Summary
Background:
The aim of this study was to determine the reorganization of the language areas in patients with tumors located near speech centers using functional magnetic resonance imaging (fMRI).
Material/Methods:
fMRI was performed prior to the surgical treatment of 11 right-handed patients with tumors located close to the Broca’s or Wernicke’s areas of the left hemisphere. The analysis included a record of the activity in four regions of interest (ROIs): Broca’s and Wernicke’s areas, and their anatomic homologues in the right hemisphere. For each patient a regional lateralization index was calculated separately for Broca’s area versus its right-hemisphere homolog and Wernicke’s area versus its right-hemisphere homolog. The results were correlated with the histopathological type of the tumor and its size.
Results:
Our fMRI examinations showed activation of the Broca’s area in the right hemisphere in 3/4 cases of low grade gliomas (LGG) localized in the left frontal lobe. In one case of the high grade glioma (HGG) only the left hemisphere Broca’s area was activated (LI=1). Activation in Wernicke’s area in both hemispheres was obtained irrespective of the size and histological type of the tumor.
All tumors localized in the left temporal lobe were HGG. We obtained activation only in the right hemisphere Wernicke’s area in 4/5 of the cases. In 4/5 of the cases activation in Broca’s area was present- in 2 cases in the left hemisphere, in 1 case in the right hemisphere and in 1 case bilateral.
Conclusions:
The presence of a neoplastic lesion in close topographic relationship to language areas induces their functional reorganization.
fMRI is an useful method for determination of language areas localization in pre-operative planning. HGG tumors localized near Wernicke’s area lead to transfer its function to the healthy hemisphere and/or to decreased activity in the affected hemisphere.
Keywords: neuroplasticity, language lateralization, functional magnetic resonance imaging, Broca, Wernicke
Background
Brain tumors localized in the area of speech centers, of clinical importance, situated respectively – in the back of the inferior frontal gyrus and posterior parts of the superior temporal gyrus, are challenge for decision making in neurosurgery. Surgery to remove lesions in this area can lead to speech disorders in patients.
Several brain mapping techniques are used to localize language centers. However, most of them are invasive i.e. Wada test, [1], intraoperative stimulation of cortical centers (ICSM) [2] or less accessible, i.e. positron emission tomography (PET) [3], magnetoencephalography (MEG) [4]. This makes the functional functional magnetic resonance (fMRI) because of its availability and non-invasive nature a potentially widely useful tool for determining language lateralization and localization of speech centers. fMRI data has a quite satisfactory time resolution and quite precise association with the anatomical structures of the brain. In addition, tests may be repeated as many times as it is needed, which allows to apply the required number of paradigms [5,6]. fMRI study can also serve as a tool for prediction of occurrence and resolution of postoperative aphasia in patients, due to the determination of the activity in areas adjacent to the tumor and homologous areas in the opposite hemisphere responsible for language functions [7–10]. fMRI study may be able to assess the plastic abilities of the brain.
The aim of our study was to determine the effect of the presence of proliferative processes in the area of speech centers on their functional reorganization.
Material and Methods
The study protocol was approved by the Commission of Bioethics, Medical University of Lodz (Decision no. RNN/123/09/KE).
11 patients with tumors around eloquate areas were qualified into the study – 8 men and 3 women, aged from 22 to 64 years old, median age was 47 years old. Patients were treated at the Department of Neurosurgery, University Hospital No. 1 in Lodz in 2010–2012.
The patients were qualified into the study according to the following criteria: 1) tumor located in Broca’s area or Wernicke’s area of the left hemisphere 2) right handedness of patients 3) neuropsychological status of patients, allowing for planned, standardized procedures.
All patients were right-handed, as determined by the inventory test. Patients were divided into two groups according to the location of the tumor in the left hemisphere of the brain. One group consisted of 6 patients with a tumor located in the frontal lobe and the other other group consisted of 5 patients with tumor located in the temporal lobe or on the border. Characteristics of patients is included in Table 1.
Table 1.
Characterization of the patients group including gender, age and tumor localization, size, histopathological type of the tumor.
| Patient | Gender | Age [years] | Tumor localization in the left hemisphere of the brain | Tumor size V [cm3] | Histopathological type of the tumor | |
|---|---|---|---|---|---|---|
| F.A. | F | 30 | Frontal lobe | 54.65 | Glioblastoma | HGG |
| B.T. | M | 64 | Frontal lobe | 31.86 | Carcinoma nonmicrocellulare | HGG |
| S.K. | M | 22 | Frontal lobe | 10.95 | Astrocytoma pilocyticum | LGG |
| G.J. | M | 57 | Frontal lobe | 22.85 | Oligoastrocytoma | LGG |
| Si.A. | F | 32 | Frontal lobe | 16.63 | Astrocytoma fibrillare | LGG |
| Ma.K. | F | 31 | Frontal lobe | 21.89 | Astrocytoma fibrillare | LGG |
| N.S. | M | 47 | Temporal-occipital border | 21.77 | Glioblastoma | HGG |
| K.S. | M | 55 | Temporal-parietal-occipital border | 37.42 | Glioblastoma | HGG |
| K.Z. | M | 57 | Temporal lobe | 39.48 | Gliosarcoma | HGG |
| St.A. | M | 49 | Temporal lobe | 24.00 | Glioblastoma | HGG |
| Mi.K. | M | 44 | Temporal lobe | 24.68 | Glioblastoma | HGG |
F – female, M – male; HGG – high grade glioma; LGG – low grade glioma.
Total or subtotal resection of the tumors was performed on all patients. During the same hospitalization speech disorders were assessed before and after surgery by clinical neuropsychologist.
All postsurgical tumors were examined histologically. 4 of all tumors were classified as low-grade tumors (low-grade glioma, LGG) – all belonged to primary neuroepithelial tumors, 7 tumors were classified as high grade (high-grade glioma, HGG) – 6 primary neuroepithelial tumors and 1 metastatic tumor of non-small cell lung cancer.
In patients with tumors located close to the Broca’s area, 4 were classified as LGG, 2 as HGG. All Wernicke’s area tumors were classified as HGG.
Prior to the surgical treatment, an fMRI was performed in all patients using a 1.5 T magnetic Resonance scanner (Siemens, Avanto). Morphological, three-dimensional T1-weighted sequences were obtained according to the following protocol: FOV=256×256 mm, matrix =512×512, TR=8.8 ms, TE=4.8 ms, TA=5′07. Each volume acquired contained 160 slices of 1 mm thick. The functional examination included echoplanar imaging (EPI) sequences: TR=3000 ms, TE=50 ms, FOV=1680×1680 mm, matrix 64×64, TA=5′11, thirty-eight 3 mm thick slices.
The analysis of the data was conducted using the statistical program SPM 2, running in MATLAB (http://www.fil.ion.ucl.ac.uk/spm/). Data were analyzed for p=0.05
All patients were informed about the exact course of the study prior 30 minutes before it starts. The used paradigm was word generation (WG) [11]. The study was divided into five blocks, each containing 10 acquisitions. Pattern of stimulation proceeded in ABABABABAB block diagram, where A is the rest (control), and B represents stimuli. Two different methods of stimulation were used. Three patients respectively chose words in the reference category, e.g. furniture. The other eight patients were ordered to pronounce in periods of stimulation, non-repetitive names of male and female. The choice of paradigm was dependent on intellectual abilities of patients. Creation of names was considered an easier task in comparison with the generation of nouns of different categories.
Using radiological anatomy atlases four regions of interest (ROIs) were designated:
Broca’s area in the left inferior frontal gyrus (Brodmann area, BA 44, BA 45),
the area anatomically homologous to the a region in the right hemisphere,
Wernicke’s area in the left superior temporal gyrus (BA 22) and adjacent: superior temporal sulcus, middle temporal gyrus (BA 21), angular gyrus (BA 39) and supramarginal gyrus (BA 40),
the area anatomically homologous to the region c located in the right hemisphere [12].
Based on data from the literature it is known that it is possible to calculate the lateralization index (LI) on the basis of differences in levels of activation or activation volume between left and right hemispheres [13,14]. The most frequently used variables describing the among of the activation is the number of activated voxels. Lateralization index can be calculated for the whole hemisphere, or for individual ROIs. In our study, lateralization index was determined based on the number of activated voxels in the cluster for the four defined above areas responsible for language functions. Such limitation enabled to avoid analysis of non-specific activity or activity of non-linguistic, activated as a consequence of sensory or motor stimuli [13]. Lateralization index was calculated according to the formula: LI=(L–R)/(L+R), separately for the Broca’s area and its right-sided homologous area and Wernicke’s area and its right-sided homologous area [12]. The obtained results were in the range from −1 to 1. Positive value of LI was synonymous with left-sided laterality, and values from 0.5 to 1 clearly showed a strong lateralization, and values from 0.25 to 0.5 a weak lateralization. Negative value of the LI meant right-sided lateralization, the strong one for values of −1 to −0.5, and weak one for values of −0.5 to −0.25. The values from −0.25 to 0.25 was considered to be symmetrical activation [11].
Results
Tumors located in the frontal lobe of the left hemisphere
In the case of patients with LGG located in the left frontal lobe near the frontal operculum of insula in 3/4 cases we obtained the activation of Broca’s area in the right hemisphere. As far as half the LGG tumors are concerned LI showed a strong right-sided lateralization (LI=−1), whereas in the second half the LI was left-sided (strong: LI=1 and weak: LI=0.28) (Figure 1). In the case of two patients classified to HGG in one case we obtained the activation only in Broca’s area of the left hemisphere (LI=1), in one case we did not obtain any activation in the hemisphere.
Figure 1.

BOLD activation map (p<0.05) for patient M.K. Tumor in the left frontal lobe. Broca’s areas activation.
Regardless of the size and histological type of tumor we obtained the activation in Wernicke’s area both in the left and the right hemispheres. For patients from LGG group in 2 cases, the LI was right-sided (strong: LI=−0.8 and weak: LI=−0.28), in one case the LI was left-sided, and 1 case demonstrated symmetrical activation of both hemispheres (LI=−0.04). In the case of HGG tumors lateralization was in both cases left-sided (strong: LI=0.71 and weak: LI=0.3).
Tumors located in the temporal lobe of the left hemisphere
In the case of tumors located in temporal area of the left hemisphere near Wernicke’s area in 4/5 cases we obtained activation in Wernicke’s area contralateral to the tumor (LI=−1) (Figure 2). In one case we obtained activation in Wernicke’s area of the left hemisphere (LI=1). In 4/5 cases we obtained activation in Broca’s area in 2 cases in the left hemisphere, in one case in the right hemisphere, and in one case in both sides. In Broca’s area in 3/5 cases LI was left-sided (in 2 cases strong: LI=1, and in one case weak: LI=0.48).
Figure 2.
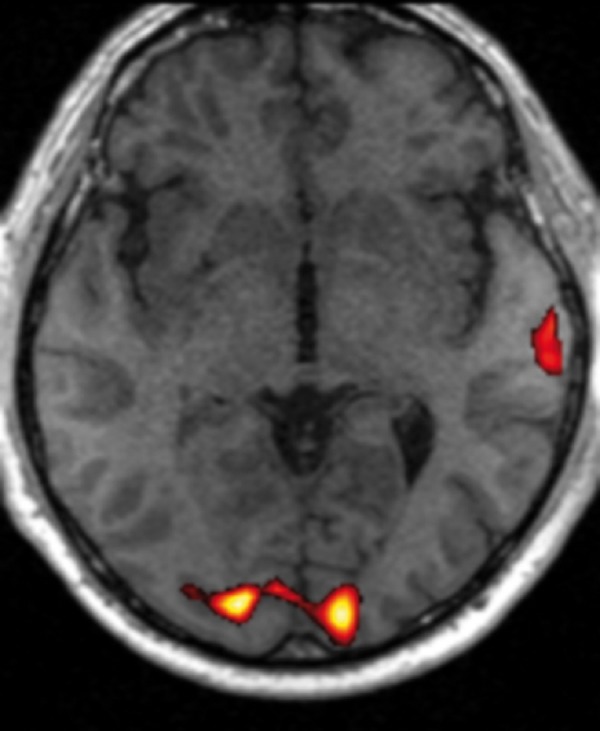
BOLD activation map (p<0.05) for patient K.Z. with left temporal lobe tumour. Wernicke’s area activation in the right hemisphere.
The obtained values of t-statistics of the intensity of activation (T) and the number of activated voxels in clusters (ke) did not differ significantly depending on the used paradigm, location and histological type of tumors.
A detailed list of results are shown in the Tables 2 and 3.
Table 2.
Value of t-statistics (T) and number of activated voxels within each cluster (ke) in patients with left frontal lobe tumors.
| Patient | Broca’s area | Wernicke’s area | ||||||
|---|---|---|---|---|---|---|---|---|
| Left hemisphere | Right hemisphere | Left hemisphere | Right hemisphere | |||||
| T | ke | T | ke | T | ke | T | ke | |
| F.A. | 6.15 | 95 | – | – | 7.47 | 280 | 3.27 | 47 |
| B.T. | – | – | – | – | 9.17 | 388 | 7.76 | 207 |
| S.K. | 5.08 | 31 | – | – | 6.71 | 94 | 4.42 | 16 |
| G.J. | – | – | 6.36 | 237 | 5.68 | 141 | 6.03 | 252 |
| Si.A. | – | – | 4.04 | 154 | 6.87 | 401 | 8.25 | 436 |
| Ma.K. | 9.24 | 142 | 4.94 | 80 | 4.13 | 23 | 6.32 | 209 |
Table 3.
Value of t-statistics (T) and number of activated voxels within each cluster (ke) in patients with left temporal lobe tumors.
| Patient | Broca’s area | Wernicke’s area | ||||||
|---|---|---|---|---|---|---|---|---|
| Left hemisphere | Right hemisphere | Left hemisphere | Right hemisphere | |||||
| T | ke | T | ke | T | ke | T | ke | |
| N.S. | – | – | – | – | – | – | 4.42 | 15 |
| K.S. | 3.44 | 2 | – | – | – | – | 5.14 | 33 |
| K.Z. | 7.48 | 65 | – | – | – | – | 7.72 | 63 |
| St.A. | – | – | 4.02 | 42 | – | – | 4.68 | 61 |
| Mi.K. | 6.58 | 116 | 3.92 | 44 | 4.86 | 113 | – | – |
Summary of speech disorders with the calculated LI for Broca’s and Wernicke’s area is illustrated in the Table 4.
Table 4.
Pre- and postoperative impairment of language ability in patients with brain tumors compared with calculated laterality index (LI) for Broca’s and Wernicke’s areas separately.
| Patient | Preoperative speech disorders | Postoperative speech disorders | LI Broca’s area | LI Wernicke’s area |
|---|---|---|---|---|
| F.A. | Lack | Lack of data | 1 | 0.71 |
| B.T. | Lack | Lack | – | 0.3 |
| S.K. | Two episodes of dysphasia | Mild dysphasia | 1 | 0.71 |
| G.J. | Average degree of dysphasia | Complete motor aphasia | −1 | −0.28 |
| Si.A. | Lack | Lack | −1 | −0.04 |
| Ma.K. | Lack | Lack | 0.28 | −0.8 |
| N.S. | Lack | Lack | – | −1 |
| K.S. | Lack | Mild dysphasia | 1 | −1 |
| K.Z. | Two episodes of dysphasia | Lack | 1 | −1 |
| St.A. | Lack | Lack | −1 | −1 |
| Mi.K. | Mixed aphasia without motor deficit | Periodical dysphasia, no motor deficit | 0.45 | 1 |
Discussion
The concept of brain plasticity is defined as the ability to modify the structural and functional neural networks that are to lead to the acquisition of specific functions of the brain centers between them. Incentives to stimulate such changes may be the factors of both external origin – such as surgical resection of brain tissue, as well as internal origin – such as growing tumors of the CNS. Two concepts explaining the mechanism of compensatory remodeling of the CNS were created. The first one assumes that in two hemispheres of the brain there are homologous regions responsible for exactly the same function (redundancy region). The second one claims that some adjacent areas can take on the role of centers that were destroyed (regional degenerative) [15]. The evaluation of these mechanisms in the fMRI study might be ambiguous. Some authors conclude that the observed activity only in Broca’s or Wernicke’s areas in the healthy hemisphere is not the result of functional plasticity of the centers, but may be due to destruction by lesion of specialized neurons and damaged neuronal networks responsible for the inhibition of the activity centers of the opposite hemisphere [16–18]. The reduced BOLD signal (Blood-Oxygen Level Dependent) in the areas of the hemisphere with lesion, may be due to compression of the tumor mass and surrounding edema on the lumen of vessels, which reduces or prevents the formation of activity in fMRI study [19,20]. In addition, neovas-cularization and necrotic changes in the tumor may affect the BOLD signal [21].
However, studies conducted in healthy volunteers showed that with an increase in the relative difficulty of the task, the activity of homologous non-dominant hemisphere areas increase as well. For this reason it is likely that it is progressive decline of intellectual capacity with tumor growth, and thus the relative increase in task difficulty activates additional areas of non-dominant hemisphere [22].
A number of different paradigms for mapping of language centers in fMRI study is proposed. The literature emphasizes that the use of only one type of paradigm cannot afford to detect all language areas [7–10]. On the other hand, in patients with organic amendments one aims at minimizing the testing time. fMRI study method should not be burdensome for the patient. Patients with brain tumors are often less neurologically and neuropsychologically skilled compared to healthy people. They are not able to perform lengthy tasks while maintaining the appropriate focus, and thus restrict the execution of a larger number of paradigms.
In our work we used only one type of paradigm – generation of nouns in two levels of difficulty, and break as a control. In comparison with the rest, such incentive enables to detect stimulation of the centers responsible for the semantics, phonology, articulation of speech and memory. Reports in the literature indicate that while using such a paradigm, an increase in signal intensity was observed among others in the inferior frontal gyrus of the left hemisphere and close to the posterior part of the left temporal lobe, which was consistent with the methodology established through the region of interest (ROIs) [23].
In our study, in the case of tumors in the left frontal lobe in two patients G.J. and Si.A., we obtained the activation only in right hemisphere in homologous area for Broca’s area, which may indicate the acquisition of function by this hemisphere. In these cases, also for Wernicke’s area LI was negative, although only one case of lateralization was right-sided, as in the second case the LI=−0.04 that was the evidence of a symmetrical distribution of the function in both hemispheres in this case. These tumors belong to a group of low-grade tumors. As far as patient Si.A. is concerned, no impairment of speech neither in the period before nor after surgery were found. The obtained results confirm reports of other investigators, which showed the processes of reorganization in the case of low-grade tumors [24–26]. Patient G.J. preoperatively suffered from dyphasia and postoperatively developed a complete motor aphasia. This may indicate the presence of suppressive effect of lesion on BOLD signal.
For two patients with tumors of the left frontal lobe: F.A. and S.K., lateralization indexes for both Broca’s and Wernicke’s areas were left-sided. This may indicate that there was no process of functional reorganization of the brain in these patients. It is worth noting that one of these tumors was characterized by both low malignance, and the smallest volume among all analyzed tumors. For these reasons it could not affect the acquisition of function by right-sided areas. In this patient in the postoperative period there was a mild dysphasia. In many studies the authors emphasize that the reorganization of centers takes time, which may explain the fact that in the second, fast-growing tumor from the HGG group, we did not obtain the activation in the areas of right hemisphere. The results of postoperative neuropsychological testing were not available for this patient.
In one patient with a tumor of the frontal lobe – B.T., and in one patient with a tumor of the temporal lobe – N.S., we did not obtain activation for Broca’s area, which may be due to several reasons. The first reason may be the methodology of the study, because the paradigm did not equally stimulate the eloquent areas in all patients. Another reason may be overtaking the function of Broca’s area by an area of the brain beyond the ROIs identified by us, which may indicate the lack of symptoms of speech disorders in this patient. This is confirmed by observations of other researchers who reported cases of activation of areas adjacent to the lesion [27,28]. As a possible cause of inactivity, we rejected the lack of cooperation from the patient and other technical reasons due to derived activity centers in the temporal area. For patient B.T. lateralization index for Wernicke’s area was weak left-sided and for patient NS right-sided, which may indicate that processes of functional reorganization are present here.
For tumors of the left temporal lobe, all of the HGG group, in 4/5 of the patients lateralization for Wernicke’s area was right-sided (LI=−1). Of these patients, in two: K.S. and K.Z., lateralization for Broca’s area was left-sided (LI=1). In right-handed patients this could be a confirmation of taking over the function of Wernicke’s left area by homologous areas of the opposite hemisphere, which is consistent with observations of other researchers [29]. Of this group, there was dysphasia only in 1 patient (LI=1, LI=−1). In one case, even for Broca’s area lateralization was right-sided. However, reorganization of both eloquent areas in this patient is unlikely. Studies in healthy volunteers indicate that up to 7.5% of right-handed patients may show a right-sided lateralization, which seems more plausible explanation for the obtained result [30]. In one patient, lateralization indexes for both Broca’s and Wernicke’s areas were left-sided, which is for non-occurrence of reorganization process of speech centers in one patient in this group. In this patient presurgery there was a mixed aphasia and after surgery there was dysphasia without periodic motor deficit.
In the future the study of functional magnetic resonance imaging may be useful in determining the location of speech centers. However, until standards are developed for used testing protocols and the interpretation of results, fMRI studies cannot be the sole method of mapping of language centers. It is extremely important to summaries neuropsychological test results with the results of fMRI studies.
Our studies were carried out on a small group of patients, which limits the possibility of generalization of the results. However, observations were carried out at a fairly homogeneous groups – very difficult to establish in clinical practice because of the extraordinary variety of CNS tumors in relation to their location and histological type. This entitles to highlight interesting trends, particularly in terms of clinical applications.
Conclusions
The presence of proliferative processes in close topographical relations with centers of speech has an effect on their functional reorganization.
fMRI study is a method useful in the presurgery assessment of the location of language centers, helpful in planning surgical treatment.
HGG tumors located in the Wernicke’s area cause the transfer of its activities on the healthy hemisphere and/or reduction of activities in the in the affected hemisphere.
References:
- 1.Loring DW, Meador K, Lee G, et al. Amobarbital Effects and Lateralized Brain Function: The Wada Test. Springer-Verlag; New York: 1992. pp. 1–23. [Google Scholar]
- 2.Ojemann G, Ojemann J, Lettich E, et al. Cortical language localization in left, dominant hemisphere: An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71(3):316–26. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- 3.Pardo JV, Fox PT. Preoperative assessment of the cerebral hemispheric dominance for language with CBF PET. Hum Brain Mapp. 1993;1(1):57–68. [Google Scholar]
- 4.Breier JI, Simos PG, Zouridakis G, et al. Language dominance determined by magnetic source imaging: a comparison with the Wada procedure. Neurology. 1999;53(5):938–45. doi: 10.1212/wnl.53.5.938. [DOI] [PubMed] [Google Scholar]
- 5.FitzGerald DB, Cosgrove GR, Ronner S, et al. Location of language in the cortex: a comparison between functional MR imaging and electrocortical stimulation. AJNR Am J Neuroradiol. 1997;18(8):1529–39. [PMC free article] [PubMed] [Google Scholar]
- 6.Rutten GJ, van Rijen PC, van Veelen CW, et al. Language area localization with three-dimensional functional magnetic resonance imaging matches intrasulcal electrostimulation in Broki’s area. Ann Neurol. 1999;46(3):405–8. doi: 10.1002/1531-8249(199909)46:3<405::aid-ana17>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y, Vikingstad EM, George KP, et al. Cortical language activation in stroke patients recovering from aphasia with functional MRI. Stroke. 1999;30(11):2331–40. doi: 10.1161/01.str.30.11.2331. [DOI] [PubMed] [Google Scholar]
- 8.Thulborn KR, Carpenter PA, Just MA. Plasticity of language-related brain function during recovery from stroke. Stroke. 1999;30(4):749–54. doi: 10.1161/01.str.30.4.749. [DOI] [PubMed] [Google Scholar]
- 9.Calvert GA, Brammer MJ, Morris RG, et al. Using fMRI to study recovery from acquired dysphasia. Brain lang. 2000;71(3):391–99. doi: 10.1006/brln.1999.2272. [DOI] [PubMed] [Google Scholar]
- 10.Rosen HJ, Petersen SE, Linenweber MR, et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55(12):1883–94. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- 11.Lehericy S, Cohen L, Bazin B, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54(8):1625–33. doi: 10.1212/wnl.54.8.1625. [DOI] [PubMed] [Google Scholar]
- 12.Stippich C, Rapps N, Dreyhaupt J, et al. Localizing and lateralizing language in patients with brain tumors: feasibility of routine preoperative functional MR imaging in 81 consecutive patients. Radiology. 2007;243(3):828–36. doi: 10.1148/radiol.2433060068. [DOI] [PubMed] [Google Scholar]
- 13.Rutten GJ, Ramsey NF, van Rijen PC, et al. Reproducibility of fMRI-determined language lateralization in individual subjects. Brain lang. 2002;80(3):421–37. doi: 10.1006/brln.2001.2600. [DOI] [PubMed] [Google Scholar]
- 14.Benson RR, FitzGerald DB, LeSueur LL, et al. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology. 1999;52(4):798–809. doi: 10.1212/wnl.52.4.798. [DOI] [PubMed] [Google Scholar]
- 15.Tononi G, Sporns O, Edelman GM. Measures of degeneracy and redundancy in biological networks. Proc Natl Acad Sci. 1999;96(6):3257–62. doi: 10.1073/pnas.96.6.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller RA, Rothermel RD, Behen ME, et al. Brain organization of language after early unilateral lesion: a PET study. Brain Lang. 1998;62(3):422–51. doi: 10.1006/brln.1997.1931. [DOI] [PubMed] [Google Scholar]
- 17.Netz J, Ziemann U, Homberg V. Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res. 1995;104(3):527–33. doi: 10.1007/BF00231987. [DOI] [PubMed] [Google Scholar]
- 18.Heiss WD, Thiel A, Kessler J, et al. Disturbance and recovery of language function: correlates in PET activation studies. Neuroimage. 2003;20(Suppl.1):S42–49. doi: 10.1016/j.neuroimage.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Holodny AI, Schulder M, Liu WC, et al. Decreased BOLD functional MR activation of the motor and sensory cortices adjacent to a glioblastoma multiforme: implications for image-guided neurosurgery. AJNR Am J Neuroradiol. 1999;20(4):609–12. [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiber A, Hubbe U, Ziyeh S, et al. The influence of gliomas and nonglial space-occupying lesions on blood-oxygen-level-dependent contrast enhancement. AJNR Am J Neuroradiol. 2000;21(6):1055–63. [PMC free article] [PubMed] [Google Scholar]
- 21.Schlaier J, Fellner C, Schwerdtner J, et al. The quality of functional MR images in patients with brain tumors: influences of neurological disorders and tumor location. Comput Med Imag Grap. 1999;23(5):259–65. doi: 10.1016/s0895-6111(99)00023-3. [DOI] [PubMed] [Google Scholar]
- 22.Mattay VS, Weinberger DR. Organization of the human motor system as studied by functional magnetic resonance imaging. Eur. J Radiol. 1999;30(2):105–14. doi: 10.1016/s0720-048x(99)00049-2. [DOI] [PubMed] [Google Scholar]
- 23.Yetkin FZ, Swanson S, Fischer M, et al. Functional MR of frontal lobe activation: comparison with Wada language results. AJNR Am J Neuroradiol. 1998;19(6):1095–98. [PMC free article] [PubMed] [Google Scholar]
- 24.Robles SG, Gatignol P, Lehericy S, et al. Long-term brain plasticity allowing a multistage surgical approach to World Health Organization Grade II gliomas in eloquent areas. J Neurosurg. 2008;109(4):615–24. doi: 10.3171/JNS/2008/109/10/0615. [DOI] [PubMed] [Google Scholar]
- 25.Duffau H. Brain plasticity: from pathophysiological mechanisms to therapeutic applications. J Clin Neurosci. 2006;13(9):885–97. doi: 10.1016/j.jocn.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 26.Holodny AI, Schulder M, Ybasco A, et al. Translocation of Broca’s area to the contralateral hemisphere as the result of the growth of a left inferior frontal glioma. J Comput Assist Tomogr. 2002;26(6):941–43. doi: 10.1097/00004728-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Thiel A, Herholz K, Koyuncu A, et al. Plasticity of language networks in patients with brain tumors: a positron emission tomography activation study. Ann Neurol. 2001;50(5):620–29. doi: 10.1002/ana.1253. [DOI] [PubMed] [Google Scholar]
- 28.Ackermann H, Riecker A. The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain Lang. 2004;89(2):320–28. doi: 10.1016/S0093-934X(03)00347-X. [DOI] [PubMed] [Google Scholar]
- 29.Petrovich NM, Holodny AI, Brennan CW, et al. Isolated translocation of Wernicke’s area to the right hemisphere in a 62-year-man with a temporo-parietal glioma. AJNR Am J Neuroradiol. 2004;25(1):130–33. [PMC free article] [PubMed] [Google Scholar]
- 30.Knecht S, Deppe M, Drager B, et al. Language lateralization in healthy right-handers. Brain. 2000;123(1):74–81. doi: 10.1093/brain/123.1.74. [DOI] [PubMed] [Google Scholar]


