Journal of Medicine. 1954;16(1):80–97. doi: 10.1016/0002-9343(54)90325-3. [DOI] [PubMed] [Google Scholar]
- 23.Mead J, Whittenberger JL. Physical properties of human lungs measured during spontaneous respiration. Journal of Applied Physiology. 1953;5:779–796. [Google Scholar]
- 24.Hyatt RE. The interrelationships of pressure, flow, and volume during various respiratory maneuvers in normal and emphysematous subjects. American Review of Respiratory Disease. 1961;83:676–683. doi: 10.1164/arrd.1961.83.5.676. [DOI] [PubMed] [Google Scholar]
- 25.Leaver DG, Pride NB. Flow-volume curves and expiratory pressures during exercise in patients with chronic airways obstruction. Scandinavian Journal of Respiratory Diseases. Supplementum. 1971;77:23–27. [PubMed] [Google Scholar]
- 26.Ingram RH, Schilder DP. Effect of gas compression on pulmonary pressure, flow, and volume relationship. Journal of Applied Physiology. 1966;21(6):1821–1826. doi: 10.1152/jappl.1966.21.6.1821. [DOI] [PubMed] [Google Scholar]
- 27.Stubbing DG, Pengelly LD, Morse JLC, Jones NL. Pulmonary mechanics during exercise in subjects with chronic airflow obstruction. Journal of Applied Physiology Respiratory Environmental and Exercise Physiology. 1980;49(3):511–515. doi: 10.1152/jappl.1980.49.3.511. [DOI] [PubMed] [Google Scholar]
- 28.Younes M, Kivinen G. Respiratory mechanics and breathing pattern during and following maximal exercise. Journal of Applied Physiology Respiratory Environmental and Exercise Physiology. 1984;57(6):1773–1782. doi: 10.1152/jappl.1984.57.6.1773. [DOI] [PubMed] [Google Scholar]
- 29.D’Angelo E, Prandi E, Milic-Emili J. Dependence of maximal flow-volume curves on time course of preceding inspiration. Journal of Applied Physiology. 1993;75(3):1155–1159. doi: 10.1152/jappl.1993.75.3.1155. [DOI] [PubMed] [Google Scholar]
- 30.D’Angelo E, Prandi E, Marazzini L, Milic-Emili J. Dependence of maximal flow-volume curves on time course of preceding inspiration in patients with chronic obstruction pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1994;150(6):1581–1586. doi: 10.1164/ajrccm.150.6.7952618. [DOI] [PubMed] [Google Scholar]
- 31.Koulouris NG, Rapakoulias P, Rassidakis A, et al. Dependence of forced vital capacity manoeuvre on time course of preceding inspiration in patients with restrictive lung disease. European Respiratory Journal. 1997;10(10):2366–2370. doi: 10.1183/09031936.97.10102366. [DOI] [PubMed] [Google Scholar]
- 32.Melissinos CG, Webster P, Tien YK, Mead J. Time dependence of maximum flow as an index of nonuniform emptying. Journal of Applied Physiology Respiratory Environmental and Exercise Physiology. 1979;47(5):1043–1050. doi: 10.1152/jappl.1979.47.5.1043. [DOI] [PubMed] [Google Scholar]
- 33.Fairshter RD. Airway hysteresis in normal subjects and individuals with chronic airflow obstruction. Journal of Applied Physiology. 1985;58(5):1505–1510. doi: 10.1152/jappl.1985.58.5.1505. [DOI] [PubMed] [Google Scholar]
- 34.Wellman JJ, Brown R, Ingram RH. Effect of volume history on successive partial expiratory flow volume maneuvers. Journal of Applied Physiology. 1976;41(2):153–158. doi: 10.1152/jappl.1976.41.2.153. [DOI] [PubMed] [Google Scholar]
- 35.Beck KC, Offord KP, Scanlon PD. Bronchoconstriction occurring during exercise in asthmatic subjects. American Journal of Respiratory and Critical Care Medicine. 1994;149(2):352–357. doi: 10.1164/ajrccm.149.2.8306029. [DOI] [PubMed] [Google Scholar]
- 36.Koulouris NG, Valta P, Lavoie A, et al. A simple method to detect expiratory flow limitation during spontaneous breathing. European Respiratory Journal. 1995;8(2):306–313. doi: 10.1183/09031936.95.08020306. [DOI] [PubMed] [Google Scholar]
- 37.Boczkowski J, Murciano D, Pichot MH, Ferretti A, Pariente R, Milic-Emili J. Expiratory flow limitation in stable asthmatic patients during resting breathing. American Journal of Respiratory and Critical Care Medicine. 1997;156(3):752–757. doi: 10.1164/ajrccm.156.3.9609083. [DOI] [PubMed] [Google Scholar]
- 38.Jones MH, Davis SD, Kisling JA, Howard JM, Castile R, Tepper RS. Flow limitation in infants assessed by negative expiratory pressure. American Journal of Respiratory and Critical Care Medicine. 2000;161(3):713–717. doi: 10.1164/ajrccm.161.3.9807135. [DOI] [PubMed] [Google Scholar]
- 39.Dimitroulis J, Bisirtzoglou D, Retsou S, et al. Effect of posture on expiratory flow limitation in spontaneously breathing stable COPD patients. American Journal of Respiratory and Critical Care Medicine. 2001;163(5) Abstract no. A410. [Google Scholar]
- 40.Murciano D, Ferretti A, Boczkowski J, Sleiman C, Fournier M, Milic-Emili J. Flow limitation and dynamic hyperinflation during exercise in COPD patients after single lung transplantation. Chest. 2000;118(5):1248–1254. doi: 10.1378/chest.118.5.1248. [DOI] [PubMed] [Google Scholar]
- 41.Baydur A, Milic-Emili J. Expiratory flow limitation during spontaneous breathing: comparison of patients with restrictive and obstructive respiratory disorders. Chest. 1997;112(4):1017–1023. doi: 10.1378/chest.112.4.1017. [DOI] [PubMed] [Google Scholar]
- 42.O’Donnell DE, Sanii R, Anthonisen NR, Younes M. Effect of dynamic airway compression on breathing pattern and respiratory sensation in severe chronic obstructive pulmonary disease. American Review of Respiratory Disease. 1987;135(4):912–918. doi: 10.1164/arrd.1987.135.4.912. [DOI] [PubMed] [Google Scholar]
- 43.Dueck R. Assessment and monitoring of flow limitation and other parameters from flow/volume loops. Journal of Clinical Monitoring and Computing. 2000;16(5-6):425–432. doi: 10.1023/a:1011492710070. [DOI] [PubMed] [Google Scholar]
- 44.Johnson BD, Beck KC, Zeballos RJ, Weisman IM. Advances in pulmonary laboratory testing. Chest. 1999;116(5):1377–1387. doi: 10.1378/chest.116.5.1377. [DOI] [PubMed] [Google Scholar]
- 45.Pellegrino R, Brusasco V. Lung hyperinflation and flow limitation in chronic airway obstruction. European Respiratory Journal. 1997;10(3):543–549. [PubMed] [Google Scholar]
- 46.Ninane V, Leduc D, Kafi SA, Nasser M, Houa M, Sergysels R. Detection of expiratory flow limitation by manual compression of the abdominal wall. American Journal of Respiratory and Critical Care Medicine. 2001;163(6):1326–1330. doi: 10.1164/ajrccm.163.6.2004150. [DOI] [PubMed] [Google Scholar]
- 47.Abdel Kafi S, Sersté T, Leduc D, Sergysels R, Ninane V. Expiratory flow limitation during exercise in COPD: detection by manual compression of the abdominal wall. European Respiratory Journal. 2002;19(5):919–927. doi: 10.1183/09031936.02.00217602. [DOI] [PubMed] [Google Scholar]
- 48.Dellacà RL. Measurement of respiratory system impedances. In: Aliverti A, Brusasco V, Macklem PT, Pedotti A, editors. Mechanics of Breathing. Milan, Italy: Springer; 2002. pp. 157–171. [Google Scholar]
- 49.Dellacà RL, Santus P, Aliverti A, et al. Detection of expiratory flow limitation in COPD using the forced oscillation technique. European Respiratory Journal. 2004;23(2):232–240. doi: 10.1183/09031936.04.00046804. [DOI] [PubMed] [Google Scholar]
- 50.Dellacà RL, Duffy N, Pompilio PP, et al. Expiratory flow limitation detected by forced oscillation and negative expiratory pressure. European Respiratory Journal. 2007;29(2):363–374. doi: 10.1183/09031936.00038006. [DOI] [PubMed] [Google Scholar]
- 51.Ma S, Hecht A, Varga J, et al. Breath-by-breath quantification of progressive airflow limitation during exercise in COPD: a new method. Respiratory Medicine. 2010;104(3):389–396. doi: 10.1016/j.rmed.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 52.Thomas HM, Koulouris NG, Valta P, et al. Expiratory flow limitation during tidal breathing. European Respiratory Journal. 1995;8(9, article 1624) [PubMed] [Google Scholar]


