Summary
Background:
Mast cells, owing to diversity of secreted mediators, play a crucial role in the regulation of inflammatory response. Together with basophils, mast cells constitute a central pathogenetic element of anaphylactic (IgE-dependent) and anaphylactoid (IgE-independent) reactions. In severe cases, generalized degranulation of mast cells may cause symptoms of anaphylactic shock. The influence of the classical, iodine-based contrast media on mastocyte degranulation has been fully described. Our objective was to determine the influence of the gadolinium-based MRI contrast media on histamine release from mast cells and to compare the activity of ionic and non-ionic preparations of contrast media.
Material/Methods:
To determine the intensity of mast cell degranulation, we used an experimental model based on mastocytes isolated from rat peritoneal fluid. Purified suspensions of mast cells were incubated with various concentrations of Gd-DTPA and Gd-DTPA-BMA, and solutions of PEG 600 which served as a non-toxic osmotic stimulus. The intensity of mast cell activation was presented as mean percentage of histamine released from cells after incubation.
Results/Conclusions:
The obtained results demonstrate that both ionic and non-ionic preparations of the MRI contrast media are able to induce mast cell degranulation in vitro. It was also proved that the non-ionic MRI contrast media stimulate mast cells markedly more weakly than ionic contrast media at identical concentration. The aforementioned results may suggest a more profitable safety profile of the non-ionic contrast preparations. We may also conclude that triggering of mast cell degranulation after incubation with the solutions of MRI contrast media results from non-specific osmotic stimulation and direct toxicity of free ionic residues.
Keywords: mast cell, histamine, MRI contrast media, anaphylactic reaction
Background
Mast cells (mastocytes) may be found in almost all tissues of the human body. They may be found in high numbers mainly in close vicinity of blood vessels, peripheral nerves, in mucosal membranes, skin and subcutaneous tissue. Multiple secretory granules contained within the cytoplasm of mast cells have unique histochemical properties and are a characteristic structural element of those cells. Mast cell granules store multiple mediators, which are responsible for various functions of mastocytes in both physiological and pathological processes.
The key mechanism involved in the activation of mast cells is cross-bridging of IgE molecules bound to FcER receptors located on the cell membrane. In allergic reactions, IgE cross-bridging is mediated by polyvalent antigen molecules named allergens. Allergen-induced mast cell degranulation is the basis of anaphylactic reactions.
Activation of mast cells by endogenous and exogenous factors, which do not involve IgE/FcER-related mechanism, is called anaphylactoid reaction. Endogenous factors which may activate mast cells in the anaphylactoid mechanism include: complement fragments C3a, C4a, C5a (also known as anaphylotoxins); substance P; angiotensin II; endorphins; eosinophilic proteins MBP and ECP; interleukins IL-1 and IL-3; TNF [1–3]. Despite the heterogeneous expression of mast cell complement receptors for C3a and C5a, the fact that complement-related activation of mastocytes is the main factor responsible for hypersensitivity reactions to multiple pharmaceuticals remains uncontroversial. Contrast media belong to a large group of substances which have the ability to activate the complement [4–6].
Degranulation of mast cells releases mediators previously stored in mast cell granules (preformed mediators) and induces de novo synthesis of various biologically active substances with proinflammatory activity. The action of these mediators is clinically observed as immediate and late-phase allergic reaction (LPR).
Nuclear magnetic resonance-based techniques bring a new quality to diagnostic imaging. Unfortunately, the initial expectations that MRI would be a totally non-invasive method could not be fulfilled. Rapid progress in magnetic resonance imaging techniques confirmed that the use of infusion contrast media may improve the sensitivity and specificity of that method. Contrast media utilized in MRI techniques may be divided into ionic and non-ionic. Ionic contrast media are characterized by the presence of ionic bonds in the chemical structure of the molecule and dissociate in water to form solutions of high osmolality. The presence of active ions in solution can additionally increase the toxicity and reduce the biological tolerance of pharmacological preparations. Non-ionic contrast media do not dissociate to form active ions and are characterized by low osmolality of water solutions. The reduction of osmolality alone may improve their safety profile.
The contrast media most commonly used in MRI-based diagnostics belong to the group of gadolinium chelates. Based on clinical experiences, gadolinium chelates are considered to be safe. Adverse events after administration of such pharmaceuticals are observed in 0.17–0.5% of all patients, are usually benign and resemble mild allergic reactions. The most common MRI contrast media-related adverse events include: nausea and vomiting (67% of all cases) and allergic skin reactions (33% of all cases) [7,8]. Only one case of fatal complications associated with anaphylactic reaction after the administration of Gd-DTPA has been described so far [9]. However, there are a lot of reports describing generalized anaphylactic reactions, which have been successfully treated [10–13].
Publications mentioned above clearly prove that hypersensitivity reactions may be observed after parenteral administration of gadolinium chelates. Anaphylactic reactions are rare, but may be life-threatening. The aim of our study was to demonstrate that the gadolinium-based contrast media may trigger mast cell degranulation in vitro and to compare the effect of ionic and non-ionic MRI contrast media on mast cell activation.
Material and Methods
Animals
Male Wistar rats weighing 200–300 g were used as a source of peritoneal mast cells. All animals were maintained on a 12-hour light/dark cycle and allowed access to food and water ad libitum. The study protocol was approved by the Local Ethical Committee of Medical University in Lodz.
Mast cells isolation and activation
Animals were sacrificed by exposition to carbon dioxide and oxygen mixture in a special chamber. After exsanguination, 10ml of HEPES buffer (pH 6.9) were injected into the peritoneal cavity and abdominal walls were massaged for 90 seconds. Fluid containing a mixture of peritoneal cells was collected after opening the abdominal cavity and then purified by centrifugation in density gradient of Percoll. The cellular suspension obtained after centrifugation contained almost pure mast cells. The isolated cells were placed in incubation tubes (5000 in each tube) and incubated for 30 minutes with equal concentrations of Gd-DTPA (ionic contrast media), Gd-DTPA-BMA (non-ionic contrast media), or PEG 600 (osmotically active substance without direct cellular toxicity). The following osmolalities of the studied substances were tested: 300, 400, 500, 600, 700, 800, and 900 mOsm/kg H2O. Incubation with pure buffer served as a negative control, while incubation with compound 48/80 (non-specific degranulation inducer) at the concentration of 1 mcg/ml was a positive control. Incubation with each concentration of the tested substances was repeated 15 times using cell suspensions derived from different animals.
Histamine concentration assessment
Histamine concentration in supernatants and sediments of the incubated cultures was assessed using the spectrofluorometric method described by Shore, based on the condensation of histamine with ortoftalide aldehyde (OPT). The degree of mast cell activation was expressed as the percentage of total cellular histamine released from mast cells after incubation with the tested substances.
Statistics
All statistical analyses were conducted with the statistical analysis package Statistica licensed to Medical University in Lodz. Data are presented as means ± standard deviations. As most variables were non-normally distributed, a non-parametric Wilcoxon’s test was used for the evaluation of group differences. Significant associations were defined by a probability level of 0.05.
Results
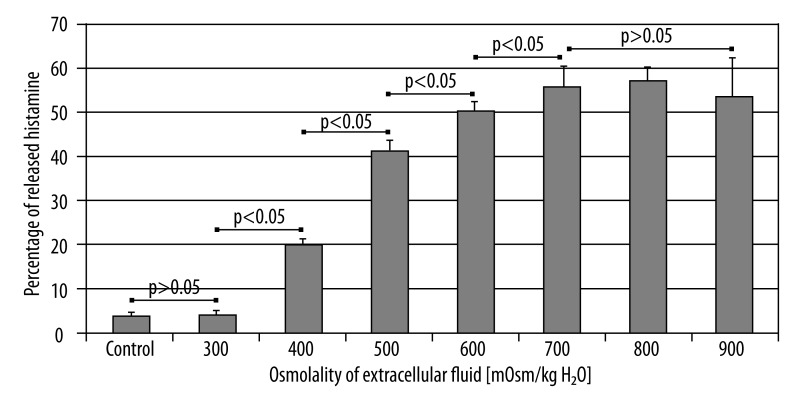
Figure 1 shows the relationship between the osmolality of incubation medium and the degree of histamine release from mast cells. Significant increase in histamine secretion in comparison to the control was observed for 400 mOsm/kg H2O of incubation medium osmolality (20.18% ±1.51). Rising release of histamine was observed for the consecutively studied osmolalities, up to 700 mOsm/kg H2O (55.78% ±4.82). Further increase in osmolality did not significantly increase the release of histamine from the incubated mastocytes.
Figure 1.
Influence of extracellular fluid osmolality on histamine release from mast cells.
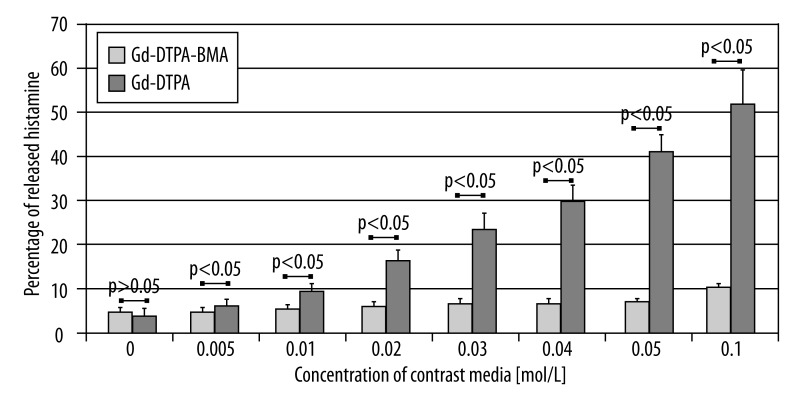
In the series of our experiments, Gd-DTPA, as well as Gd-DTPA-BMA induced mast cell degranulation in a concentration-dependent manner. For Gd-DTPA, significant augmentation of mastocyte degranulation was observed after exposition to the lowest concentration of the tested contrast media (0.01 mol/L). Incubation of mast cells with Gd-DTPA at the concentration of 0.1 mol/L resulted in mean histamine release of 50.01%, which was higher than histamine release in the positive control.
Markedly weaker activation of mast cells was observed in the samples incubated with Gd-DTPA-BMA. Significant increase in histamine release in comparison to control was not observed until the concentration of contrast media reached 0.04 mol/L.
The comparison of histamine release from mast cells under the influence of Gd-DTPA and Gd-DTPA-BMA at identical concentrations revealed that ionic contrast media are stronger histamine liberators than non-ionic ones. This relationship was visible especially for the highest concentration of the tested substances: for the concentration of 0.1 mol/L histamine release from mastocytes was five-fold higher in the samples incubated with Gd-DTPA in comparison to the samples incubated with Gd-DTPA-BMA. The data illustrating the influence of Gd-DTPA and Gd-DTPA-BMA at various concentrations on histamine release from mast cells are shown in Figure 2.
Figure 2.
Comparison of histamine release under the influence of Gd-DTPA and Gd-DTPA-BMA.
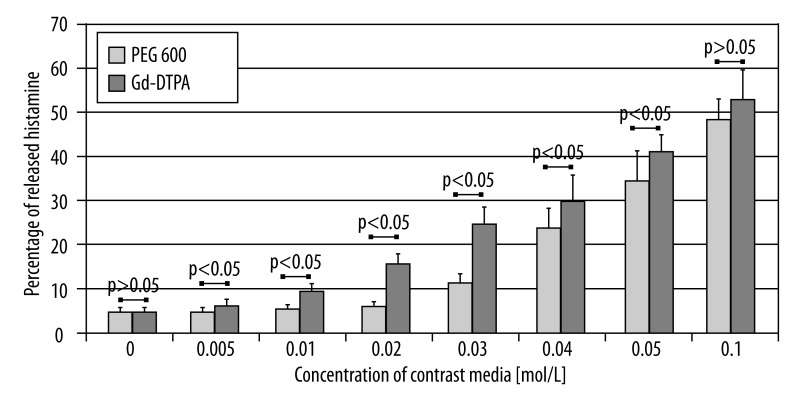
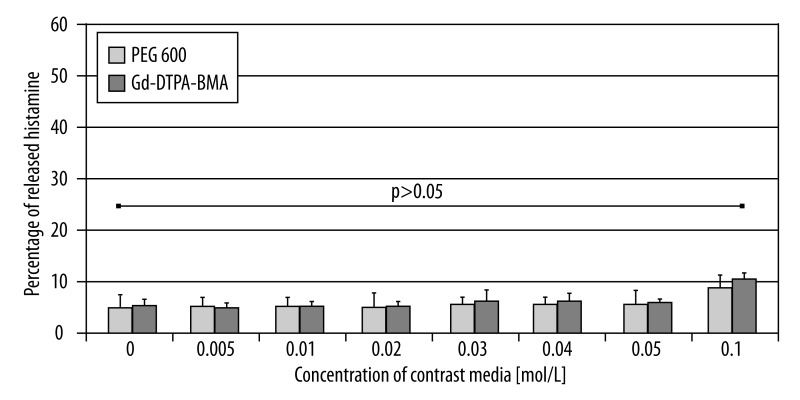
Statistically significant difference in the activation of mast cells between the samples stimulated with PEG 600 solutions at osmolality similar to the tested contrast media and the negative control (spontaneous histamine release) may be observed for concentrations 0.03 mol/L and higher in the Gd-DTPA series, and 0.05 mol/L and higher in the Gd-DTPA-BMA series. The comparison of degranulation intensity between the samples incubated with Gd-DTPA and PEG600 at similar osmolalities shows a significantly higher release of histamine for the samples incubated with the contrast media. Such a relationship may be observed for the majority of the tested concentrations. Only the samples incubated with higher concentrations of Gd-DTPA and PEG 600 did not bring statistically significant results (Figure 3). In the series comparing the release of histamine under the influence of Gd-DTPA-BMA and PEG 600 at similar osmolalities, no significance was found for any of tested concentrations (Figure 4).
Figure 3.
Comparison of histamine release from mast cells after incubation with the PEG 600 and Gd-DTPA solutions.
Figure 4.
Comparison of histamine release from mast cells after incubation with the PEG 600 and Gd-DTPA-BMA solutions.
Discussion
Degranulation of mast cells induced by various exogenous factors may cause multiple symptoms and complications related to the mechanisms of immediate and delayed hypersensitivity. The severe type of generalized hypersensitivity reaction may result in an abrupt decline in the regulatory mechanisms of blood pressure control and a collapse of peripheral tissue perfusion, thus giving rise to a life-threatening condition named anaphylactic shock. The presented results clearly prove that both ionic and non-ionic MRI contrast media are able to induce mast cell degran-ulation in vitro. In the experimental model designed for our study, animals did not contact contrast media before mast cell isolation, therefore they could not be sensitized to produce specific IgE antibodies. This leads to a conclusion that the mechanism responsible for the induction of degranulation belongs to the group of IgE-independent anaphylactoid reactions. Although the activation of mast cells could be induced by the cross-reactivity of other antibodies with contrast media molecules, the previous reports did not reveal any immune reactivity to Gd-DTPA or Gd-DTPA-BMA. Nonetheless, autoantibodies against the classical, iodine-based contrast media were identified in several groups of patients with allergic reactions [14]. It is also possible that the tested substances may act as haptens [15].
The available literature lacks unequivocal descriptions of the mechanism by which the MRI contrast media could induce mast cell degranulation, but it may be suspected that such a mechanism is at least partially similar to those described for the classical iodine-based contrasts and should include:
direct interaction of hyperosmolal solutions of contrast media [16];
complement system activation followed by the production of anaphylotoxins (C3a and C5a) [4,17].
Hageman’s factor activation followed by the stimulation of the kinin system may also participate in the pathogenesis of adverse reactions to the iodine-based contrast media, such as bronchoconstriction, increased volume or permeability of blood vessels. Vasomotor reactions may be related to increased NO production observed in some patients after the infusion of contrast media [18–20].
However, it should be emphasized that the iodine based contrast media are different from the gadolinium chelates in their chemical structure and physical properties. Therefore a direct transposition of the results obtained for the classical contrast media to those for the MRI contrasts appears to be hazardous. Yet from another point of view, the correspondence between the mechanisms of action is indicated by the highest risk of anaphylactic reaction after the infusion of the gadolinium-based contrast media in a group of patients with a history of adverse reactions to the iodine-based contrast media [21,22]. Despite a considerable dissimilarity in the chemical structure of the classical and MRI contrast media, they have one common property: water solutions of both groups of contrasts are characterized by high osmolality. This chemical property arises from high concentration of pharmaceutical substance in solution, which is required to properly contrast specific tissues and improve imaging. The same dose of contrast media cannot be injected into an adequately increased volume of solvent, as it would markedly increase the time required to finish the procedure. Hypothetically, high osmolality of MRI contrast solutions may be at least partially responsible for the stimulation of mast cell activity in vitro. That hypothesis is supported by the well-known observation, that environment hyperosmolality is able activate the degranulation of mastocytes and basophils [23,24]. Our results confirm the activating effect of hyperosmolal solutions on mast cells. Incubation of mastocytes with a 700 mOsm/kg H2O PEG 600 solution resulted in a secretion of histamine comparable to that induced by high concentrations of compound 48/80 (positive control). Therefore it may be deduced that hyperosmolal solutions are able to induce massive degranulation of mast cells. From another point of view, regarding the pharmacokinetics of Gd-DTPA and Gd-DTPA-BMA, even administration of the highest approved dose of contrast media (0.3 mmol/kg b.w.) cannot increase the osmolality of extracellular fluid to the levels tested in our study. Sufficient concentrations of pharmaceuticals may only be achieved locally, in the site of injection or, in the case of extravasation, to the surrounding tissues. The aforegoing statement is verified by the results by Runge et al. which confirm the increased inflammatory activity or even formation of necrosis after subcutaneous injection of the MRI contrast media in animal model [25]. Similarly, markedly elevated focal reactions may be suspected in the sites characterized by altered permeability of blood vessels, but that supposition cannot be confirmed by experimental data or clinical observations.
Introduction of non-ionic contrast media to diagnostic imaging has improved the safety of radiological procedures. The incidence and severity of adverse events observed after the injection of non-ionic contrast media is markedly lower in comparison to that of ionic preparations [26,27]. The preferable safety profile of non-ionic contrast media arises predominantly from the lower osmolality of their solutions. However, some authors demonstrated that the toxicity of contrast media may be reduced by blocking free carboxyl groups in the molecule, without changing the osmolality [28]. Multiple reports prove that the iodine-based ionic contrast media are definitely more potent mast cell degranulation activators than non-ionic ones in both in vitro and in vivo models [29–31]. Our experiments show similar results for the gadolinium-based contrast media widely used in NMR imaging. Our observations exhibit that Gd-DTPA induces higher release of histamine from mast cells than Gd-DTPA-BMA at the same concentration. A statistically significant difference was already observed for the lowest tested concentration of contrast media (0.005 mol/L), and that difference was even more prominent for higher concentrations of the tested solutions. In the highest tested concentration group (0.1 mol/L), histamine release from mast cells was five-fold higher for ionic than for non-ionic contrast media. This confirms the initial hypothesis, that non-ionic contrast media are characterized by a more favourable safety profile than ionic ones. The aforementioned observations may be explained by the differences in osmolality between the solutions of Gd-DTPA and Gd-DTPA-BMA. The ionic structure of Gd-DTPA entails that the osmolality of a 0.1 mol/L solution amounts to 600 mOsm/kg H2O, while the osmolality of a similarly concentrated solution of Gd-DTPA-BMA is only 380 mOsm/kg H2O. Thus solely the differences in osmolality may be responsible for the disparity in histamine release from mast cells after incubation with ionic and non-ionic contrast media.
The comparison of mast cell activation after stimulation with the tested pharmaceuticals and solutions of PEG 600 at identical osmolality yields very interesting conclusions. PEG 600, a medium molecular weight polyethylene glycol, is believed to be an osmotically active substance devoid of any biological activity and cellular toxicity. PEG 600 does not penetrate into the cells through the cell membrane and is characterized by chemical and physical properties congruent to those of the MRI contrast media. In the series of our experiments we failed to find a significant difference in histamine release under the influence of the Gd-DTPA-BMA and PEG 600 solutions at equal osmolalities. The above mentioned results lead to a conclusion that the activation of mast cells by the non-ionic MRI contrast media results exclusively from non-specific osmotic challenge. It appears that the blockade of free ionic groups in the DTPA molecule by BMA entirely suppressed the direct toxicity of the chemical complex to mast cells. This explains the clinical safety of non-ionic contrast media, which are unable to increase the osmolality of extracellular fluid to a level adequate to stimulate tissue mastocytes and lack the toxicity related to the presence of free ionic residues. These observations gain significance in light of the comparison between the histamine release from mast cells after stimulation by the Gd-DTPA and PEG 600 solutions at equal osmolalities.
It was elucidated that Gd-DTPA liberates higher amounts of histamine than PEG 600 solutions. Therefore a conclusion can be made that the mechanism of mast cell activation by Gd-DTPA engages osmotic challenge as well as another, additional factor. The presence of free ionic residues, which dissociate in aqueous environment, may disturb the water-electrolyte balance of the extracellular and intracellular fluid, leading to direct cellular toxicity of ionic contrast media. Another factor of potentially great importance for the mechanism of MRI contrast media toxicity is the process named “transmetallation” defined as a spontaneous substitution of gadolinium molecules in chelates by zinc or copper ions [32,33]. This results in the liberation of free gadolinium ions which are highly toxic and may trigger mast cell degranulation. However, ionic preparations of gadolinium chelates are more stable, which stands against the higher toxicity of Gd-DTPA in comparison to the non-ionic Gd-DTPA-BMA, observed in our results [34]. Finally, it should be claimed that the mechanism of mast cell activation by various types of contrast media remains unexplained and requires further investigations.
Conclusions
The observation that the non-ionic MRI contrast media activate mastocytes markedly more weakly than ionic preparations in vitro may support the higher clinical safety profile of the non-ionic MRI contrast agents, at least in the scope of anaphylactoid reactions. Our results draw an analogy between ionic and non-ionic preparations of the MRI contrast media and the classical iodine-based contrasts.
Footnotes
Source of support: Researches was financed from statutory fund of General and Experimental Pathology Department
References:
- 1.Brzezinska-Blaszczyk E, Zalewska A. Sensitivity of human cutaneous mast cells to anaphylactic and nonimmunological stimuli. Arch Immunol Ther Exp. 1997;45:55–59. [PubMed] [Google Scholar]
- 2.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–79. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 3.Brzezinska-Blaszczyk E, Forczmanski M, Pietrzak A. The action of tumor necrosis factor-alpha on rat mast cells. J Interferon Cytokine Res. 2000;20:377–82. doi: 10.1089/107999000312315. [DOI] [PubMed] [Google Scholar]
- 4.Szebeni J. Hypersensitivity reactions to radiocontrast media: the role of complement activation. Curr Allergy Asthma Rep. 2004;4:25–30. doi: 10.1007/s11882-004-0038-9. [DOI] [PubMed] [Google Scholar]
- 5.Morcos SK, Thomsen HS, Exley CM. Contrast media: interactions with other drugs and clinical tests. Eur Radiol. 2005;15:1463–68. doi: 10.1007/s00330-004-2600-1. [DOI] [PubMed] [Google Scholar]
- 6.Deftereos S, Giannopoulos G, Kossyvakis C, et al. Effect of radiographic contrast media on markers of complement activation and apoptosis in patients with chronic coronary artery disease undergoing coronary angiography. J Invasive Cardiol. 2009;21:473–77. [PubMed] [Google Scholar]
- 7.Murphy KJ, Brunberg JA, Cohan RH. Adverse reactions to gadolinium contrast media: a review of 36 cases. AJR Am J Roentgenol. 1996;167:847–49. doi: 10.2214/ajr.167.4.8819369. [DOI] [PubMed] [Google Scholar]
- 8.Runge VM. Safety of approved MR contrast media for intravenous injection. J Magn Reson Imaging. 2000;12:205–13. doi: 10.1002/1522-2586(200008)12:2<205::aid-jmri1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Jordan RM, Mintz RD. Fatal reaction to gadopentetate dimeglumine. AJR Am J Roentgenol. 1995;164:743–44. doi: 10.2214/ajr.164.3.7863905. [DOI] [PubMed] [Google Scholar]
- 10.Li A, Wong CS, Wong MK, et al. Acute adverse reactions to magnetic resonance contrast media – gadolinium chelates. Br J Radiol. 2006;79:368–71. doi: 10.1259/bjr/88469693. [DOI] [PubMed] [Google Scholar]
- 11.Kalogeromitros DC, Makris MP, Aggelides XS, et al. Anaphylaxis to gadobenate dimeglumine (Multihance): a case report. Int Arch Allergy Immunol. 2007;144:150–54. doi: 10.1159/000103227. [DOI] [PubMed] [Google Scholar]
- 12.Singer BD, Woodrick RS, Pedicano JB. Severe adverse drug reaction to gadobenate dimeglumine. Scientific World Journal. 2009;9:363–65. doi: 10.1100/tsw.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prince MR, Zhang H, Zou Z, et al. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol. 2011;196:W138–43. doi: 10.2214/AJR.10.4885. [DOI] [PubMed] [Google Scholar]
- 14.Mita H, Tadokoro K, Akiyama K. Detection of IgE antibody to a radiocontrast medium. Allergy. 1998;53:1133–40. doi: 10.1111/j.1398-9995.1998.tb03832.x. [DOI] [PubMed] [Google Scholar]
- 15.Laroche D, Namour F, Lefrancois C, et al. Anaphylactoid and anaphylactic reactions to iodinated contrast material. Allergy. 1999;54(Suppl.58):13–16. [PubMed] [Google Scholar]
- 16.Canter LM. Anaphylactoid reactions to radiocontrast media. Allergy Asthma Proc. 2005;26:199–203. [PubMed] [Google Scholar]
- 17.Szebeni J. Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology. 2005;216:106–21. doi: 10.1016/j.tox.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Freyria AM, Lasser EC. Primers of contact system activity in asthmatic patients and contrast material reactors. Invest Radiol. 1988;23(Suppl.1):S197–99. doi: 10.1097/00004424-198809001-00037. [DOI] [PubMed] [Google Scholar]
- 19.Lasser EC, Lamkin GE, Lyon S. A role for nitric oxide in X-ray contrast material toxicity. Acad Radiol. 1995;2:559–64. doi: 10.1016/s1076-6332(05)80115-1. [DOI] [PubMed] [Google Scholar]
- 20.Meth MJ, Maibach HI. Current understanding of contrast media reactions and implications for clinical management. Drug Saf. 2006;29:133–41. doi: 10.2165/00002018-200629020-00003. [DOI] [PubMed] [Google Scholar]
- 21.Niendorf HP, Dinger JC, Haustein J, et al. Tolerance data of Gd-DTPA: a review. Eur J Radiol. 1991;13:15–20. doi: 10.1016/0720-048x(91)90049-2. [DOI] [PubMed] [Google Scholar]
- 22.Nelson KL, Gifford LM, Lauber-Huber C, et al. Clinical safety of gadopentetate dimeglumine. Radiology. 1995;196:439–43. doi: 10.1148/radiology.196.2.7617858. [DOI] [PubMed] [Google Scholar]
- 23.Findlay SR, Dvorak AM, Kagey-Sobotka A, et al. Hyperosmolar triggering of histamine release from human basophils. J Clin Invest. 1981;67:1604–13. doi: 10.1172/JCI110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eggleston PA, Kagey-Sobotka A, Lichtenstein LM. A comparison of the osmotic activation of basophils and human lung mast cells. Am Rev Respir Dis. 1987;135:1043–48. doi: 10.1164/arrd.1987.135.5.1043. [DOI] [PubMed] [Google Scholar]
- 25.Runge VM, Dickey KM, Williams NM, et al. Local tissue toxicity in response to extravascular extravasation of magnetic resonance contrast media. Invest Radiol. 2002;37:393–98. doi: 10.1097/00004424-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Wolf GL, Mishkin MM, Roux SG, et al. Comparison of the rates of adverse drug reactions. Ionic contrast agents, ionic agents combined with steroids, and nonionic agents. Invest Radiol. 1991;26:404–10. doi: 10.1097/00004424-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep. 2005;5:28–31. doi: 10.1007/s11882-005-0051-7. [DOI] [PubMed] [Google Scholar]
- 28.Bush WH, Swanson DP. Acute reactions to intravascular contrast media: types, risk factors, recognition, and specific treatment. AJR Am J Roentgenol. 1991;157:1153–61. doi: 10.2214/ajr.157.6.1950858. [DOI] [PubMed] [Google Scholar]
- 29.Peachell PT, Morcos SK. Effect of radiographic contrast media on histamine release from human mast cells and basophils. Br J Radiol. 1998;71:24–30. doi: 10.1259/bjr.71.841.9534695. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez RM, Gueant JL, Gastin IA, et al. Comparison of effects of ioxaglate versus iomeprol on histamine and tryptase release in patients with ischemic cardiomyopathy. Am J Cardiol. 2001;88:185–88. doi: 10.1016/s0002-9149(01)01620-4. [DOI] [PubMed] [Google Scholar]
- 31.Simon MR, Reher RL, Long PM, et al. The nonionic radiocontrast medium iopromide does not release endothelin-1 or activate cardiac mast cells during coronary angiography. Int Arch Allergy Immunol. 2003;131:53–56. doi: 10.1159/000070435. [DOI] [PubMed] [Google Scholar]
- 32.Wedeking P, Kumar K, Tweedle MF. Dissociation of gadolinium chelates in mice: relationship to chemical characteristics. Magn Reson Imaging. 1992;10:641–48. doi: 10.1016/0730-725x(92)90016-s. [DOI] [PubMed] [Google Scholar]
- 33.Corot C, Idee JM, Hentsch AM, et al. Structure-activity relationship of macrocyclic and linear gadolinium chelates: investigation of transmetallation effect on the zinc-dependent metallopeptidase angiotensin-converting enzyme. J Magn Reson Imaging. 1998;8:695–702. doi: 10.1002/jmri.1880080328. [DOI] [PubMed] [Google Scholar]
- 34.Morcos SK. Extracellular gadolinium contrast agents: differences in stability. Eur J Radiol. 2008;66:175–79. doi: 10.1016/j.ejrad.2008.01.025. [DOI] [PubMed] [Google Scholar]