Abstract
Transportation of rodents has repeatedly been demonstrated to potentially affect research outcomes. In addition, rapid acceleration and deceleration have marked physiologic effects. The current study determined the accelerative forces associated with common types of animal transportation within the institution and means of reducing these effects. A rodent-sized (24 g) accelerometer was placed in a standard polycarbonate mouse cage, which then was hand-carried or loaded onto a plastic, small metal, or large metal cart. The cage then moved along a set path that included several flooring types and obstacles. Accelerative forces within the mouse cage varied by as much as 35 m/s2 in as little as 1 s, primarily along the vertical axis (Z-axis). Measured acceleration was greatest with the plastic cart and lowest during hand-carrying. The placement of a towel under the cage dampened in-cage acceleration due to cart use by more than 50%, whereas a similarly located underpad had no significant effect. These data document that small rodents typically are exposed to considerable motion during transportation. The resulting physical and physiologic effects could affect study outcomes.
Many studies have demonstrated negative effects of transportation on laboratory rodents. Even noninvasive handling procedures, such as weighing a rodent or lifting it to clean its cage or weighing it, are associated with robust physiologic responses.15 Minor tasks, such as transferring a mouse cage into a new room, can cause a rapid increase in corticosterone levels, exploratory activity, and feeding but reduce grooming behavior.2,7-9,16 Reports in rodents and other species have suggested acclimation periods of between 2 h and 6 wk after transportation for normalization of physiologic and behavioral responses.6,9,10,12,14-16 Even traveling short distances has been shown to significantly increase plasma glucose concentrations.15 Other studies have noted a correlation between duration of transport and weight loss.5 All of these changes can have significant effects on animal health, welfare, and study outcomes.
Acceleration refers to the rate of change in velocity over time (that is, the rate at which an object speeds up or slows down). A substantial body of work documents deleterious effects of rapid acceleration and deceleration, as this property describes the events associated with car crashes, concussions, traumatic brain injury, and so on.1,3,11 These injuries can result in axonal stretching and tearing,3 even without direct cranial impact.11 Similarly, rapid acceleration and deceleration can cause increased production of prostaglandins, tissue plasminogen activator, and nitric oxide.1
Although previous studies have examined the physiologic outcomes of transportation, they have not focused on the actual methods of transport. Different modes of transportation are likely to have different and important effects on the animals transported. No known studies have examined acceleration associated with specific modes of transportation of animals, either by hand or on a cart. Further, few data exist concerning how to mitigate these effects. The current study was designed to document the accelerative forces experienced during routine cage transportation by using several different cart types and hand carrying. Additional work was done to find a simple means to minimize these forces.
Materials and Methods
No animals were used in this study. To approximate the motion that a mouse would be exposed to during typical transport, an accelerometer (catalog no. VS303, Vibration Sentry, Convergence Instruments, Sherbrooke, Quebec, Canada) was used to measure motion along 3 axes (X, forward and backward; Y, side to side; Z, up and down). The accelerometer measured 33 mm × 33 mm × 15.5 mm and weighed 24 g. The device collected measurements along all 3 axes 105 times per second. Data were reported from the device as average, maximal, and minimal acceleration levels. This device was chosen because its weight was similar to that of a typical laboratory mouse, allowing for a reasonable approximation of the motion a mouse may experience during transport.
Prior to each daily use, the accelerometer was calibrated along all 3 axes. For data collection, the device was placed into the middle of a polycarbonate mouse cage (189 mm × 297 mm × 128 mm), which was filled approximately 1-cm deep with 1/4-in. corncob bedding and had a metal wire-bar lid and polycarbonate filter top. Control data were collected by placing the cage into 3 rodent housing systems: a low-capacity system, with ventilation units mounted on top of the rack (Enviro-Gard A System, Lab Products, Seaford, DE); a high-capacity system, with ventilation units remote to the racks (Enviro-Gard Multiplex System, Lab Products); and on stainless steel shelving. Three locations (top, middle, and bottom) on each rack were tested for each control system. Each test period was 5 min in duration.
After the collection of control data, vibration due to hand-carrying of cages and their transportation on 3 different types of carts (2 metal carts, 1 plastic; Table 1) was assessed. Each cart had 2 shelves and hard rubber casters. The casters in the front of the cart were fixed but swiveled at the back of the cart. Except for their diameters, all casters were the same in composition. The 2 metal carts had flat shelves spot-welded to hollow-tube, vertical struts (diameter, 2 cm), whereas the plastic cart had a tray (depth, 2 in.) fastened to L-shaped vertical struts (7 cm per side) by multiple bolts.
Table 1.
Characteristics of carts used
| Cart | Height (m) | Weight (kg) | Wheel diameter (mm) |
| Plastic 2-shelf | 0.93 | 17.6 | 125 |
| Large steel 2-shelf | 1.14 | 25.7 | 125 |
| Small steel 3-shelf | 0.61 | 12.8 | 100 |
Throughout the study, the same cage was used, the accelerometer was placed in approximately the same location in the middle of the cage, and the same person walked the data collection route. For cart transportation, the cage and sensor were set on the top shelf of each cart. Hand carrying involved holding the cage in a horizontal position while it was cradled under the right arm of the transporter. The route started with a 10-m walk on epoxy resin floor to an elevator, followed by a 4-floor descent. On exit from the elevator, the route then followed a circuitous route covering several hallways, one down ramp, one up ramp, approximately 30 m of commercial carpet flooring, and 220 m of linoleum tile. The route concluded with a final entry into the elevator, a 4-floor ascent, and then repetition of the 10-m walk on the epoxy resin floor. The entire 270-m route was walked in about 5.5 min, at a pace of approximately 2.9 km/h. The same route was used for each round of data collection, and it was performed 6 times for each cart type. The data were averaged to account for variability occurring during transport, such as striking irregularities on the ground or changes in speed or direction to avoid obstacles.
After each cart was tested, 2 types of padding were chosen for placement beneath the cage. A double-folded bath towel (approximately 2.5 cm thick) and a disposable, cotton–polymer-filled underpad (approximately 0.5 cm thick; Covidien, Mansfield, MA) were selected to address the possibility of reducing vibration by using inexpensive and readily available materials. The data collection method and route were completed 4 times for each of the 3 carts.
In this report, acceleration refers to the increase in velocity in a direction away from the initial location of the accelerometer, whereas deceleration refers to the change in velocity as the device returns to its base position. Vibration refers to the waveform combination of acceleration and deceleration. Data from this project were recorded and processed by using Vibration Sentry Manager software (Convergence Instruments). Mean acceleration for test runs with each transportation method were averaged and then compared by using ANOVA and post hoc analysis by the least-squares method. Different methods of vibration reduction were compared by using paired t-test analysis for each cart type. Data were analyzed by using Statistica (StatSoft, Tulsa, OK). Group differences that yielded a P value of less than 0.05 were considered significant.
Results
Under control conditions, virtually no motion was detected in any of the housing systems, and acceleration greater than 0.1 m/s2 was not detected along any of the 3 axes.
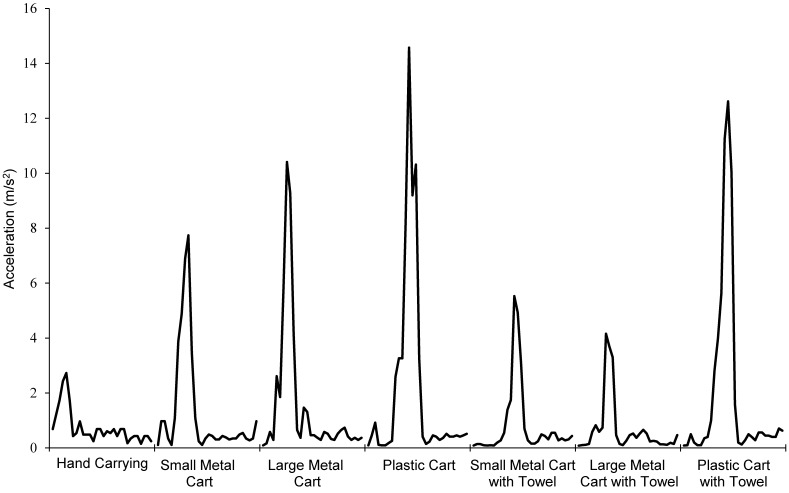
In all other test scenarios, average X- and Y-axis acceleration was approximately 2 m/s2. Z-axis acceleration (both mean and peak) varied widely depending on the mode of transportation (Figure 1). Use of the plastic cart resulted in the highest mean acceleration levels and the highest peak acceleration (Table 2). Spikes in the data correspond to events including entering or exiting an elevator, going over a bump, negotiating a change in flooring type, and starting or stopping. Crossing the threshold of an elevator consistently produced the most pronounced peaks in acceleration with all cart types, regardless of the presence of padding (Figure 2). Maximal and mean Z-axis acceleration were significantly (P < 0.05) lower for hand-carrying than for cart transportation without padding.
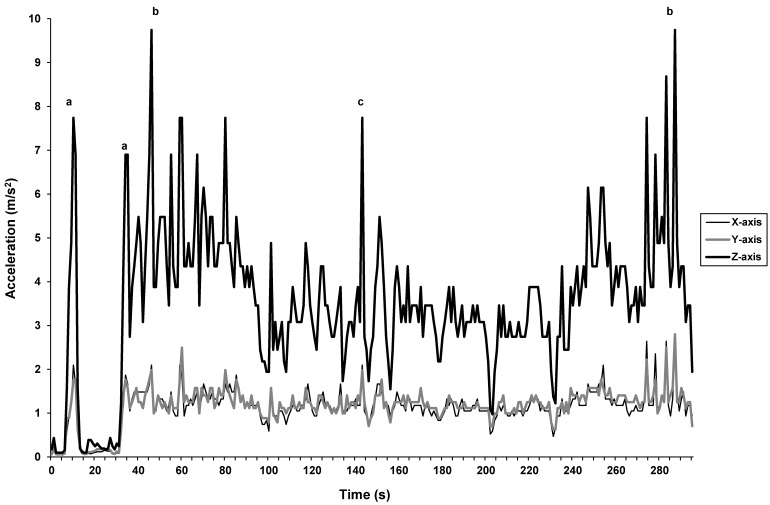
Figure 1.
Vibration associated with transporting a cage along a 270-m route by using the small metal cart. Acceleration spikes are associated with the following events during transportation: (A) entering or exiting an elevator; (B) transition from commercial carpeting to tiled linoleum flooring; and C) crossing a metal threshold.
Table 2.
Z-axis acceleration during routine cage transportation
| Transportation method | Mean Z-axis acceleration (m/s2) over the entire route | Maximal Z-axis acceleration (m/s2) at any point along the route |
| Hand | 1.98 ± 0.22 | 6.22 |
| Plastic cart | 8.60 ± 0.50ac | 17.31 |
| Large steel cart | 2.25 ± 0.33c | 13.67 |
| Small steel cart | 3.66 ± 0.15c | 13.76 |
| Plastic cart with towel | 5.70 ± 0.66 | 14.16 |
| Large steel cart with towel | 1.24 ± 0.10b | 9.71 |
| Small steel cart with towel | 2.00 ± 0.16 | 11.03 |
| Plastic cart with underpad | 5.89 ± 0.79 | 15.47 |
| Large steel cart with underpad | 1.73 ± 0.77 | 12.62 |
| Small steel cart with underpad | 3.16 ± 2.61 | 13.89 |
In all trials (that is control, towel, and underpad), the mean Z-axis acceleration was significantly greater than that for either of the steel carts.
Greater (P < 0.01) mean acceleration than that for all other transportation methods
Less (P < 0.01) mean acceleration than that for all cart types
Greater (P < 0.01) mean acceleration than that for the same cart type but with a towel between the cage and cart
Figure 2.
Changes in peak acceleration associated with entering an elevator by using different methods of transportation. Note the decrease in peak acceleration due to placement of a towel between the cage and cart.
Two methods to decrease the transfer of vibration from the cart to the cage were examined. Placing a folded bath towel beneath the cage had the greater effect on decreasing acceleration, especially along the Z-axis. The average Z-axis acceleration of the plastic cart, small metal cart, and large metal cart was decreased 33.7%, 44.9%, and 45.4%, respectively, when a towel was used compared with not used (Table 2). The maximal Z-axis acceleration also decreased when a towel was placed (Table 2). Transporting the cage on the large metal cart with a towel underneath the cage produced significantly (P < 0.05) less average acceleration than did any other form of transportation. However, the maximal Z-axis acceleration was significantly (P < 0.05) greater for the large cart and towel than for hand-carrying.
Using an underpad beneath the cage did not significantly decrease the vibration to the cage (Table 2) but showed a trend (P < 0.1), in the plastic and large metal cart, toward decreased average Z-axis acceleration compared with that of the same carts without padding. There was no change in maximal Z-axis acceleration with the addition of an underpad.
Discussion
The control data for this study shows that cages are exposed to a very low average level of vibration when housed on either ventilated or nonventilated racks. Whatever housing method was used, the cages did not experience any acceleration of greater than 0.1 m/s2 in any direction. These data are consistent with the findings of another recent study.13
Transportation of all forms significantly increased vibration. In all of the test scenarios, the average acceleration along the X- and Y-axes was similar (approximately 2 m/s2), roughly corresponding to the walking speed of the tester. Acceleration along the Z-axis produced the most striking effects during transportation. The data suggest that every time a cart crossed a bump or other obstacle, an animal in the cage would be subjected to very rapid acceleration and deceleration (Figure 2). Entering or exiting an elevator during cart transportation produced the highest rates of acceleration (as high as 17 m/s2). Whether the rates of acceleration demonstrated in this study would result in clinical changes is unclear; however rapid acceleration and deceleration (approximately 35 m/s2) has been shown to affect neurologic and vascular physiology in nonrodent species.1,4,11 The maximal rate of acceleration is likely great enough to momentarily propel a mouse off the cage floor. If this acceleration occurred when a mouse was close to a structure in the cage, the mouse likely would collide with the structure. The resulting physical and physiologic effects could affect study outcomes and potentially pose animal welfare issues.
The data seem to suggest some relationship between cart weight and Z-axis acceleration (both mean and maximal), although only when the 2 metal carts were compared. Z-axis acceleration associated with the plastic cart was significantly greater than that for either metal cart, even though the plastic cart was heavier than the smaller metal cart. We theorize that this difference was due to the rigid construct of the plastic cart, its large vertical struts, and the solid attachment of its wheels, allowing for direct transmission of vibration from the floor to the cage. Conversely, the metal carts had hollow-tube struts with spot-welded shelves, a design that may have contributed to the dampening of vibrations transmitted to the cage.
When 2 different readily available padding options were tested to reduce vibration during transport, the towel was clearly superior in decreasing Z-axis vibration (both mean and maximal). This success is likely due to the fact that the folded towel was approximately 5 times as thick as the underpad, allowing for significant dampening of the vibration transferred from the cart. The cage easily compressed the pad, leaving only a thin barrier between the cage and cart to absorb vibration. Although the data suggest that a thicker and softer barrier between the cage and cart would be more effective in dampening motion, at some point the greater thickness or softness might actually increase instability of the cage.
Although hand-carrying a cage did not produce the lowest levels of acceleration, it was among the lowest. Further, hand-carrying produced the lowest peak acceleration and dampened overall vibration. Although these data seem to suggest that hand-carrying a cage exposes an animal to less vibration, these results are likely to be dependent on the walking style of the person carrying the cage. In this study, great care was taken to keep the cage as level along the X–Y-axis as possible during hand-carrying. Anecdotal observations of other people hand-carrying cages under their arms during this study suggested that the cages are often tipped off the horizontal plane when carried during routine transportation.
This study had several limitations. The different permutations of vibration and acceleration associated with cart type, wheel size and composition, and flooring within a single institution are nearly limitless. Although each of these factors has a specific effect on cage motion, the general findings of this study likely are applicable to many laboratory animal facilities. The goal of this study was not to model all possible conditions but to demonstrate potential sources of stress when transporting animals from their housing room, either by choice or as needed to perform experiments in a lab or make use of resources outside of the animal room. As noted previously, we took great care during the hand-carrying of cages in this study, and we speculate that routine practices of hand-carrying cages may result in much greater motion along all axes. The effects of transporting multiple cages were not examined, because the interaction between the cages would add considerable variability to the study.
Although this study did not involve live animals, the size of the accelerometer was consistent with that of a mouse, suggesting that the findings were relevant. The data support the potential importance of factors, such as cart composition, size and dimensions of thresholds, and flooring type during animal transportation. Most importantly, the results suggest a simple means of dampening the vibration to near-normal levels if transportation is needed.
References
- 1.Adams JA, Wu H, Bassuk JA, Arias J, Uryash A, Kurlansky P. 2009. Periodic acceleration (pGz) acutely increases endothelial and neuronal nitric oxide synthase expression in endomyocardium of normal swine. Peptides 30:373–377 [DOI] [PubMed] [Google Scholar]
- 2.Armario A, Montero JL, Balasch J. 1986. Sensitivity of corticosterone and some metabolic variables to graded levels of low intensity stresses in adult male rats. Physiol Behav 37:559–561 [DOI] [PubMed] [Google Scholar]
- 3.Barth JT, Freeman JR, Broshek DK, Varney RN. 2001. Acceleration–deceleration sport-related concussion: the gravity of it all. J Athl Train 36:253–256 [PMC free article] [PubMed] [Google Scholar]
- 4.Bayly PV, Cohen TS, Leister EP, Ajo D, Leuthardt EC, Genin GM. 2005. Deformation of the human brain induced by mild acceleration. J Neurotrauma 22:845–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown SN, Knowles TG, Edwards JE, Warriss PD. 1999. Behavioural and physiological responses of pigs to being transported for up to 24 hr followed by 6 hr recovery in lairage. Vet Rec 145:421–426 [DOI] [PubMed] [Google Scholar]
- 6.Capdevila S, Giral M, Ruiz de la Torre JL, Russell RJ, Kramer K. 2007. Acclimatization of rats after ground transportation in a new facility. Lab Anim 41:255–261 [DOI] [PubMed] [Google Scholar]
- 7.Castelhano-Carlos MJ, Baumans V. 2009. The impact of light, noise, cage cleaning, and inhouse transport on welfare and stress of laboratory rats. Lab Anim 43:311–327 [DOI] [PubMed] [Google Scholar]
- 8.Dallmann R, Steinlechner S, von Hörsten S, Karl T. 2006. Stress-induced hyperthermia in the rat: comparison of classical and novel recording methods. Lab Anim 40:186–193 [DOI] [PubMed] [Google Scholar]
- 9.Gartner K, Buttner D, Dohler K, Friedel R, Lindena J, Trautschold I. 1980. Stress response of rats to handling and experimental procedures. Lab Anim 14:267–274 [DOI] [PubMed] [Google Scholar]
- 10.Hoorn EJ, McCormick JA, Ellison DH. 2011. High tail-cuff blood pressure in mice 1 week after shipping: the need for longer acclimation. Am J Hypertens 24:534–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maxwell WL, Watt C, Graham DI, Gennarelli TA. 1993. Ultrastructural evidence of axonal shearing as a result of lateral acceleration of the head in nonhuman primates. Acta Neuropathol 86:136–144 [DOI] [PubMed] [Google Scholar]
- 12.Moura PJ, Venkitaramani DV, Tashev R, Lombroso PJ, Xavier GF. 2011. Transport of animals between rooms: a little-noted aspect of laboratory procedure that may interfere with memory. Behav Processes 88:12–19 [DOI] [PubMed] [Google Scholar]
- 13.Norton JN, Kinard WL, Reynolds RP. 2011. Comparative vibration levels perceived among species in a laboratory animal facility. J Am Assoc Lab Anim Sci 50:653–659 [PMC free article] [PubMed] [Google Scholar]
- 14.Obernier JA, Baldwin RL. 2006. Establishing an appropriate period of acclimatization following transportation of laboratory animals. ILAR J 47:364–369 [DOI] [PubMed] [Google Scholar]
- 15.Tabata H, Kitamura T, Nagamatsu N. 1998. Comparison of effects of restraint, cage transportation, anaesthesia, and repeated bleeding on plasma glucose levels between mice and rats. Lab Anim 32:143–148 [DOI] [PubMed] [Google Scholar]
- 16.Tuli JS, Smith JA, Morton DB. 1995. Stress measurements in mice after transportation. Lab Anim 29:132–138 [DOI] [PubMed] [Google Scholar]