Summary
Background:
Spinal infection (discitis; spondylodiscitis) presents a wide spectrum of pathologies. The method of choice for spondylodiscitis imaging is magnetic resonance (MR). It provides detailed anatomical information, especially concerning epidural space and spinal cord. The main aim of this article is the description and evaluation of spondylodiscitis morphological variation visible in magnetic resonance imaging.
Material/Methods:
In this article we retrospectively analysed the patients diagnosed at the Department of Radiology of the Provincial Hospital No 2 in Rzeszów between October 2009 and October 2011. The subjects involved a group of five women aged 41–74 (mean 56.3 years) and eight men aged 46–69 (mean 61,3 years). All patients had spondylodiscitis symptoms. All patients underwent MRI examination before and after the contrast enhancement. In three patients additional CT examination was performed.
Results:
Following the MRI procedure all patients were diagnosed with typical symptoms of spondylodiscitis. It also revealed a number of pathologies resulting from morphological spondylodiscitis variation. Other pathologies found on the MR images of the study group patients involved epidural intra-canal spinal pathological masses causing spinal cord compression, lung abscess, pyothorax, paravertebral abscesses and epidural empyemas, abscess between adjacent vertebral bodies, abscesses beneath anterior longitudinal ligament, and iliopsoas muscle abscesses. In all cases a destruction of vertebral bodies with end plates loss restriction and cortical layer discontinuity was observed. Moreover, one person was diagnosed with pathological vertebral body fractures and liquefactive necrosis of the vertebral body.
Conclusions:
Spondylodiscitis manifests itself in a great number of morphological variations visible on the radiological images. Apart from ordinary features of vertebral bodies and discs, progressive spinal destruction is observed together with reactive bone changes and soft tissue infiltration. The latter leads to a number of complications e.g. abscesses or even fistulas and also to the formation of obstacles in radiological images. The knowledge of radiological images together with overall evaluation of clinical and laboratory features enables a proper diagnosis.
Keywords: spondylodiscitis, discitis, discovertebral junction
Background
Infective inflammation of the spine and/or intervertebral disc (discitis, spondylodiscitis) covers a wide spectrum of pathologies including intervertebral disc inflammation, intervertebral arthritis, intervertebral arthritis and discitis, pyogenic arthropathy of the articular surfaces and infections of the surrounding soft tissues and epidural space. The inflammation can be caused by a bacterial infection or pathological conditions of granulomatous, autoimmune, idiopathic or iatrogenic character. Spondylodiscitis incidence rate is growing due to increasing number of vulnerable patients (reactivation of latent tuberculosis infections, patients with immunodeficiency disorders or after surgeries). Spondylodiscitis diagnosis remains difficult and is often delayed or missed due to nonspecific clinical symptoms. Correct diagnosis requires meeting two main criteria: the presence of characteristic lesions in the spine and a pathogen isolation from blood or the infected place. Therefore, the radiological assessment is so important both for the diagnosis of spon-dylodiscitis and for further planning and monitoring its treatment. The method of choice for spondylodiscitis imaging is the magnetic resonance imaging (MRI). It provides detailed anatomical information especially concerning epidural space and spinal cord [1,2].
The aim of the study is a description and evaluation of spondylodiscitis morphological variations observed on the MR images.
Material and Methods
The study is based on a retrospective analysis of patients diagnosed at the Department of Radiology of Provincial Hospital No. 2. of St. Queen Jadwiga in Rzeszów between October 2009 and October 2011. The clinical material comprises 13 patients, including five women, aged 41–74 (mean 56.3 years) and 8 men aged 46–69 (mean 61.3 years). All patients experienced clinical symptoms suggesting inflammation of the spine and/or intervertebral disc. They underwent magnetic resonance imaging before and after the administration of a contrast agent. In three patients additional CT examination was performed.
Results
MR images revealed in all examined patients spondylodiscitis features within the vertebral bodies and/or intervertebral discs: high signal intensity of vertebral body marrow, and/or intervertebral discs on T2-weighted images and signal enhancement after contrast administration on T1-weighted images. In 7 patients contrast administration resulted in enhanced visibility of inter-canal pathological masses compressing the thecal sac and the spinal cord (Figures 1, 2).
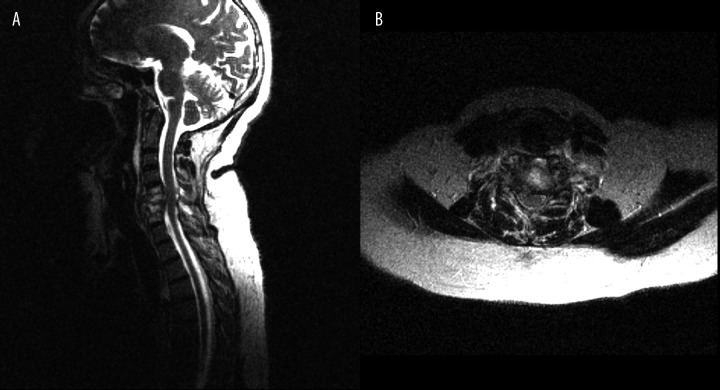
Figure 1.
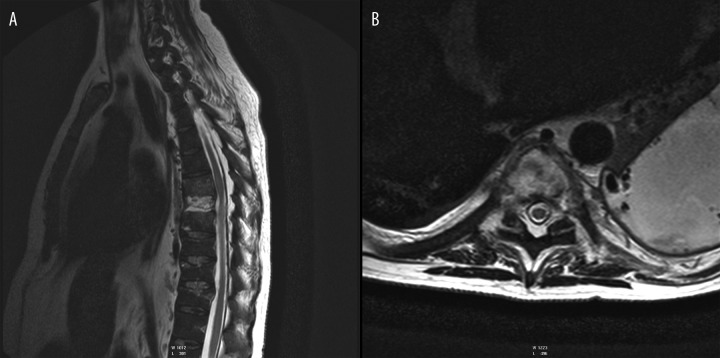
(A) T2-weighted image, sagittal plane. (B) T2-weighted image, transverse plane. C5–C7 spondylodiscitis, high signal intensity of the bone marrow of vertebral bodies and high signal intensity of intervertebral discs with destruction of C6 vertebral body, spinal cord compression.
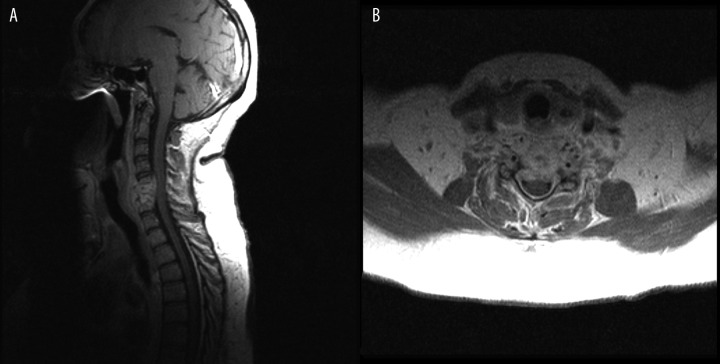
Figure 2.
(A) T1-weighted + CM image, sagittal plane. (B) T1-weighted + CM image, transverse plane. Contrast enhancement of paravertebral and epidural intra-canal spinal pathological mass.
The lesions found within the spine were accompanied by other pathological changes.
In one patient an abscess-like ring-enhancement lesion was detected in the apex of the left lung in a form of a round fluid filled cavity (Figure 3).
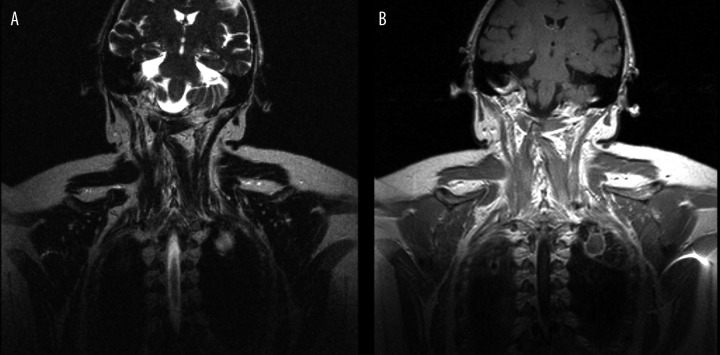
Figure 3.
(A) T2-weighted image, frontal plane. (B) T1-weighted + CM image, frontal plane. Cavity in the apex of the left lung.
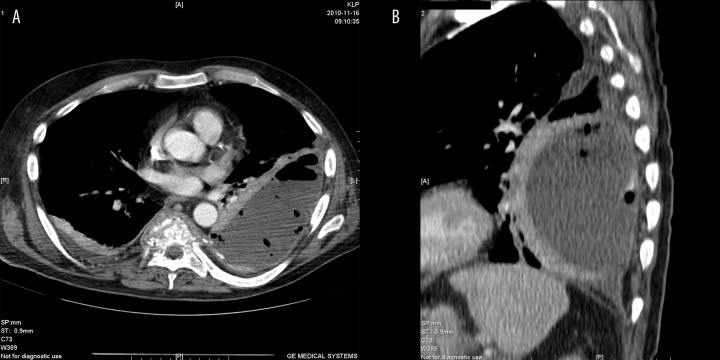
In three patients some fluid of pleural empyema character was noticed at the level of spinal inflammatory changes. In two cases it was unilateral (Figures 4, 5), and the third patient was diagnosed with bilateral pleural empyema (Figure 6).
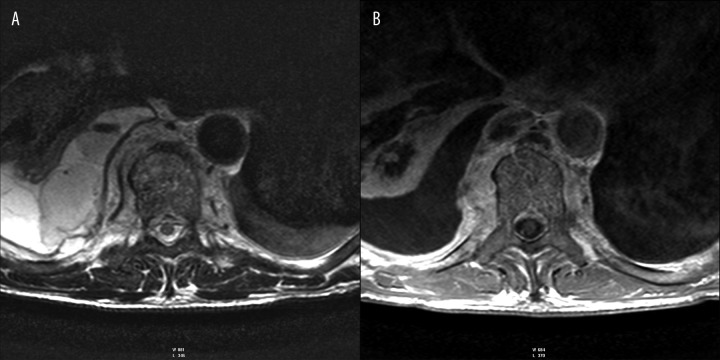
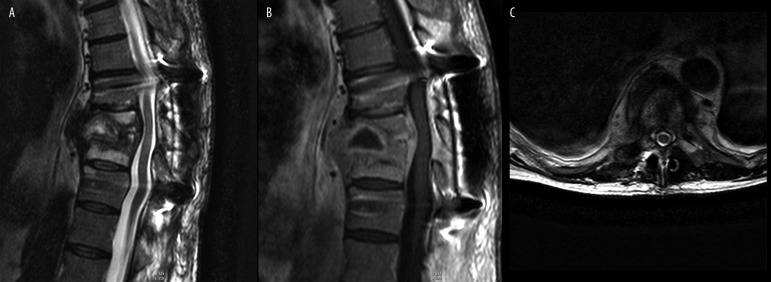
Figure 4.
(A) T2-weighted image, transverse plane. (B) T1-weighted + CM image, transverse plane. Right pyothorax. Contrast enhancement of paravertebral pathological mass.
Figure 5.
CT + CM. (A) transverse plane. (B) MPR reconstruction. Left pyothorax.
Figure 6.
(A) T1-weighted + CM image, sagittal plane. (B) T1-weighted + CM image, transverse plane. (C) T1-weighted + CM image, frontal plane. Contrast enhancement of paravertebral and epidural intra-canal pathological mass. Bilateral pyothorax.
The images from three patients showed fluid filled areas around spine and epidural space corresponding to a par-avertebral abscess (Figure 7) and epidural empyemas (Figure 8).
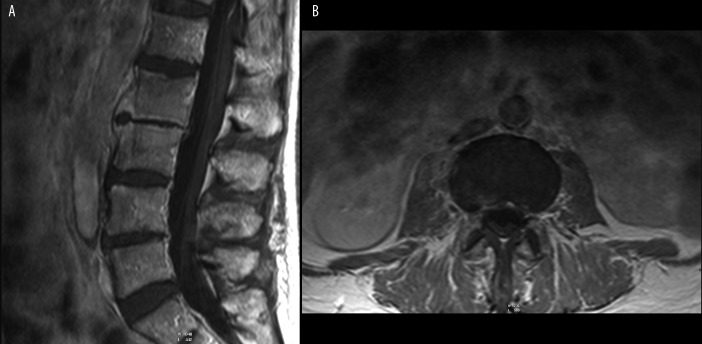
Figure 7.
(A) T1-weighted + CM image, sagittal plane. (B) T1-weighted + CM image, transverse plane. L2-L3 spondylodiscitis. Prevertebral abscess.
Figure 8.
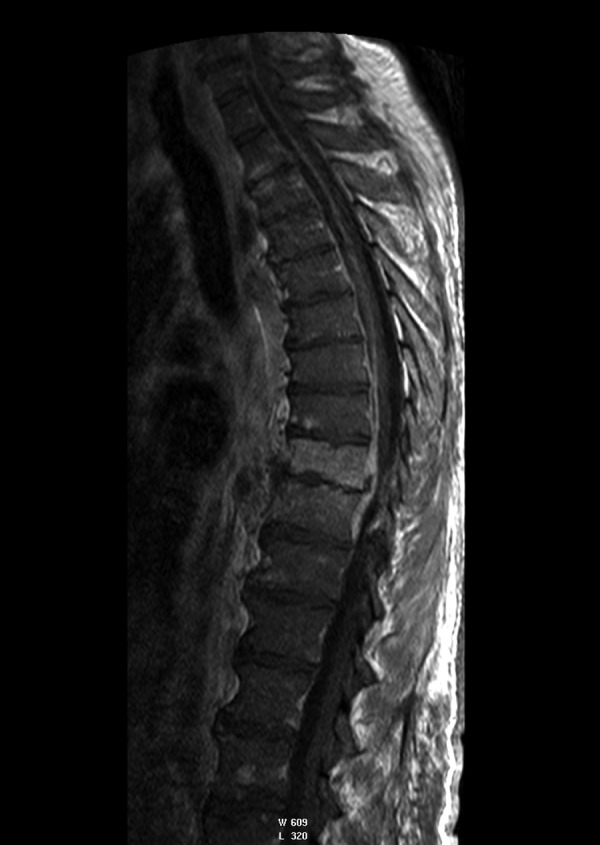
T1-weighted + CM image, sagittal plane. Contrast enhancement of paravertebral pathological mass. Epidural empyema in the cervical and thoracic part of spinal.
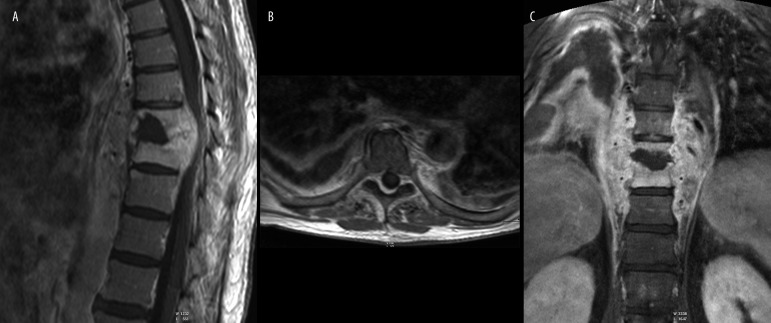
In one person from the study group a limited, ring-enhancement fluid lesion between the vertebral bodies – an intervertebral abscess (Figure 9) was diagnosed.
Figure 9.
(A) T2-weighted image, sagittal plane. (B) T1-weighted + CM image, sagittal plane. (C) T2-weighted image, transverse plane. Follow-up after surgery. Spinal cord oedema, abscess between Th8 and Th9.
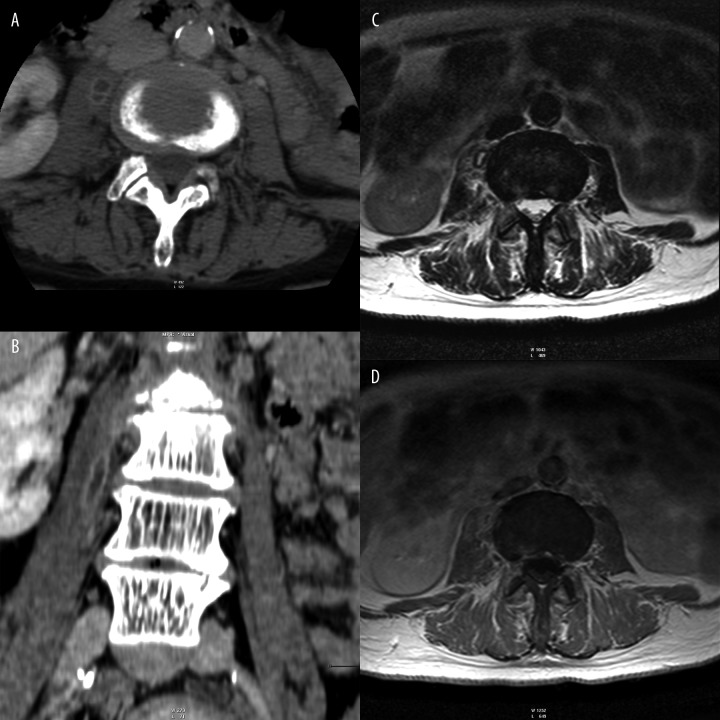
In one patient fluid streaks visible at the level of inflammatory spinal changes turned out to be abscesses under the anterior longitudinal ligament, and in another patient - iliopsoas muscle abscesses (Figure 10).
Figure 10.
CT – (A) transverse plane. (B) MPR reconstruction. (C) T2-weighted image, transverse plane. (D) T1-weighted + CM image, transverse plane. Right iliopsoas muscle abscesses.
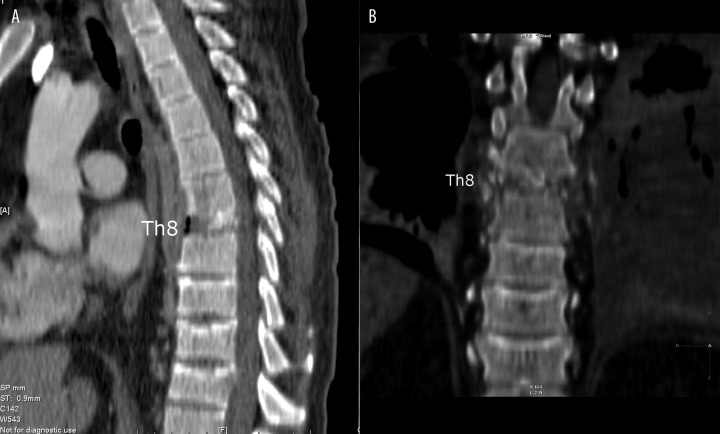
All patients were diagnosed with various degrees of destruction of the affected vertebral bodies and the loss of end plate reduction and discontinuity of the cortical layer. Moreover, pathological fractures of vertebral bodies (Figure 11) and liquefactive necrosis within one vertebral body were found in one of the patients (Figure 12).
Figure 11.
CT – (A) (B) MPR reconstructions. Subtotal destruction of Th8 vertebral body – vertebra plana. Compressive fractures of Th10 – Th12 vertebral bodies.
Figure 12.
(A) T2-weighted image, sagittal plane. (B) T2-weighted image, transverse plane. Th7–Th9 spondylodiscitis, high signal intensity of the bone marrow of Th7 and Th9 vertebral bodies and high signal of intervertebral discs, high signal – liquefactive necrosis – of subtotally destructed Th8 vertebral body – spinal cord compression.
The lesions were located within the cervical spine (3 patients), the thoracic spine (5 patients) or the lumbar spine (2 patients). In one person the lesions affected both cervical and thoracic spine.
Three patients underwent conservative treatment, and nine underwent surgical treatment (Figure 13).
Figure 13.
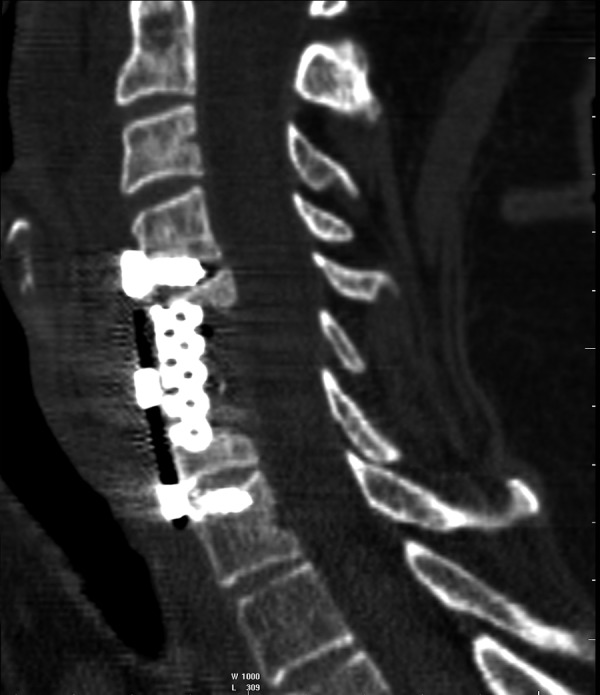
Follow-up CT after surgery. MPR reconstruction.
Discussion
Spondylodiscitis (SD, discitis) is a septic inflammation of the spine and/or intervertebral disc, involving the intervertebral space, adjacent vertebral bodies and often penetrating into soft paravertebral tissues. Various definitions are used in the subject literature, depending on the extent of inflammatory infiltration – when the intervertebral disc is affected it is called discitis, and when the inflammatory infiltration affects also the adjacent vertebral bodies it is defined as spondylodiscitis or osteomyelitis. The earliest references to the disease date back to ancient times, while the first description of pyogenic osteomyelitis was published by the French physician Lannelongue in 1897 [1,3–5].
The disease incidence rate is estimated at 0.4–2 cases per 100 000 per year. Spondylodiscitis distribution in the population has a bimodal character with two peaks: for patients below 20 and between 50 and 70 years. The condition shows a male preponderance, with a male to female ratio of 1.5–2:1. Men are also a larger group among our patients (approximately 63%).
The incidence rate increases with age. In people over 70 it is as high as about 6 cases per 100 000 per year. Due to negative demographic trends in the population we can expect more patients diagnosed with this disorder. Mortality rate ranges from 18% to 31% [1,2,6].
Infections of the spine and/or intervertebral disc can be classified based on the microorganism responsible for their development. A broad term of spondylodiscitis includes inflammation of the spine and/or intervertebral disc caused by bacterial infections, both specific and nonspecific. The most important etiological factors of non-specific bacterial infections of the spine and intervertebral disc include: Staphylococcus aureus accounting for more than half of non-tuberculous cases (20–84%), Enterobacteriaceae, Klebsiella, Staphylococcus epidermidis, Streptococcus viridans, Escherichia coli, Pseudomonas aeruginosa, Pneumococci, Clostridium perfringens, Proteus mirabilis. A pathogen most commonly associated with postoperative inflammation of the intervertebral disc is Propionibacterium acnes. The most important etiological factors of specific bacterial infections of the spine and intervertebral disc include: Mycobacterium tuberculosis, Mycobacterium leprae, Brucella bacteria, Salmonella. Tuberculosis is the leading cause of spondylodiscitis worldwide and it accounts for 9–46% cases in developed countries. It usually affects patients under 40.
In immunocompromised or immunosuppressed patients, or patients treated in intensive care units the infections of the spine and/or intervertebral discs can be caused by viruses, fungi or parasites [1–3,6,7].
Predisposing factors for spondylodiscitis usually involve diabetes, chronic renal failure, AIDS, immunosuppression, chemotherapy, advanced age, alcoholism, intravenous injections, cancer, rheumatic diseases, cirrhosis, and previous spinal surgery. Less common causes of the spine and intervertebral disc inflammation include direct penetrating trauma or a gunshot wound [1,2,6].
The spine and intervertebral disc infection can spread via four main routes. The predominant route is the circulation system, paravertebral arteries and veins (Batson’s paravertebral venous plexuses), complications of general infections in a form of sepsis, infections of urogenital system, pelvis, and respiratory tract, gastrointestinal diseases, skin and soft tissue diseases, and even tonsillectomy. Lymphatic route of the infection spread is less common. The infection can also spread by direct infiltration from the adjacent infectious foci such as paravertebral abscesses, infected aortic graft, ruptured esophagus, psoas muscle abscess, epidural abscess, retropharyngeal abscess. The last route is a direct one, being the result of a penetrating trauma or infection during surgery and minimally invasive endovascular procedures [1,2,6,8].
In adults, inflammatory lesions initially begin with a marginal part of the vertebral body or under the margin plate and then spread to the intervertebral disc. The inflammation results in a disc destruction, progressive destruction of adjacent vertebral bodies, narrowing of the intervertebral space and intervertebral foramen. Further consequences may involve compression fractures leading to spinal deformity and instability as well as a risk of damage to the adjacent neural structures and the spinal cord compression. Uncontrolled infection can spread to the paravertebral space and further to anterior prevertebral space, lumbar muscles and into the lumen of the spinal canal, forming an epidural abscess. Sometimes the inflammatory changes within the intervertebral disc and vertebral body are accompanied by paravertebral abscess formation [1,3,9].
Axial skeleton is a frequent site of hematogenous and pyogenic infections, accounting for about 2–7% of cases. The inflammatory process is usually localized within the lumbar spine, and less frequently within the thoracic and cervical spine, the proportion is 58%, 30%, 11%, respectively. In rare cases, the spine and/or intervertebral disc infection has a multifocal character. Extension to the adjacent vertebrae is usually the result of the infection spread along the anterior longitudinal ligament [1–3].
Development of the inflammation focus causes remodeling and destruction of bony structures and formation of erosions. At the initial stage the erosion affects the subchondral bone layer. With time it spreads and results in destruction of the entire vertebral body. Chronic inflammatory process may lead to several complications. The most often one is the formation of paravertebral abscesses within the soft tissues. When the abscesses are located within the dorsal spine they can perforate into the parietal pleura and cause pleural empyema. If not properly treated the infection can spread beneath the anterior longitudinal ligament along a few vertebral segments. When the inflammatory process spreads within the ligament, epidural empyema is formed. Untreated empyema may in turn cause inflammation of the spinal cord and/or meningitis. A rare complication, usually associated with tubercular infection, is the formation of fistulas to the skin surface or to the abdominal cavity [7,10,11]. The inflammatory process is accompanied by a progression of pathological lesions.
Clinical symptoms are nonspecific and involve mainly severe back pain, fever and elevated values of inflammatory parameters (CRP, BSR, leukocytes). Pain is usually accompanied by spontaneous mobility restriction of the affected spinal section and distinct muscular defense, and sometimes forced body position. In some patients the pain may radiate to a specific dermatome, causing referred pain experienced e.g. as abdominal pain. This significantly hampers diagnosis, and can even lead to unjustified laparatomy. Inter-canal infiltration of the pathological mass, causing thecal sac and spinal cord compression, may result in neurological complications associated with sensory-motor deficits (paraplegia, sensory deficits, paralysis). Untreated infection can cause significant deformity of the spine (kyphoses, tubercular kyphoses). If the infection of the cervical spine is accompanied by retropharyngeal abscess, a patient may have difficulty with swallowing [1–3,6,9].
Results of laboratory tests depend on a etiotropic factor. In most cases leukocytosis and increased CRP and ESR values are observed [2].
Collecting the material for microbiological examination directly from the pathologically changed spot is most valuable diagnostic procedure. A biopsy is usually performed in patients with negative blood culture, when there is no response to empirical treatment and for infections of fungal or mycobacterial etiology [1,3].
Sensitivity and specificity of classical radiography is low. Usually early stages of the disease are not manifested by pathological changes, and a negative result does not rule out infectious lesions within the discs. After about 2–3 weeks of the inflammatory process a narrowing of the intervertebral space and blurred outlines of the vertebral bodies surface may appear. Prolonged inflammatory process results in lowering of the vertebral body, loss of the end plate margin, and osteolysis of the vertebral body part adjacent to the intervertebral disc. Later stages are characterized by the appearance of reactive changes such as subchondral sclerosis of adjacent vertebrae, osteophyte formation, kyphotic deformity and bone ankylosis [1–3,12,13].
A useful, though not very specific examination is a bone scintigraphy with the use of different tracers, particularly technetium (Tc-99m). Inflammatory changes in the early stages of the disease are visible as spots of increased radi-otracer accumulation. This test confirms a high uptake of the radiotracer in the period of bone remodeling and repair until complete healing, and sometimes also some time after the infection. 67-galium citrate is a useful compound for detecting infection in the surrounding soft tissues. It is less sensitive to the bone remodeling and provides better results in case of an active inflammation. It should be remembered that degenerative changes can produce false positive results. Promising results and very high sensitivity are offered by positron emission tomography using 18 fluorodeoxyglucose (FDG-PET). It allows to effectively distinguish infection from degenerative changes in the spine, even if the MRI did not give unambiguous results [2,3,14].
Computed tomography plays a minor role in the diagnosis of the spine and/or intervertebral disc inflammation. This examination, especially if performed with multi-row apparatus and using volume and multiplanar rendering, allows to show the disease progress within the spinal bone structures, early destruction and subsequent sequestration of the margin plate, cortical layer erosions, sclerosis, degenerative changes of the intervertebral disc and edema of paravertebral soft tissues. However, this technique falls short of magnetic resonance imaging of the nervous structures and the accompanying changes in the surrounding soft tissues, especially abscesses. Computed tomography is now frequently used for targeted biopsy of the spine [1,2].
Magnetic resonance (MR) of the affected area of the spine is the method of choice in the diagnosis of the spine and/or intervertebral disc inflammation. Its sensitivity is about 96%, specificity 93% and accuracy 94%. A correct diagnosis is often based on detecting the presence of spondylodiscitis specific symptoms in MRI scans. MR protocol may include a number of sequences, namely spin echo sequences (T1 and T2-weighted), gradient echo sequences (T2-weighted), inversion recovery, especially STIR and spin echo sequences using contrast enhancement (with and without fat signal suppression). Inflammation of the spine leads to increased hydrogen density (protons), which makes the STIR method suitable for obtaining high contrast between the yellow bone marrow and pathological lesions. Fat suppression sequences after administration of Gd-DTPA are the technique of choice for the correct evaluation of paraspinal abscesses. Abscesses in T1-weighted images with fat saturation after contrast administration show strong ring enhancement with central, non-enhanced fluid space. MRI allows a precise determination of the area of inflammatory infiltration within the spongy substance and disintegration of the bone cortical layer. The presence of inflammatory lesions causes a significant signal reduction in T1-weighted images. Specific signs of infection in the T1-weighted images are the areas of low signal within the vertebral body with its enhancement after contrast administration, loss of end plate margin, disruption of cortical layer continuity and destruction of its edge. In the T2-weighted images the inflammatory tissue generates a much stronger signal. In case of intervertebral disc inflammation the discs are seen as strongly hyperintense in T2-weighted images, so called hot discs. However, it should be remembered that in spine inflammation of fungal etiology there is often no signal increase in T2-weighted images and the signal enhancement after contrast administration may be low or even undetectable [3,6,7,15,16].
The diagnosis of the spine and/or intervertebral disc inflammation is not easy, especially if the infection process is in an early stage of development and it is set in an unusual location. Contrast enhancement significantly increases the specificity and accuracy of an examination, both in case of the intervertebral disc pathology and vertebrae and surrounding soft tissue lesions. Contrast enhancement of the affected area is the earliest pathognomonic symptom of acute inflammation of the spine and/or intervertebral disc. Prolonged inflammatory process features progressive destruction of a vertebra and the loss of cortical layer continuity in the adjacent end plates as well as soft tissue infiltration. There appear reactive bone changes such as osteosclerosis, loss of vertebral body height, kyphosis, or ankylosis [2].
Interpretative difficulties may be encountered if a patient experiences also degenerative changes or, less frequently, neoplasms of myeloma or chordoma type. In patients with degenerative disease the disc in T2-weighted images is usually hypointense due to the dehydration process, in contrast to infectious changes, where the intervertebral discs are highly hyperintense (hot disc). Differentiation between infection and cancer in early spondylodiscitis may be difficult, as the intervertebral disc is not affected yet or, in rare cases, the rear part of the vertebra is affected. In these cases, a thorough clinical comparison of a patient’s history, laboratory tests and imaging results is necessary [7].
Magnetic resonance imaging usually allows to make a final diagnosis of spondylodiscitis. However, multiple factors make the changes in infectious inflammation of a vertebra and/or intervertebral disc not always unambiguous, and their morphological image is highly variable. Depending on diverse etiology the spondylodiscitis images may be very different. For example in tuberculous spondylitis, where in contrast to purulent inflammation, an intervertebral disc remains intact between the affected vertebral bodies. MR image variations are supported by the process evolution and the tendency of the inflammatory focus to spread. This causes both bone remodeling and destruction of bone structures and quite often soft tissue complications. In our group of 13 patients we found degenerative changes and compression fractures of vertebral bodies, lytic lesions, as well as liquefactive necrosis of vertebral bodies, blurring and destruction of end plates, reduction of vertebral height, and vacuum phenomenon. The spread of inflammatory changes caused further morphological differentiation of MR images.
In our group of 13 patients we observed lung abscess, pleural empyema, atelectatic changes in the lung parenchyma, and intra-canal epidural and paravertebral pathological masses with or without compression of the spinal cord. We also diagnosed an abscess near posterior longitudinal ligament, intervertebral abscess, and iliopsoas muscle abscess. Given the nonspecific clinical symptoms and quite common occurrence of back pains in the general population, the diagnosis of osteomyelitis is often delayed. MRI is performed in various stages of the inflammatory process, and thus the morphological variability of the evolving inflammation image makes the diagnosis even more difficult. The diagnosis of a vertebra and/or intervertebral disc inflammation is particularly difficult in patients with concomitant degenerative diseases or spinal cancers. In neoplastic processes affecting more than one vertebra an important clue is unaffected intervertebral disc.
The treatment aim is to eradicate the infection, restore and preserve the structure and function of the spine, as well as pain management. Appropriate treatment gradually reduces the edema within adjacent vertebral bodies and the mass in the paraspinal soft tissues. Conservative treatment includes antimicrobial drug therapy and drug-free treatment such as immobilisation and physiotherapy. Surgical treatment is introduced in case of symptoms suggesting compression of the nerve structures, spinal instability caused by bone destruction, severe kyphosis, and failure of conservative treatment. Sometimes it is also recommended in case of persistent and incessant pain [1,17].
Conclusions
Septic inflammation of the spine and/or intervertebral disc is a fairly rare disease. Diagnosis of spondylodiscitis is difficult due to infrequent occurrence of the spine and/or intervertebral disc inflammation, nonspecific clinical symptoms, insidious course of the disease, and high incidence of back pain in the general population. The method of choice in spondylodiscitis diagnostics is the magnetic resonance imaging. Spondylodiscitis provides highly variable radiological images. Apart from characteristic lesions within the vertebral body and / or intervertebral disc there also occur a progressive destruction of the spine, reactive bone changes and soft tissue infiltration. Soft tissue infiltration leads to a number of complications such as abscesses or even fistulas that make the interpretation of the radiological image more difficult. Knowledge of radiological images and comprehensive evaluation of clinical and laboratory results facilitate the correct diagnosis.
References:
- 1.Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65(3):11–24. doi: 10.1093/jac/dkq303. [DOI] [PubMed] [Google Scholar]
- 2.Tali ET. Spinal infections. Eur J Radiol. 2004;50(2):120–33. doi: 10.1016/j.ejrad.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Łukjanowicz M, Bohatyrewicz A, Brzosko M. Zapalenie kręgosłupa wywołane przez drożdże z rodzaju Candida – przegląd piśmiennictwa. Ann Acad Med Stetin. 2007;53(3):128–33. [in Polish] [PubMed] [Google Scholar]
- 4.Hennequin C, Bouree P, Hiesse C, et al. Spondylodiskitis due to Candida albicans: report of two patients who were successfully treated with fluconazole and review of the literature. Clin Infect Dis. 1996;23(1):176–78. doi: 10.1093/clinids/23.1.176. [DOI] [PubMed] [Google Scholar]
- 5.Miller DJ, Mejicano GC. Vertebral osteomyelitis due to Candida species: case report and literature review. Clin Infect Dis. 2001;33(4):523–30. doi: 10.1086/322634. [DOI] [PubMed] [Google Scholar]
- 6.Titlic M, Josipovic-Jelic Z. Spondylodiscitis. Bratist Lek Listy. 2008;109(8):345–47. [PubMed] [Google Scholar]
- 7.Longo M, Granata F, Gaeta M, et al. Contrast-enhanced MR imaging with fat suppression in adult-onset septic spondylodiscitis. Eur Radiol. 2003;13(3):626–37. doi: 10.1007/s00330-002-1411-5. [DOI] [PubMed] [Google Scholar]
- 8.Bruzzese V. Spondylodiscitis as the only clinical manifestation of the onset of psoriatic spondyloarthritis. Reumatismo. 2011;63(1):38–43. doi: 10.4081/reumatismo.2011.38. [DOI] [PubMed] [Google Scholar]
- 9.Grammatico L, Besnier JM. Infection spondylodiscitis. Rev Prat. 2007;57(9):970–78. [PubMed] [Google Scholar]
- 10.Jinkins JR, Bazan C, III, Xiong L. MR of disc protrusion engendered by infectious spondylitis. J Comput Assist Tomogr. 1996;20(5):715–18. doi: 10.1097/00004728-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Reijnierse M, Dijkmans BA, Hansen B, et al. Neurologic dysfunction in patients with rheumatoid arthritis of the cervical spine. Predictive value of clinical, radiographic and MR imaging parameters. Eur Radiol. 2001;11(3):467–73. doi: 10.1007/s003300000557. [DOI] [PubMed] [Google Scholar]
- 12.Chelsom J, Solberg CO. Vertebral osteomyelitis at a Norwegian university hospital 1987–97: clinical features, laboratory findings and outcome. Scand J Infect Dis. 1998;30(2):147–51. doi: 10.1080/003655498750003537. [DOI] [PubMed] [Google Scholar]
- 13.Jevtic V. Vertebral infection. Eur Radiol. 2004;14(3):43–52. doi: 10.1007/s00330-003-2046-x. [DOI] [PubMed] [Google Scholar]
- 14.Gemmel F, Dumarey N, Palestro CJ. Radionuclide imaging of spinal infections. Eur J Nucl Med Mol Imaging. 2006;33(10):1226–37. doi: 10.1007/s00259-006-0098-2. [DOI] [PubMed] [Google Scholar]
- 15.Maiuri F, Iaconetta G, Gallicchio B, et al. Sondylodiscitis. Clinical and magnetic resonance diagnosis. Spine. 1997;22(15):1741–46. doi: 10.1097/00007632-199708010-00012. [DOI] [PubMed] [Google Scholar]
- 16.Ledermann HP, Schweitzer ME, Morrison WB, et al. MR imaging findings in spinal infections: rules or myths? Radiology. 2003;228(2):506–14. doi: 10.1148/radiol.2282020752. [DOI] [PubMed] [Google Scholar]
- 17.Hadjipavlou AG, Katonis PK, Gaitanis IN, et al. Percutaneous transpedicular discectomy and drainage in pyogenic spondylodiscitis. Eur Spine J. 2004;13(8):707–13. doi: 10.1007/s00586-004-0699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]