Abstract
Fur mites were diagnosed in a colony of mice at our research institution. In the current study, we compared the effectiveness of PCR and tape test in a small population of mice at the onset of diagnosis and throughout treatment. Samples were collected 1 d prior to treatment with permethrin impregnated cotton balls and 6 and 12 wk after treatment. PCR confirmed the presence of Myocoptes musculinus and Radfordia affinis or Myobia musculi, but tape test confirmed only the presence of Myocoptes spp. The results of the PCR and tape test agreed 97.2% of the time during active infection on day 1, but only 59.5% and 48.4% of results coincided at 6 and 12 wk after treatment, respectively. At 6 wk, 11 of the 37 samples were PCR-negative but tape-test–positive, compared with 9 of the 31 samples at 12 wk. Our results show that PCR is a reliable diagnostic method during active fur mite infection but that false-negative results are possible after treatment. Negative PCR results after treatment should be interpreted carefully, and a secondary diagnostic method should be considered.
Effective diagnosis of the common murine fur mites Myocoptes musculinus, Myobia musculi, and Radfordia affinis continues to be a topic of study because of the persistence of infection, their potential research complications, challenges of diagnosis, and numerous treatment options and their varying degrees of success. The fur mite identified most often in laboratory mouse colonies is Myocoptes musculinus.7,16 This mite frequently is observed as part of a mixed infection with Myobia spp. and may crowd out Myobia mites during a heavy infestation.7,8 Radfordia spp. is another common fur mite that looks similar to Myobia spp. and that can occur as part of a mixed infestation.7
The adult Myocoptes spp. mite typically infects the inguinal region, abdomen, and dorsum but can also be found on the head and neck.1,7 Myocoptes spp. mites are oval in shape, and the body is heavily chitinized.7 Microscopically, Myocoptes can be differentiated from other mite species because the adult has a third and fourth pair of legs that are pigmented, and tarsal suckers are present.7,8 Myobia spp. and Radfordia spp. mites typically inhabit the head and neck.8,13 Myobia spp. and Radfordia spp. are morphologically similar but can be differentiated by the characteristic bulge between the legs of Radfordia spp. and by the terminal tarsal claws seen in Radfordia spp. but lacking in Myobia spp.8
Clinical symptoms associated with infestations can have profound effects on the overall health of mice in a research colony. Signs associated with infestations can vary from inapparent infections to alopecia, pruritis, self-excoriation, ulceration, and secondary pyoderma.8,13,17,22 In addition, unthriftiness, lymphadenopathy, reduced weight gain, and decreased life span have been associated with acariasis.3,8,26
Many reports in the literature describe diagnostic methods for fur mites, including skin scrapes, cellophane tape impressions of the dorsal fur, hair pluck, stereomicroscopic examination of cooled pelts, and a sticky paper technique.5,8,9,15,23 Each diagnostic method presents its own challenges. All methods are dependent on mite burden, which can be variable and will depend on the length of infestation, mouse strain, age, grooming patterns, hair cycle length, and housing density.1,6,11,15,18,23 All of these tests can be labor-intensive and depends on the experience of the personnel collecting the specimens and their skill in mite identification.23
Recently, one of our conventionally housed mouse colony rooms experienced a fur mite outbreak. The infestation was identified during routine quarterly sentinel health screening of CD1 mice. Mites were identified by direct examination of the pelage at necropsy, and Myocoptes musculinus was confirmed through microscopic examination. Cellophane tape tests and PCR samples of the infected mice were collected following initial diagnosis of the infestation and then at 6 and 12 wk after treatment of the mice with permethrin-impregnated cotton balls.
In the current study, we compared a commonly used diagnostic test for fur mites, the tape test, with a PCR assay. We hypothesized that the PCR assay would be a more sensitive indicator than microscopic tape impression examination after treatment compared with initial diagnosis.
Materials and Methods
Mice and husbandry.
The animals in the fur mite-infested study room were male or female C57BL/6 mice or various transgenic strains. The mice were obtained from The Jackson Laboratory (Sacramento, CA) or Taconic (Cambridge City, IN). Animals were bred at our facility and enrolled in neurologic studies. The total number of mice treated and tested was estimated to be 140. Mice were housed at 2 to 4 animals per cage in ventilated polysulfone cages (Techniplast, Buguggiate, Italy) or conventional polysulfone cages with isolator tops.
Female Crl:CD1(ICR) mice (n = 24) were obtained from our breeding colony. These mice were enrolled in our sentinel program and served as control animals. The control mice were housed in the same facility as those in the fur mite-infested study room, but the control animals were located in 5 different Animal Biosafety Level 2 study rooms on a different floor of the facility. Mice were housed in similar ventilated caging. Each cage contained corncob bedding, and 2 mice were housed per cage. Animals in our sentinel program are screened quarterly for specific pathogens, which includes assessment for fur mites via direct microscopic exam of pelage after euthanasia. The control rooms had no history of fur mite infestations.
Mice in the fur-mite–infested study room and control mice were seronegative for lymphocytic choriomeningitis, mouse hepatitis virus, mouse rotavirus, ectromelia virus, minute virus of mice, mouse parvovirus, murine norovirus, pneumonia virus of mice, Sendai virus, reovirus type 3, Theiler mouse encephalomyelitis virus, K virus, mouse adenovirus, mouse thymic virus, polyoma virus, mouse cytomegalovirus, Haantan virus, lactic dehydrogenase elevating virus, cilia-associated respiratory bacillus, and Mycoplasma pulmonis. The mice were also free of Helicobacter spp. and ecto- and endoparasites. All mice in the study were at least 3 wk old. Mice were fed rodent chow (Laboratory Rodent Diet 5001, Mouse Diet 5015 or 5058, or PicoLab Mouse Diet 20, PMI Nutrition International, Richmond, IN). All study rooms were kept on a 12:12-h light:dark cycle. Cages were changed every 2 wk in all study rooms. Soiled bedding from cages was transferred to the sentinel cages every 2 wk at cage change.
The animal care and use program at University of California–Davis is AAALAC-accredited. Animal handling and care was in accordance with the Guide for the Care and Use of Laboratory Animals12 and IACUC-approved protocols.
Treatment.
Cotton bedding impregnated with Mitarrest (7.4% permethrin, EcoHealth, Boston, MA) was placed in each mouse cage according to the manufacturer's instructions on day 1 after initial sample collection. All animals in the fur mite-infested room were treated. Briefly, 2 cotton balls per rodent were placed in each cage weekly for a total of 6 wk. During cage changes, the used cotton balls were replaced with fresh treated cotton balls. For weeks during which cage changes did not occur, fresh cotton balls were placed in cages on the same day of the week as for cage changes; used cotton balls were not removed.
Sample collection and analysis.
Control mice were sampled first to prevent cross-contamination from the fur mite-infested room. For sampling, each mouse was removed from its cage. For tape test evaluation, an approximately 5 cm × 2 cm piece of cellophane tape was pressed several times against the back, neck, and abdomen of each mouse in the cage to collect a pooled tape impression. The tape was pressed onto a glass slide.
For PCR testing, individually wrapped sterile polyester swabs with a plastic shaft were used for sample collection. A swab was passed multiple times against the grain of the fur over the dorsum, head, abdomen, and inguinal region of each mouse in the cage.21 A single swab was used for each cage of mice. Each polyester swab was placed into a separate sterile microfuge tube, and the swab tip was broken off for sample transport and submission. The polyester swab samples were maintained at ambient temperature.
Samples were collected on day 1 prior to treatment and at 6 and 12 wk after treatment prior to the biweekly cage change. At each time point, 11 (day 1) or 12 (6 and 12 wk) pooled tape-test and PCR samples were collected from the control mice. On day 1, pooled tape-test and PCR samples were collected from 36 cages in the fur mite-infested colony. At 6 and 12 wk after treatment a total of 37 and 31 samples, respectively, were collected from the fur mite-infested colony. The difference in the sample sizes were based on the availability of mice for collection in the respective study rooms. In the fur mite-infested room, tape-test and PCR samples were collected from all cages except those with litters, to avoid any potential adverse effect of sample handling on the ongoing experimental breeding in the treated room.
The cellophane tape impressions were evaluated (Comparative Pathology Laboratory, University of California–Davis) microscopically at 100× magnification according to a grid pattern. The cellophane tape impressions were analyzed by an experienced medical technologist, who was blinded to the source of the samples at each time point. Slides were numbered sequentially without reference to the room from which the sample originated; the key was generated by the persons who collected the samples. A positive tape-test result was defined as the presence of at least one egg or evidence of adult mites (whole or in part); a negative result was defined as any sample that lacked eggs or evidence of adult mites. No attempt was made to quantify the number of eggs or mites present. Posttreatment samples that were deemed positive by tape test and negative by PCR were reevaluated by a veterinary pathologist.
The samples for PCR were submitted to a commercial laboratory (Research Animal Diagnostic Laboratory, Columbia, MO) for analysis. The first PCR assay performed confirmed whether fur mites were present. If this assay provided a positive result, a second assay was performed to confirm these results and differentiate the mite species.19 A positive PCR result was defined as any sample that yielded the presence of fur mites and confirmed the presence of either Myocoptes spp. alone or with Radfordia spp. and Myobia spp. Because the sequences evaluated in this PCR assay are about 98.8% identical between Radfordia spp. and Myobia spp., these results are reported collectively.20 A negative PCR result was defined as any sample that did not yield the presence of fur mites in the first assay.
Statistical analysis.
The marginal probabilities of obtaining positive or negative results for the PCR and tape test at each time point were compared by using an exact McNemar test for paired data. A P value less than or equal to 0.05 was considered statistically significant. The proportions of positive and negative results for each diagnostic test at each time point and their 95% confidence intervals were calculated. Data were analyzed by using StatXact-8 (Cytel Software, Cambridge, MA). In addition, the specificity of PCR was calculated. Samples positive by both tests were considered to have yielded true-positive results.
Results
At all time points during the study, the pooled samples collected from the control mice were negative by both tape-test and PCR. On day 1 (before treatment), only one fur-mite–infested sample was negative by tape test (Table 1). All other samples yielded positive results in both diagnostic tests. PCR revealed the presence of Myocoptes spp., Radfordia spp., and Myobia spp. Three tape tests samples from day 1 had discernible adult mites that could be speciated microscopically as male and female Myocoptes spp. mites. On all other day 1 tape-test slides, mite species could not be differentiated because hair shafts obstructed visibility or because only parts of adult mites were present. Eggs along hair shafts were identified on all slides.
Table 1.
Percentages of samples that were fur-mite positive (+) or negative (–) by each test at each time point
| Mean % (95% confidence interval) |
||||
| PCR+ and tape test+ | PCR– and tape test– | PCR+ and tape test– | PCR– and tape test+ | |
| Day 1 (pretreatment) | 97.2% (85.5% to 99.9%) | 0 | 2.8% (0.1% to 14.5%) | 0 |
| Week 6 | 40.5% (24.8% to 57.9%) | 16.2% (8.4% to 28.7%) | 10.8% (3.0% to 25.4%) | 32.4% (19.6% to 48.7%) |
| Week 12 | 29.0% (14.2% to 48.0%) | 19.4% (7.5% to 37.5%) | 22.6% (10.0% to 41.1%) | 29.0% (14.2% to 48.0%) |
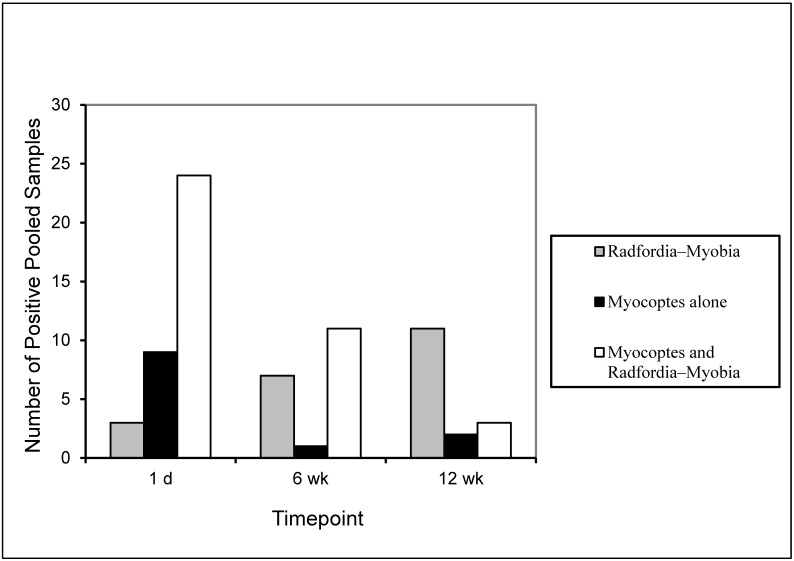
Figure 1.
The number of mite-positive PCR samples by species on day 1 before treatment and at 6 and 12 wk after treatment. The PCR assay used cannot differentiate Radfordia spp. and Myobia spp., which are closely related.
After treatment, both tests yielded negative results. The number of false-negative tape-test results increased from 2.8% on day 1 to 10.8% at 6 wk and 22.6% at 12 wk (Table 1). Approximately 29% (6 wk) and 32.4% (12 wk) of the samples after treatment were false-negative by PCR. The corresponding tape tests for these samples showed the presence of mite eggs, intact adult mites, or parts of adult mites (Table 2). PCR confirmed the presence of all 3 fur mite species in the positive posttreatment samples; the number of positive samples decreased over time for Myocoptes spp. only, whereas the numbers of Radfordia spp. and Myobia spp. collectively increased.
Table 2.
No. of posttreatment samples positive for fur mite eggs or adults or both by tape test and confirmed by positive PCR results
| Week 6 |
Week 12 |
|||
| Observation of tape test | Tape test | PCR | Tape test | PCR |
| Eggs only | 23 | 15 | 13 | 6 |
| Eggs + adults (whole or part) | 4 | 0 | 2 | 2 |
| Adults only | 0 | — | 3 | 1 |
The sensitivity of the PCR assay before treatment was calculated to be 100% (95% confidence interval, 92.6% to 100.0%). Given the reasonable assumption that all PCR-positive results are true positives, the specificity of PCR was 100%. There was no significant difference in the proportions of tests that were mite-positive or -negative by either PCR or tape test before or at 6 or 12 wk after treatment.
Discussion
Deciding on a single diagnostic method for murine fur mite evaluation can be difficult because the literature is contradictory regarding which diagnostic test is the most reliable and accurate for fur mites. Skin scrape has been reported to be the most accurate and sensitive diagnostic for identification of fur mites.5,23 In one study, 5 diagnostic tests were evaluated and ranked from most to least effective diagnostically: skin scrape was considered the most effective, followed by stereomicroscopy, tape test, hair pluck, and finally observation for clinical signs.5 Another study compared stereoscopic examination of the whole animal to hair pluck and concluded that accuracies of these diagnostics are comparable.2 A recent report compared the sticky-paper technique to the hair pluck test. For the sticky-paper technique, the mice were euthanized and placed on a piece of sticky paper overnight; the sticky-paper technique was more sensitive for fur-mite diagnosis than were hair plucks.15
The results of our study show that PCR has high sensitivity during active fur mite infection. The PCR and tape test results coincided 97.2% of the time on day 1, but this value decreased to 59.5% and 48.4% at 6 and 12 wk after treatment, respectively. According to the commercial laboratory, the PCR test has a sensitivity of less than 10 gene copies, potentially providing a more sensitive diagnostic method than the tape test for laboratories to use.19,20 With the PCR method, a swab is used to collect cellular debris and mite fragments;19 presumably mite DNA arising from any mixture of live adult mites or mite parts, dead adult mites or mite parts, eggs, or excrement would yield positive results in the PCR assay. If PCR detects both live and dead mites, then positive results are not necessarily indicative of active infection after treatment. The length of the hair cycle is as long as 8 mo in some strains of mice,24 suggesting that mite eggs or mite parts could still be attached to the hair shaft for this length of time and could result in a positive PCR until the hair is shed, even though an active infection is not present. At 6 wk after treatment, eggs and adult mites or mite parts were visualized on 4 tape tests, and none of these samples were positive by PCR. At 12 wk, a total of 5 tape-test samples had either adults only or eggs and intact or partial adult mites; and only 3 of these samples were positive by PCR. The reason for the false-negative results in our study are unclear but may be related to the reduction of mite-associated materials present from treatment, grooming patterns, or routine cage changes or to the collection method, especially given that PCR was negative when adult mites (whole or parts) were present by tape test after treatment. During preliminary evaluation of the fur-mite PCR assay, a total of 5 cages each containing a single fur-mite–infested mouse housed with 3 confirmed fur-mite–negative mice was tested by the commercial laboratory. The PCR assay was more sensitive than was fur pluck, tape test, and pelage exam, but no claims were made regarding the sensitivity or efficacy of the PCR assay after treatment.20
Several groups consider the dorsal tape test, in which cellophane tape is placed on the back of a euthanized mouse for 6 h, to be the most reliable diagnostic method.1,7,8 Tape test continues to be a common method in many laboratories because it is relatively quick, can be done on live animals, and is cost effective.5,10,13 In addition, tape test of the pelage has a reported estimated sensitivity of 84% for detecting Myocoptes spp.; however, some authors claim that the test is robust only during a heavy infestation.10,16 At our institution, the tape test is a common diagnostic tool. In the current study, the fur-mite–infested mice were part of a neurologic study; we therefore needed a reliable diagnostic method that allowed us to sample live animals. An advantage of the tape test, as with hair pluck, skin scrape, and PCR, is that these tests can be performed quickly on awake animals with minimal distress, whereas pelt examination and the sticky-paper technique require anesthetized or euthanized mice.2,5,8,13
PCR identified both Myobia–Radfordia spp. and Myocoptes spp. during infestation, whereas direct visualization of tape tests did not confirm the presence of Myobia spp. and Radfordia spp. Over the course of the study, the number of pooled samples containing only Myocoptes spp. decreased over time, suggesting that the treatment was effective for this species. In contrast, the number of cages positive for Radfordia–Myobia spp. increased over time. Myocoptes spp. can crowd out Myobia spp. during heavy infestations.7,8 These results may suggest that as the Myocoptes spp. burden was decreasing, Myobia spp. and Radfordia spp. were able to flourish and increase in numbers; however, the susceptibility of these mites to treatment is unknown. Following these mice past 12 wk to see whether the numbers of cages infected with the different mite species continued to increase or decline was not possible because they were euthanized for experimental purposes and their pelts were not available for further examination. Further studies are required to address the meaning of these results.
Permethrin (Mitarrest) was chosen as the treatment method for this study because of concerns of potential toxicity with ivermectin. Ivermectin is potentially toxic to young animals,25 and we wanted to limit any interference with the neurologic studies. Variable efficacy of different treatments for fur mites has been noted.2,4,16-18,22,24 The efficacy of permethrin depends on several factors including length of treatment, repeat use for treatment, and husbandry standards.3,14 Although we did not calculate the efficacy of the treatment we used, we had several samples that were confirmed to be mite-positive by both PCR and tape test at 12 wk after treatment. These positive results, however, do not confirm whether an active infection was still present. The choice of treatment may affect the results of the diagnostic test used.
One limitation of our study is the sample size, which was relatively small and constrained by the number of mice available in the control and mite-infested rooms. The fur mite-infested room had several breeding cages. Cages with litters were not sampled to avoid any adverse effect of handling on the experimental breeding and to prevent adding another variable that might have affected testing. Breeding and ongoing experiments led to considerable turnover of the population in the mite-infested room, and additional cages were selected randomly for testing to maintain a similar sample size throughout the study. Approximately 64% to 80% of the cages in the fur-mite–infested room were sampled during the study. It was not possible to increase the sample size without infecting additional animals. Although the sample size is small and did not include the breeding cages, the current study still reflects a typical infestation of a mouse colony in which only one room is affected and has a small number of cages.
In the current study, the head, neck, and abdomen of mice were sampled by tape test; the inguinal region was not sampled. We chose this collection method because it closely resembles the current procedure at our institution, where the pelage is collected at necropsy to assess for fur mites. Although we did not sample the inguinal region by tape test, this diagnostic method still provided positive results at all time points. The entire body of the mice, including the inguinal region, was sampled for the PCR assay, and negative results were obtained after treatment when the corresponding tape test was positive. All of the commonly used fur mite diagnostic tests rely on visualization of adult mites or eggs, and false-negatives are possible with inadequate sampling, low mite burdens, enzootic populations, early stage of infestation, or mature mice with stable or suppressed burdens.10 The advent of a PCR-based assay provides an additional diagnostic tool that has a relatively quick and efficient collection method for detecting fur mites on awake animals or in their environment (for example, cages and bedding).19,20 Although PCR may be costly, pooling samples (that is, a single swab for multiple animals), as we performed here, can help decrease the overall cost of the test.
To our knowledge, this study is the first to compare a PCR assay with the tape test in the context of a natural infection by fur mites. Our findings indicate that PCR is a reliable diagnostic method during active infection prior to treatment for fur mites. This assay is highly specific for murine fur mites and can identify the mite species once an infestation is confirmed. Negative results should be interpreted carefully if fur mites are suspected, and a secondary diagnostic method should be considered to supplement PCR results after treatment. The presence of mite eggs, intact adult mites, or adult mite parts during the secondary diagnostic evaluation may be indicative of infection. Clinical assessment of the animals and collection of follow-up samples would need to be considered to determine whether the infection is active (or not). Additional studies with a larger and more controlled sample size, additional time points, and comparison with other diagnostic techniques are required to better assess whether PCR assays could effectively replace our current diagnostics for murine fur mites.
Acknowledgments
We acknowledge Eugene Dunn and Marion Derby (Comparative Pathology Laboratory, University of California, Davis, CA) for their help reviewing the tape test slides. We also thank Saleda Braggs, Carolyn Smith, and Jaime Amaral (Campus Veterinary Services, University of California) for their help with sample collection, Dr Rebecca Sammak for her help with sample preparation, and Drs Phil Kass and Amir Ardeshir for statistical analysis.
References
- 1.Baker DG, 2007. Flynn's parasites of laboratory animals. Ames (IA): Blackwell Publishing
- 2.Baumans V, Havenaar R, van Herck H. 1988. The use of repeated treatment with Ivomec and Neguvon spray in the control of murine fur mites and oxyurid worms. Lab Anim 22:246–249 [DOI] [PubMed] [Google Scholar]
- 3.Bean-Knudsen DE, Wagner JE, Hall RD. 1986. Evaluation of the control of Myobia musculi infestations on laboratory mice with permethrin. Lab Anim Sci 36:268–270 [PubMed] [Google Scholar]
- 4.Bornstein DA, Scola J, Rath A, Warren HB. 2006. Multimodal approach to treatment for control of fur mites. J Am Assoc Lab Anim Sci 45:29–32 [PubMed] [Google Scholar]
- 5.Burdett EC, Heckmann RA, Ochoa R. 1997. Evaluation of 5 treatment regimens and 5 diagnostic methods for murine mites (Myocoptes musculinus and Myobia musculi). Contemp Top Lab Anim Sci 36:73–76 [PubMed] [Google Scholar]
- 6.Dawson DV, Whitmore SP, Bresnahan JF. 1986. Genetic control of susceptibility to mite-associated ulcerative dermatitis. Lab Anim Sci 36:262–267 [PubMed] [Google Scholar]
- 7.Fox J, Anderson LC, Loew FM, Quimby FW, 2002. Laboratory animal medicine, 2nd ed. San Diego (CA): Academic Press
- 8.Fox J, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL, 2007. The mouse in biomedical research, vol 2: diseases. San Diego (CA): Academic Press
- 9.Hendrix CM.1998. Diagnostic veterinary parasitology. St Louis (MO): Mosby.
- 10.Huerkamp MJ, Zitzow LA, Webb S, Pullium JK. 2005. Cross-fostering in combination with ivermectin therapy: a method to eradicate murine fur mites. Contemp Top Lab Anim Sci 44:12–16 [PubMed] [Google Scholar]
- 11.Iijima OT, Takeda H, Komatsu Y, Matsumiya T, Takahashi H. 2000. Atopic dermatitis in NC/Jic mice associated with Myobia musculi infestation. Comp Med 50:225–228 [PubMed] [Google Scholar]
- 12.Institute for Laboratory Animal Research. 1996. Guide for care and use of laboratory animals. Washington (DC): National Academies Press.
- 13.Lindstrom KE, Carbone LG, Kellar DE, Mayorga MS, Wilkerson JD. 2011. Soiled-bedding sentinels for the detection of fur mites in mice. J Am Assoc Lab Anim Sci 50:54–60 [PMC free article] [PubMed] [Google Scholar]
- 14.Mather T, Lausen NCG. 1990. A new insecticide delivery method for control of furmite infestations in laboratory mice. Lab Anim 19:25–29 [Google Scholar]
- 15.Metcalf Pate KA, Rice KA, Wrighten R, Watson J. 2011. Effect of sampling strategy on the detection of fur mites within a naturally infested colony of mice (Mus musculus). J Am Assoc Lab Anim Sci 50:337–343 [PMC free article] [PubMed] [Google Scholar]
- 16.Mook DM, Benjamin KA. 2008. Use of selamectin and moxidectin in the treatment of mouse fur mites. J Am Assoc Lab Anim Sci 47:20–24 [PMC free article] [PubMed] [Google Scholar]
- 17.Pollicino P, Rossi L, Rambozzi L, Farca AM, Peano A. 2008. Oral administration of moxidectin for treatment of murine acariosis due to Radfordia affinis. Vet Parasitol 151:355–357 [DOI] [PubMed] [Google Scholar]
- 18.Pullium JK, Brooks WJ, Langley AD, Huerkamp MJ. 2005. A single dose of topical moxidectin as an effective treatment for murine acariasis due to Myocoptes musculinus. Contemp Top Lab Anim Sci 44:26–28 [PubMed] [Google Scholar]
- 19.RADIL. [Internet]. 2010. The answer to keeping fur mites out of your animal facility—RADIL's mite PCR—the most sensitive method for mite detection! [Cited 19 December 2011]. Available at: http://wwwradilmissouriedu/userfiles/download_files/RADIL_Mites%20Flyerpdf.
- 20.RADIL. [Internet]. 2011. Defeating the mighty mite: biology, impact, and diagnosis. [Cited 19 December 2011]. Available at: http://radilmissouriedu/
- 21.RADIL. [Internet]. 2011. Video: swabbing technique for fur mites. [Cited 19 January 2012]. Available at: http://wwwradilmissouriedu/On-Line_Resources/SOPs/Video__Swabbing_technique_for_mites/indexhtml.
- 22.Ricart Arbona RJ, Lipman NS, Riedel ER, Wolf FR. 2010. Treatment and eradication of murine fur mites: I. Toxicologic evaluation of ivermectin-compounded feed. J Am Assoc Lab Anim Sci 49:564–570 [PMC free article] [PubMed] [Google Scholar]
- 23.Ricart Arbona RJ, Lipman NS, Wolf FR. 2010. Treatment and eradication of murine fur mites. II: diagnostic considerations. J Am Assoc Lab Anim Sci 49:583–587 [PMC free article] [PubMed] [Google Scholar]
- 24.Ricart Arbona RJ, Lipman NS, Wolf FR. 2010. Treatment and eradication of murine fur mites. III: treatment of a large mouse colony with ivermectin-compounded feed. J Am Assoc Lab Anim Sci 49:633–637 [PMC free article] [PubMed] [Google Scholar]
- 25.Skopets B, Wilson RP, Griffith JW, Lang CM. 1996. Ivermectin toxicity in young mice. Lab Anim Sci 46:111–112 [PubMed] [Google Scholar]
- 26.Weisbroth SH, Friedman S, Powell M, Scher S. 1974. The parasitic ecology of the rodent mite Myobia musculi. I. Grooming factors. Lab Anim Sci 24:510–516 [PubMed] [Google Scholar]