Abstract
The streptozocin-induced diabetic rat is a model of chronic pain that shows signs of hyperalgesia and allodynia and may replicate signs in diabetic humans. Here we investigated the antinociceptive effects of A803467, a highly selective blocker of Nav1.8 channels, in diabetic rats with painful neuropathy. We systemically (intraperitoneal) or locally (intraplantar) administered A803467 (or lidocaine, a nonselective sodium channel blocker, as a control) to diabetic rats with hyperalgesia and allodynia and then measured thermal latencies and mechanical thresholds. With intraperitoneal administration, A803467 led to 6-fold greater reduction of hyperalgesia and 2-fold greater reduction of allodynia than did lidocaine. Whereas the antihyperalgesic effects of lidocaine and A803467 were similar after intraplantar administration, A803467 (1 mg) was at least 2 times more effective as an antiallodynic than was lidocaine (0.5 mg). These results suggest that compared with lidocaine, systemic or local blockade of Nav1.8 channels by A803467 may more effectively relieve hyperalgesia and allodynia in diabetic neuropathy.
Abbreviation: STZ, streptozotocin
Painful neuropathy is one of the most frequent complications of diabetes mellitus. This neuropathy involves complex mechanisms that affect both peripheral nerves and the CNS and can be difficult to treat efficiently. Streptozotocin (STZ)-induced diabetes in rodents has been extensively used to study neuropathic pain and to explore the efficacies of new potential therapeutic drugs by using sensory behavioral tests. The current study used the well-characterized STZ injection model, which results in toxicity to pancreatic islet β cells and rapidly produces diabetes. Diabetic neuropathy can be easily identified and followed in this model through associated sensory dysfunctions, including prolonged (several weeks) thermal hyperalgesia and mechanical allodynia of the plantar hindpaw in rats.6,10,15
In human clinical practice, painful neuropathy is treated based pharmacologically. Sodium channels blockade is one of the best-known treatments for relieving diabetes-induced pain.14,32 Several studies have shown the importance of voltage-dependent sodium channels in the initiation and propagation of action potentials during both normal nerve conduction4,13 and the development of neuropathic states.40,44,46
At least 9 different identified voltage-dependent sodium-channel subtypes (Nav1.1 to 1.9) have been identified in the nervous system, and each subtype can be classified functionally as either tetrodotoxin-sensitive (fast-inactivating) or -resistant (slow-inactivating).7,12,46 Seven of these subtypes are expressed in sensory neurons in the dorsal root ganglion, and peripheral nerve injury can alter the expression pattern of several voltage-gated sodium channel types.2,31,38 Of the sodium channels expressed, most of the tetrodotoxin-sensitive types (that is, Nav1.1, Nav1.2, Nav1.6, and Nav1.7) and both of the tetrodotoxin -resistant types (Nav1.8 and Nav1.9) are downregulated in dorsal root ganglia after nerve damage.1,11,30
The expression of Nav1.8 is decreased in many neuropathic pain models, and Nav1.8 may play a key role in the generation of hyperalgesia and allodynia in nerve-injured animals.9,25,30 Recent work has suggested that A803467 (5-[4-chloro-phenyl]-furan-2-carboxylicacid [3, 5-dimethoxy-phenyl]-amide) is a potent and highly selective blocker of Nav1.8 channels.23,24,33 A803467 is 300- to 1000-fold more potent at blocking Nav1.8 channels than any other voltage-dependent sodium channel.23 Previous studies have demonstrated the efficacy of A803467 in a variety of animal pain models, including models of acute, inflammatory, neuropathic, and visceral pain.23-25 However, its effects on diabetes-induced painful neuropathy are unknown.
Changes in the functions of Nav1.8 channels can contribute to diabetes-induced behavioral indicators, such as thermal hyperalgesia and mechanical allodynia, in painful diabetic neuropathy. In the current study, our criteria for determining that rats had diabetic neuropathy was based on the pronounced mechanical allodynia and thermal hyperalgesia that develops after STZ injection and the subsequent rapid rise in blood glucose levels. We tested the efficacy of systemic (intraperitoneal) or local (intraplantar) injection of A803467 in reducing diabetes-induced thermal hyperalgesia and mechanical allodynia in rats and compared these effects with those of lidocaine, a nonspecific sodium-channel blocker.
Materials and Methods
The experimental protocols used were IACUC-approved and were consistent with the guidelines of the ethical committee of the International Association for the Study of Pain.45
Animals and induction of diabetes.
The study population comprised adult female albino Wistar rats (Rattus norvegicus, Hsd:WI, weight, 260 to 280 g) that were obtained from the Medical Sciences Research Centre of Cukurova University (Adana, Turkey) and that were certified free of common rat pathogens. Female rats were used because they are less aggressive than are adult male rats, easier to handle during behavioral testing, and more sensitive to many pain conditions.5 In addition, although most pain sufferers are women,18 only about 8% to 10% of available animal pain studies have been performed with female animals.5,18 Therefore, studies of female pain sensitivity could be important for the improvement of new approaches to management.
The rats were maintained in a climate-controlled and sound-isolated room (22 to 24 °C) under a 12:12-h light:dark cycle (0600 to 1800), with 40% to 60% relative humidity, 8 to 12 air changes hourly throughout the course of the study, and ad libitum provision of feed (pellets) and water. Rats were housed 4 per cage in solid-floored polycarbonate cages (46 × 25 × 20 cm) with a deep layer of poplar–aspen sawdust; cages were changed daily because of diabetes-mellitus–induced polyuria and diarrhea. Bedding materials were free of dust and contaminants, nontraumatic, moisture-absorbent, and ammonia binding.
Diabetes was induced by a single intravenous injection of STZ (45 mg/kg, with 23-gauge needle) into the tail vein after light anesthesia with 1% to 2% isoflurane in oxygen. STZ was prepared freshly by dissolving in 0.9% sterile saline. Control animals were age-matched and treated with saline rather than STZ. Induction of diabetes was confirmed after 3 d on the basis of blood glucose levels as determined by a commercial glucometer and test strips (Accutrend GCT, Roche, Mannheim, Germany) used on a small drop of blood obtained by tail prick under light isoflurane anesthesia. A blood glucose level of 300 mg/dL was defined as the minimum for a diagnosis of diabetes. Body weights and blood glucose levels were monitored every 2 wk at the same time of the day (0900 to 1000) throughout the experiment. Care was taken during the induction of diabetes to avoid general illness in the rats. General health was monitored, and all animals demonstrated appropriate behavior during the entire study.
Drugs and experimental procedures.
All chemicals used in the experiments were purchased from Sigma–Aldrich (Munich, Germany). A803467 and lidocaine were dissolved in DMSO and saline, respectively. Drug solutions were vortexed until complete dissolution. For intraperitoneal administration, a dose of 2 mL/kg was used. Intraplantar injections (volume, 100 µL) were made into the plantar side of the right hindpaw. For vehicle-only control groups, equal volumes of DMSO or saline were injected intraperitoneally or intraplantarly. All rats were handled in accordance with institutional guidelines. The experimenter (same experimenter during the all experiments) acclimated rats by handling them at least 3 times for 20 to 30 s for 3 d before the experiment. On the day of the experiment, no rats reacted adversely during handling.
Optimal hyperalgesia and allodynia in rats occurred at 4 wk after STZ injection, at which time the effects of systemic (intraperitoneal, with 25-gauge needle) or local (intraplantar, with 30-gauge hypodermic needle) administration of A803467 or lidocaine were examined. The doses of drugs administered were chosen in accordance with our previous studies34,35 and several pilot studies, thus enabling us to minimize the number of rats used in the current study. We injected 7 rats (age-matched diabetic and nondiabetic) at each dose and route: A803467, 5 and 10 mg/kg intraperitoneally and 0.5 and 1 mg intraplantarly; and lidocaine, 10 and 30 mg/kg intraperitoneally and 0.5 mg and 1 mg intraplantarly. All rats were monitored closely for potential adverse effects, including muscle paralysis, respiratory distress, sedation, and unusual behavior. All rats remained free of signs of respiratory distress, displayed symptoms of motor impairment, sedation, and paralysis at all doses and routes throughout the experiment.
Both paws of all rats (injected right paw and noninjected left paw) were tested for all experimental groups. The ipsilateral (right) hindpaw was tested to determine local effects of injected drugs and the contralateral paw left paw to determine systemic effects of drugs. Due to the obvious physical traits of the diabetic rats, the experimenter was not blind to experimental group but was blinded to drug and dose.
Sensory testing procedure.
Sensitivities of diabetic animals to noxious and nonnoxious stimulation are altered due to sensory dysfunction.6,8 To assess sensory abnormalities that include hyperalgesia to noxious thermal and allodynia to innocuous mechanical stimuli,19,26,34,43 we used thermal plantar test and a dynamic plantar aesthesiometer, respectively.
All sensory tests were performed in a quiet room maintained at 23 to 25 °C, beginning at 0900 and according to the same time schedule to avoid diurnal variation. To avoid procedure-associated stress that might affect measurements, the same experimenter performed all experiments in a test room close to the colony room. Rats were acclimated to the experimental environment for 1 wk before STZ administration. Habituation to the experimental setup was accomplished by placing the rats on the test apparatus 3 times for at least 30 min each.
Testing of thermal hyperalgesia.
The presence of thermal hyperalgesia was determined by measuring paw withdrawal latency in a thermal stimulation system consisting of a clear plastic chamber (10 × 20 × 24 cm) that sits on a clear smooth glass floor, with the temperature regulated at 30 °C.19,34 Rats were placed individually in the chamber and allowed approximately 15 min to acclimate to the testing environment.
A radiant heat source (8-V, 50-W halogen bulb) mounted on a movable holder below the glass pane was positioned to deliver a thermal stimulus to the midplantar region of the left or right hindpaw. The intensity of the heat stimulus was maintained constant throughout all experiments. When the rat felt pain and withdrew its paw, a photocell detected interruption of a light beam reflection, the infrared generator was switched off automatically, and the timer stopped, determining the withdrawal latency. This method has a precision of 0.1 s for the measurement of paw withdrawal latency. The thermal source was discontinued automatically after 25 s (cut-off latency) whenever a rat failed to withdraw its paw.
Testing of mechanical allodynia.
Mechanical allodynia was determined by quantifying the withdrawal threshold of the hindpaw in response to mechanical stimulation. An automated version of the von Frey hair test (Dynamic Plantar Aesthesiometer, Ugo Basile, Comerio, Italy) was used for the assessment of sensitivity to a nonnoxious, light touch of the paw. A significant decrease in the threshold necessary to elicit a brisk paw withdrawal in response to mechanical stimulus was interpreted as mechanical allodynia.26,34
Rats were placed in individual acrylic boxes on a stainless steel mesh floor and allowed to acclimate for at least 15 min before testing. A straight metal filament (diameter, 0.5 mm) was used to apply force (force ramp, 2.5 g/s) to the plantar surface of the hindpaw until the rat lifted its foot, at which point the paw withdrawal threshold was digitally recorded in grams. A cut-off of 50 g was imposed to prevent significant tissue damage.
Statistical analysis.
Post hoc power analysis, with an α level of 0.05, showed that a sample size of 7 rats for each experimental group was sufficient to establish significant differences for parameters measured with a power of 0.80 or greater.
In the data displayed, each point is an average of 7 rats and values are presented as mean ± 1 SD. Results were evaluated by using ANOVA (Statistical Package for Social Sciences 15.0, SPSS, Chicago, IL). Multifactor experimental data were analyzed by using 2-way ANOVA; single-factor, multiple treatment data were analyzed by using one-way ANOVA. Two-way ANOVA models were used to analyze the effects of agent treatments; thermal latency or mechanical threshold measured during testing served as the dependent variable.
To evaluate the efficacies during agent treatment, thermal latencies and mechanical thresholds were compared between drug-injected and vehicle-injected groups across all posttreatment test sessions by using Kruskal–Wallis test followed by the Dunn multiple range test. We also evaluated the effects of drug treatments by conducting one-way, repeated-measures ANOVA on the withdrawal latencies measured in the injected paw before and after drug treatment. A P value of less than 0.05 was considered statistically significant.
Results
Diabetic complications in STZ-treated rats.
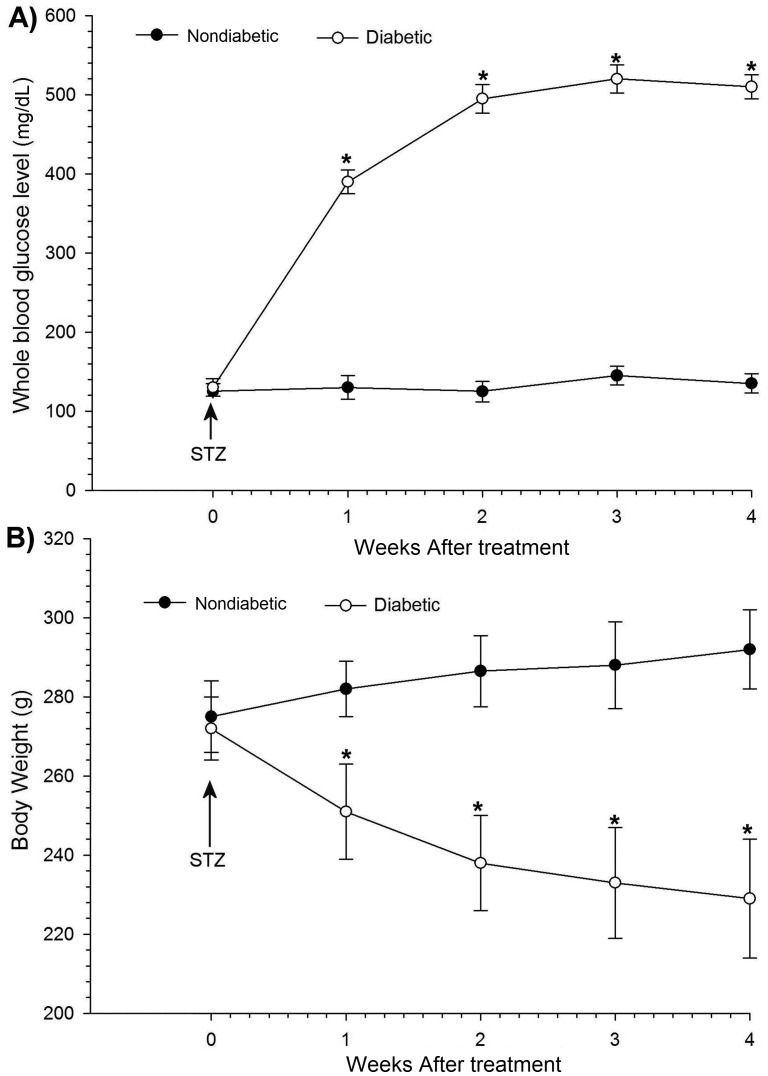
STZ-injected rats developed at least a 3-fold increase (P < 0.05) in blood glucose levels, as compared with nondiabetic weight-matched rats. STZ injection caused a rapid elevation of average whole-blood glucose levels within 2 wk, which then did not change significantly throughout the experiments (Figure 1 A). Another important sign in STZ-induced diabetes is weight loss. Compared with their starting weights, the body weights of STZ-treated rats decreased (by an average of 14.7% overall; P < 0.05), whereas nondiabetic weight-matched control rats gained weight (P < 0.05; Figure 1 B).
Figure 1.
General metabolic indicators of saline-treated (nondiabetic) and STZ-treated rats. (A) Blood glucose levels were elevated after treatment with STZ compared with those in nondiabetic rats. (B) STZ induction caused significant decreases in body weight. Each point represents the mean value of 7 rats, and the vertical bars indicate 1 SD. *, Significant (P < 0.05; repeated-measures ANOVA and the posthoc Dunn test) differences between diabetic and nondiabetic groups.
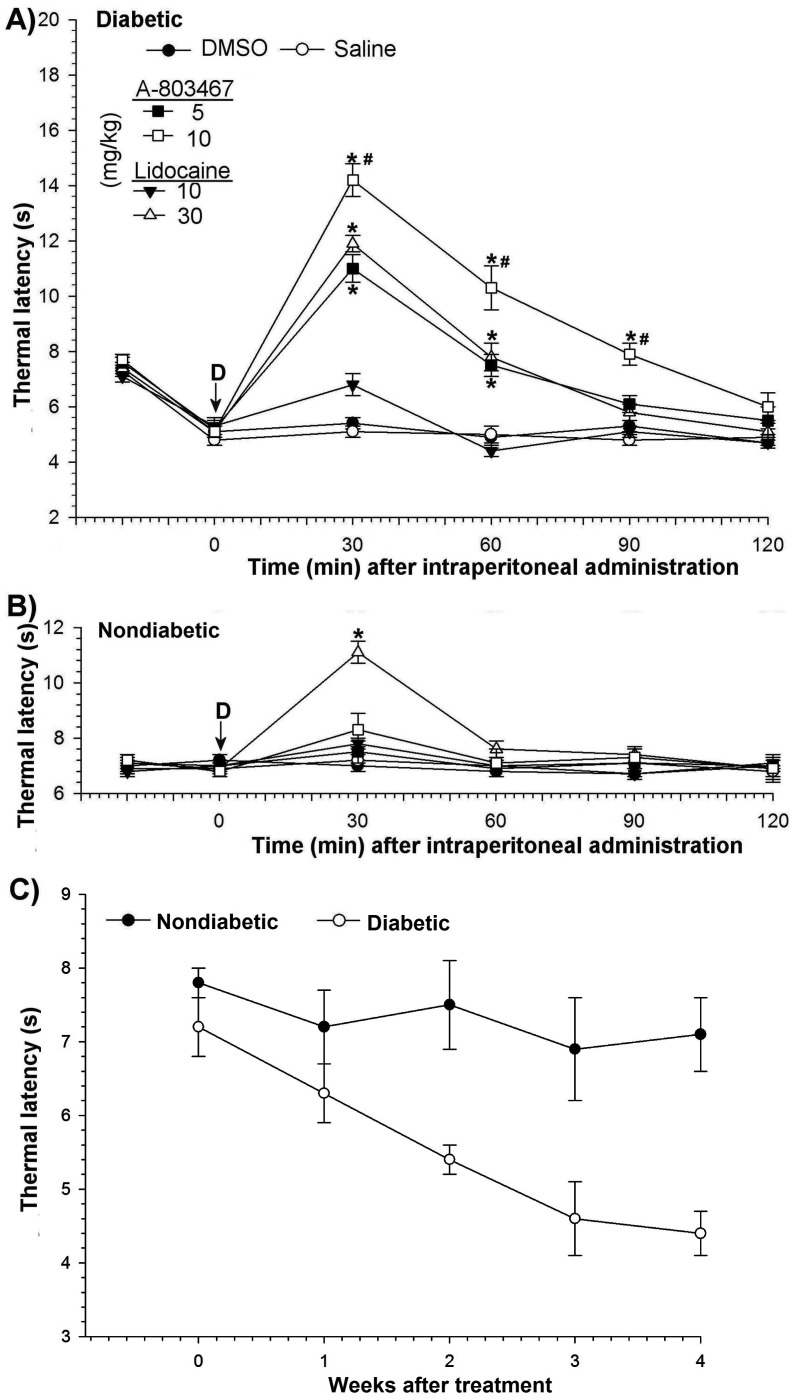
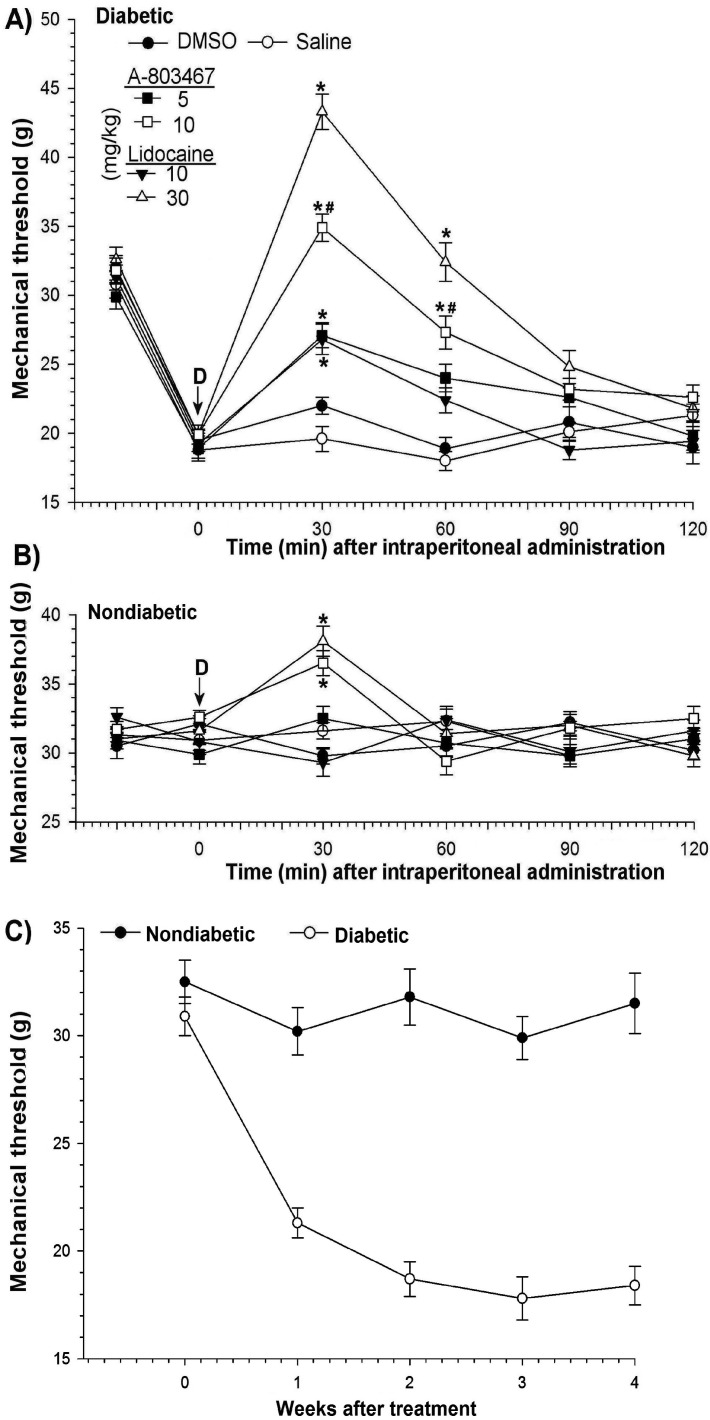
After STZ injection, rats exhibited diabetes-induced thermal hyperalgesia. To determine thermal hyperalgesia, all rats were examined for their behavioral responses to noxious thermal stimulation before and after STZ administration. Compared with those of control rats and before STZ, the paw withdrawal latency of diabetic rats was significantly (P < 0.05) reduced (Figure 2 C). The hindpaws of STZ-injected rats also became more sensitive to nonnoxious mechanical stimuli, an indicator of mechanical allodynia. The mean threshold to response was shorter (P < 0.05) in STZ-treated rats than in control rats (Figure 3 C) Nondiabetic weight-matched control rats exhibited no changes in either thermal latency or mechanical threshold.
Figure 2.
Paw withdrawal latency after thermal stimulus. Antihyperalgesic effects of intraperitoneal administration of A803467 and lidocaine to (A) diabetic and (B) nondiabetic rats. (C) Time course of thermal hyperalgesia after STZ treatment. Each point represents the mean value of 7 rats, and the vertical bars indicate 1 SD. D, time of drug injection; arrow, pretreatment value; *, significant (P < 0.05; Kruskal–Wallis repeated-measures ANOVA with Dunn multiple-comparison tests) difference from values of DMSO only and saline groups at each time point; #, significant (P < 0.05; Kruskal–Wallis repeated-measures ANOVA with Dunn multiple-comparison tests) difference from values after 10 mg/kg A803467 and 10 mg/kg lidocaine at each time point.
Figure 3.
Changes in mechanical threshold to mechanical stimulus. Antiallodynic efficacies of intraperitoneal administration of A803467 and lidocaine to (A) diabetic and (B) nondiabetic rats. (C) Time course of mechanical allodynia after STZ treatment. Each point represents the mean value of 7 rats, and the vertical bars indicate 1 SD. D, time of drug injection; arrow, pretreatment value; *, significant (P < 0.05; Kruskal–Wallis repeated-measures ANOVA with Dunn multiple-comparison tests) difference from values of DMSO only and saline groups at each time point; #, significant (P < 0.05; Kruskal–Wallis repeated-measures ANOVA with Dunn multiple-comparison tests) difference from values after 10 mg/kg A803467 and 10 mg/kg lidocaine at each time point.
Effects of intraperitoneal A803467 and lidocaine.
The thermal latencies and mechanical thresholds obtained from unmanipulated age-matched nondiabetic and diabetic rats did not differ from those of the vehicle-only control rats that received intraperitoneal DMSO or saline.
Intraperitoneal administration of A803467 to nondiabetic animals did not significantly change thermal latencies (Figure 2 B) but had transient effects (P < 0.05; maximum, 30 min) on mechanical thresholds at its highest doses (Figure 3 B). However, lidocaine at only 30 mg/kg caused transient increases (P < 0.05 at 30 min) in both the thermal latency and mechanical threshold of nondiabetic rats (Figures 2 B and 3 B).
After intraperitoneal administration, A803467 had a more pronounced antihyperalgesic effect than did lidocaine (Figure 2 A). The antihyperalgesic effect of A803467 at 10 mg/kg in diabetic rats peaked within 30 min after treatment (baseline, 5.1 ± 0.2 s; 30 min, 14.2 ± 0.6 s) and gradually returned to baseline within 120 min (Figure 2 A). A 10-mg/kg dose of lidocaine did not significantly change latency; the antihyperalgesic effect of lidocaine first appeared at a dose of 30 mg/kg. The antihyperalgesia of a 5-mg/kg dose of A803467 was similar to that of 30 mg/kg lidocaine at the all time points. These results show a 6-fold difference in doses of A803467 and lidocaine that produce the same antihyperalgesic effect.
The antiallodynic potency of intraperitoneal A803467 was higher than that of lidocaine (Figure 3 A). A803467 significantly (P < 0.05) increased the mechanical thresholds of diabetic rats with painful neuropathy in a dose- and time-dependent manner, reaching a maximum 30 min after treatment with 10 mg/kg (baseline, 19.9 ± 0.7 g; 30 min, 34.9 ± 1.1 g, and gradually returning to baseline within 90 min. Lidocaine similarly induced a maximal antiallodynic response at 30 min but at a dose of 30 mg/kg (baseline, 20.1 ± 1.1 g; 30 min, 43.3 ± 1.6 g); this effect disappeared within 90 min (Figure 3 A). At the same antiallodynic effect, the required intraperitoneal doses of A803467 (5 mg/kg) and lidocaine (10 mg/kg) differed 2-fold.
Effects of intraplantarly administered A803467 or lidocaine.
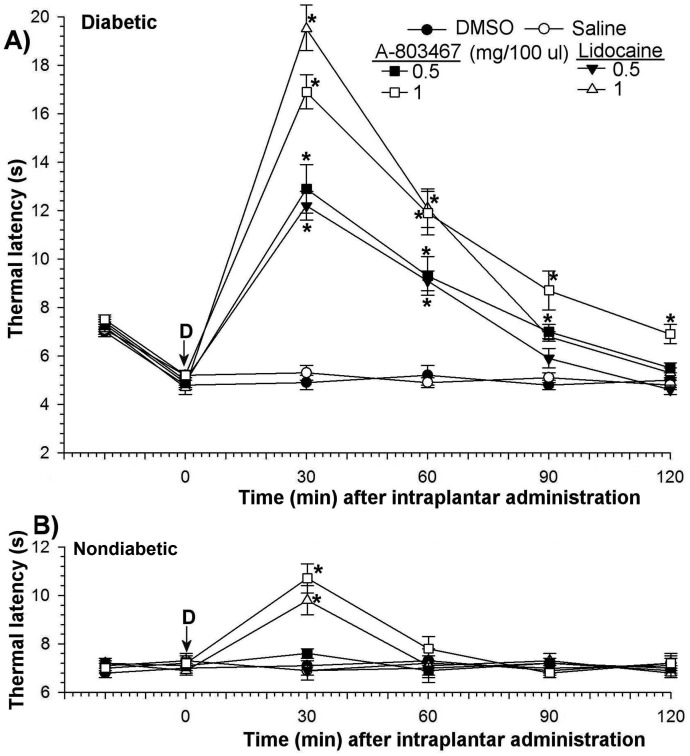
Thermal latencies and mechanical thresholds after intraplantar administration of solvent only (DMSO or saline) to diabetic or age-matched nondiabetic rats did not differ from those in unmanipulated animals. In contrast, intraplantar administration of only 1 mg/kg A803467 or lidocaine into the paws of nondiabetic rats produced significant transient increases (P < 0.05 at 30 min) in both thermal latency (Figure 4 B) and mechanical threshold (Figure 5 B).
Figure 4.
Effects of intraplantar A803467 and lidocaine on thermal hyperalgesia in diabetic (A) and nondiabetic rats (B). Each point represents the mean value of 7 rats, and the vertical bars indicate the SD. D, time of drug injection; arrow, pretreatment value; *, significant (P < 0.05; Kruskal–Wallis repeated-measures ANOVA with Dunn multiple-comparison tests) difference from values of DMSO only and saline groups at each time point.
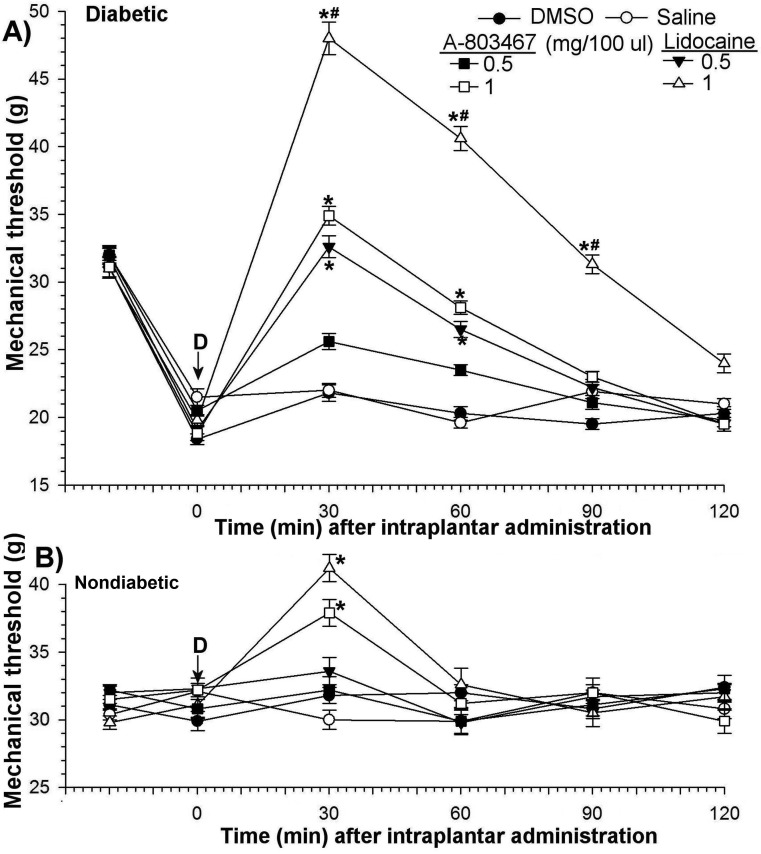
Figure 5.
Antiallodynic effects of intraplantar A803467 and lidocaine in (A) diabetic and (B) nondiabetic rats. Each point represents the mean value of 7 rats, and the vertical bars indicate the SD; D, time of drug injection; arrow, pretreatment value; *, significant (P < 0.05; Kruskal–Wallis repeated-measures ANOVA with Dunn multiple-comparison tests) difference from values of DMSO only and saline groups at each time point; #, significant (P < 0.05; Kruskal–Wallis repeated-measures ANOVA with Dunn multiple-comparison tests) difference from values after 1 mg/kg A803467 and 1 mg/kg lidocaine at each time point.
Intraplantar A803467 or lidocaine at a dose level of 0.5 mg/kg had no effect on thermal latency or mechanical threshold in nondiabetic rats but significantly (P < 0.05) decreased diabetes-induced hyperalgesia and allodynia. The antihyperalgesic potencies of A803467 and lidocaine were equivalent in diabetic rats (Figure 4 A). The antihyperalgesic efficacy of these drugs increased (P < 0.05) in a dose-dependent manner and decreased (P < 0.05) in a time-dependent manner. At 1 mg/kg, the antihyperalgesic effects of both drugs reached maximum within 30 min after treatment (baseline, 5.2 ± 0.2 s; A803467 at 30 min, 4.7 ± 0.2 to 16.9 ± 0.7 s; lidocaine at 30 min, 19.5 ± 0.9 s. These effects gradually returned to baseline within 90 min (Figure 4 A).
In contrast, the antiallodynic effects of intraplantar A803467 and lidocaine were very different (Figure 5A). The doses of A803467 (1 mg) and lidocaine (0.5 mg) that caused the same antiallodynic effect differed by 2-fold. At a dose 1 mg, the antiallodynic potency of lidocaine (baseline, 19.8 ± 0.4 g; 30 min, 48 ± 1.2 g) was greater (P < 0.05) than that of A803467 (baseline, 18.9 ± 0.5 g; 30 min, 34.9 ± 0.7 g). Whereas the antiallodynic action of lidocaine lasted for 120 min, A803467-induced increases (P > 0.05) in mechanical thresholds persisted for 60 min (Figure 5 A). Intraplantar A803467 or lidocaine did not alter paw withdrawal latencies in the contralateral control paws of diabetic rats (data not shown).
Discussion
The current study confirmed previous findings6,10,15 that STZ-induced diabetes in rats alters pain sensitivity and produces clinical signs such as allodynia and hyperalgesia. Our data suggest that Nav1.8 sodium channels contribute to the hyperalgesia and allodynia in diabetic rats with painful neuropathy. A803467, a selective blocker of Nav1.8 sodium channels, has antinociceptive effects in diabetic rats with painful neuropathy.
Behavioral and physiologic studies involving rodents have revealed indices of sensory dysfunction in animal models of diabetes that include hyperalgesia to thermal stimuli and allodynia to light touch.10,16,42,43 Tests of thermal hyperalgesia and mechanical allodynia are used widely to assess the efficacy of drugs aimed at alleviating painful neuropathy. The current study showed that rats with STZ-induced diabetes exhibited shorter withdrawal latencies to noxious thermal stimuli, illustrating thermal hyperalgesia.3,34 In addition, the paws of diabetic rats were more sensitive to nonnoxious mechanical stimuli, indicating mechanical allodynia in these animals.6,26,34
Previous studies in experimental animals and humans have shown that diabetes alters the function of many types of voltage-dependent sodium channels.20,46 Numerous factors can contribute to changes in functions of these channels in diabetic neuropathy, such as structural changes and various metabolic abnormalities. Hyperglycemia can be one such factor and is the primary pathophysiologic mechanism in various diabetes-associated conditions.28 Hyperglycemia-induced activation of the polyol pathway can cause a decrease in sodium currents due to Na+–K+ pump hypofunction in nerves.29,36 Therefore, voltage-dependent sodium channels may be an important target for the therapy of diabetic peripheral neuropathic pain.
Although nonselective sodium channel blockers such as tetrodotoxin have been important to understanding the functions of sodium channels, several clinically relevant nonselective blockers, such as lidocaine and carbamazepine, are used for the treatment of pain.17,39 These agents have complex inhibitory actions on voltage-gated sodium currents and can produce both tonic and use-dependent blockage of sodium currents.41 Although these drugs are useful clinically, they do not always show high specificity for sodium channels over other types of ion channels, and in general have only slight differences in their effects on the different channel isoforms.22,39
Changes in the activities of sodium channels, particularly tetrodotoxin-resistant Nav1.8 sodium channels, can play an important role in progression of diabetes-induced signs of pain, such as thermal hyperalgesia and mechanical allodynia.21,31 In the current study, antihyperalgesic and antiallodynic actions of systemically or locally administered A803467, a specific Nav1.8 blocker, were compared with those of lidocaine, a nonspecific voltage-dependent sodium channel blocker, in diabetic rats with painful neuropathy. Systemic A803467 was more antihyperalgesic and antiallodynic in diabetic rats than was lidocaine, suggesting that Nav1.8 channels contribute to progression of diabetes-induced pain. This hypothesis is in agreement with previous studies.20,21,36
A 2-fold difference between the antiallodynic potencies of A803467 and lidocaine was accompanied by a 6-fold difference in their antihyperalgesic potencies. These results imply that, compared with lidocaine, A803467 more effectively prevents the transmission of pain signals in unmyelinated C fibers. Mechanical allodynia is mediated by the large dorsal root ganglion cells of the myelinated Aα/β fibers and thinly myelinated Aδ fibers, whereas thermal hyperalgesia is mediated by unmyelinated C fibers.6,27 Furthermore, previous studies have shown that Nav1.8 channels are concentrated in thinly myelinated and unmyelinated nerve fibers and the terminals of central primary afferent nerves.2,13,38
Local injection of drugs into the plantar surface of the hindpaw is a useful method for determining direct pharmacologic effects on peripheral nerve endings. In the current study, withdrawal latencies in both the ipsilateral (treated, right) paw and noninjected contralateral (control, left) paw were measured after intraplantar administration in all experimental groups. Withdrawal latencies of the ipsilateral paw assess the direct effects of the agent tested, whereas those of the contralateral paw assess systemic effects of the injection. In the current study, neither intraplantar A803467 nor lidocaine had any effect on withdrawal latencies in the contralateral paw. Therefore, antinociceptive or antihyperalgesic effects of intraplantar injection were not due to systemic absorption of the drug.
Intraplantar A803467 and lidocaine both produced significant antihyperalgesic and antiallodynic activity in diabetic rats, again indicating that Nav1.8 sodium channels in peripheral nerve endings contribute to the reception and transmission of painful signals in diabetic rats with neuropathy. Although antihyperalgesic effects of intraplantar A803467 and lidocaine were the same, the lidocaine-induced antiallodynia was 2-fold higher than that of A803467. This high potency of lidocaine may indicate that sodium channels in addition to Nav1.8 are important in diabetes-induced allodynia. Furthermore, data indicated that the effects of A803467 are concordant with its systemic activity. Similarly, the antihyperalgesic efficiency of locally administered A803467 was higher than its antiallodynic action. This finding supports a key role for Nav1.8 channels in diabetes-induced hyperalgesia.
Both A803467 and lidocaine may relieve pain in diabetic peripheral neuropathy when administered systemically. Intraperitoneal administration of drugs often is preferred by clinicians for pain management during minimally invasive procedures.37 In clinical practice, systemic use of these agents may not be appropriate because of their numerous side effects.37 Instead, local administration of these same drugs may have fewer side effects and be useful for analgesia or anesthesia.
Sex-associated differences in the experience of both clinical and experimentally induced pain have been widely reported,5,18 and ratings of experimentally induced pain show some sex-associated disparity, with females generally reporting lower pain thresholds and tolerance than do males.5 Several mechanisms including psychosocial factors, familial factors, and sex hormones have been suggested. In addition, different therapies may be effective for therapy of painful conditions in men compared with women.5,18
In summary, the present results suggest that A803467, a highly selective blocker of Nav1.8 sodium channels in the nervous system, can produce effective antihyperalgesic and antiallodynic actions through both systemic and local administration to diabetic rats. These findings, together with previous observations, provide insight into the functions of Nav1.8 channels in the clinical signs of diabetes. These channels may be appropriate targets for treating the painful clinical signs of diabetes.
Acknowledgments
We acknowledge the support given by Cukurova University Research Foundation. We thank Dr Ismail Gunay and Dr Isil Ocal for their technical help.
References
- 1.Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. 1999. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci 2:541–548 [DOI] [PubMed] [Google Scholar]
- 2.Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ. 2000. Diversity of expression of the sensory-neuron-specific TTX-resistant voltage-gated sodium-ion channels SNS and SNS2. Mol Cell Neurosci 15:331–342 [DOI] [PubMed] [Google Scholar]
- 3.Andriambeloson E, Baillet C, Vitte PA, Garotta G, Dreano M, Callizot N. 2006. Interleukin-6 attenuates the development of experimental diabetes-related neuropathy. Neuropathology 26:32–42 [DOI] [PubMed] [Google Scholar]
- 4.Bean BP. 2007. The action potential in mammalian central neurons. Nat Rev Neurosci 8:451–465 [DOI] [PubMed] [Google Scholar]
- 5.Berkley KJ. 1997. Sex differences in pain. Behav Brain Sci 20:371–380 [DOI] [PubMed] [Google Scholar]
- 6.Calcutt NA. 2002. Potential mechanisms of neuropathic pain in diabetes. Int Rev Neurobiol 50:205–228 [DOI] [PubMed] [Google Scholar]
- 7.Chahine M, Ziane R, Vijayaragavan K, Okamura Y. 2005. Regulation of Nav channels in sensory neurons. Trends Pharmacol Sci 26:496–502 [DOI] [PubMed] [Google Scholar]
- 8.Chong MS, Hester J. 2007. Diabetic painful neuropathy: current and future treatment options. Drugs 67:569–585 [DOI] [PubMed] [Google Scholar]
- 9.Coggeshall RE, Tate S, Carlton SM. 2004. Differential expression of tetrodotoxin-resistant sodium channels Nav1.8 and Nav1.9 in normal and inflamed rats. Neurosci Lett 355:45–48 [DOI] [PubMed] [Google Scholar]
- 10.Courteix C, Eschalier A, Lavarenne J. 1993. Streptozocin-induced diabetic rats: behavioural evidence for a model of chronic pain. Pain 53:81–88 [DOI] [PubMed] [Google Scholar]
- 11.Cummins TR, Waxman SG. 1997. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin sensitive sodium current in small spinal sensory neurons after nerve injury. J Neurosci 17:3503–3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devor M. 2006. Sodium channels and mechanisms of neuropathic pain. J Pain 7:S3–S12 [DOI] [PubMed] [Google Scholar]
- 13.Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. 2010. Sodium channels in normal and pathological pain. Annu Rev Neurosci 33:325–347 [DOI] [PubMed] [Google Scholar]
- 14.Erichsen HK, Hao JX, Xu XJ, Munro GB. 2003. A comparison of the antinociceptive effects of voltage-activated Na channel blockers in 2 rat models of neuropathic pain. Eur J Pharmacol 458:275–282 [DOI] [PubMed] [Google Scholar]
- 15.Fox A, Eastwood C, Gentry C, Manning D, Urban L. 1999. Critical evaluation of the streptozotocin model of painful diabetic neuropathy in the rat. Pain 81:307–316 [DOI] [PubMed] [Google Scholar]
- 16.Fuchs D, Birklein F, Reeh PW, Sauer SK. 2010. Sensitized peripheral nociception in experimental diabetes of the rat. Pain 151:496–505 [DOI] [PubMed] [Google Scholar]
- 17.Gilron I, Watson CPN, Cahill CM, Moulin DE. 2006. Neuropathic pain: a practical guide for the clinician. Can Med Assoc J 175:265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ; Consensus Working Group of the Sex, Gender, and Pain SIG of the IASP 2007. Studying sex and gender differences in pain and analgesia: a consensus report. Pain 132:S26–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hargreaves K, Dubner R, Broun F, Flores C, Joris J. 1988. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88 [DOI] [PubMed] [Google Scholar]
- 20.Hirade M, Yasuda H, Omatsu-Kanbe M, Kikkawa R, Kitasato H. 1999. Tetrodotoxin-resistant sodium channels of dorsal root ganglion neurons are readily activated in diabetic rats. Neuroscience 90:933–939 [DOI] [PubMed] [Google Scholar]
- 21.Hong S, Morrow TJ, Paulson PE, Isom LL, Wiley JW. 2004. Early painful diabetic neuropathy is associated with differential changes in tetrodotoxin-sensitive and -resistant sodium channels in dorsal root ganglion neurons in the rat. J Biol Chem 279:29341–29350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huizinga MM, Peltier A. 2007. Painful diabetic neuropathy: a management-centered review. Clin Diab 25:6–15 [Google Scholar]
- 23.Jarvis MF, Honore P, Shieh CC, Chapman M, Joshi S, Zhang XF, Kort M, Carroll W, Marron B, Atkinson R, Thomas J, Liu D, Krambis M, Liu Y, McGaraughty S, Chu K, Roeloffs R, Zhong C, Mikusa JP, Hernandez G, Gauvin D, Wade C, Zhu C, Pai M, Scanio M, Shi L, Drizin I, Gregg R, Matulenko M, Hakeem A, Gross M, Johnson M, Marsh K, Wagoner PK, Sullivan JP, Faltynek CR, Krafte DS. 2007. A803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci USA 104:8520–8525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joshi SK, Honore P, Hernandez G, Schmidt R, Gomtsyan A, Scanio M, Kort M, Jarvis MF. 2009. Additive antinociceptive effects of the selective Nav1.8 blocker A803467 and selective TRPV1 antagonists in rat inflammatory and neuropathic pain models. J Pain 10:306–315 [DOI] [PubMed] [Google Scholar]
- 25.Joshi SK, Mikusa JP, Hernandez G, Baker S, Shieh CC, Neelands T, Zhang XF, Niforatos W, Kage K, Han P, Krafte D, Faltynek C, Sullivan JP, Jarvis MF, Honore P. 2006. Involvement of the TTX-resistant sodium channel Nav1.8 in inflammatory and neuropathic, but not postoperative, pain states. Pain 123:75–82 [DOI] [PubMed] [Google Scholar]
- 26.Kapur D. 2003. Neuropathic pain and diabetes. Diabetes Metab Res Rev 19:S9–S15 [DOI] [PubMed] [Google Scholar]
- 27.Khan GM, Chen SR, Pan HL. 2002. Role of primary afferent nerves in allodynia caused by diabetic neuropathy in rats. Neuroscience 114:291–299 [DOI] [PubMed] [Google Scholar]
- 28.Kitano Y, Kuwabara S, Misawa S, Ogawara K, Kanai K, Kikkawa Y, Yagui K, Hattori T. 2004. The acute effects of glycemic control on axonal excitability in human diabetics. Ann Neurol 56:462–467 [DOI] [PubMed] [Google Scholar]
- 29.Krishnan AV, Lin CS, Kiernan MC. 2008. Activity-dependent excitability changes suggest Na+/K+ pump dysfunction in diabetic neuropathy. Brain 131:1209–1216 [DOI] [PubMed] [Google Scholar]
- 30.Lai J, Gold MS, Kim CS, Bian D, Ossipov MH, Hunter JC, Porreca F. 2002. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel Nav1.8. Pain 95:143–152 [DOI] [PubMed] [Google Scholar]
- 31.Liu M, Wood JN. 2011. The roles of sodium channels in nociception: implications for mechanisms of neuropathic pain. Pain Med 12:S93–S99 [DOI] [PubMed] [Google Scholar]
- 32.Mao J, Chen LL. 2000. Systemic lidocaine for neuropathic pain relief. Pain 87:7–17 [DOI] [PubMed] [Google Scholar]
- 33.McGaraughty S, Chu KL, Scanio MJC, Kort ME, Faltynek CE, Jarvis MF. 2008. A selective Nav1.8 sodium channel blocker, A803467 [5-(4-Chlorophenyl-N-(3,5-dimethoxyphenyl)furan-2-carboxamide], attenuates spinal neuronal activity in neuropathic rats. J Pharmacol Exp Ther 324:1204–1211 [DOI] [PubMed] [Google Scholar]
- 34.Mert T, Gunay I, Ocal I, Inal TC, Sencar L, Polat S. 2009. Macrophage depletion delays progression of neuropathic pain in diabetic animals. Naunyn Schmiedebergs Arch Pharmacol 379:445–452 [DOI] [PubMed] [Google Scholar]
- 35.Mert T, Gunes Y, Gunay I. 2007. Local analgesic efficacy of tramadol following intraplantar injection. Eur J Pharmacol 558:68–72 [DOI] [PubMed] [Google Scholar]
- 36.Misawa S, Sakurai K, Shibuya K, Isose S, Kanai K, Ogino J, Ishikawa K, Kuwabara S. 2009. Neuropathic pain is associated with increased nodal persistent Na+ currents in human diabetic neuropathy. J Peripher Nerv Syst 14:279–284 [DOI] [PubMed] [Google Scholar]
- 37.Ng A, Smith G. 2002. I: Intraperitoneal administration of analgesia: is this practice of any utility? Br J Anaesth 89:535–537 [DOI] [PubMed] [Google Scholar]
- 38.Novakovic SD, Tzoumaka E, McGivern JG, Haraguchi M, Sangameswa-ran L, Gogas KR, Eglen RM, Hunter JC. 1998. Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions. J Neurosci 18:2174–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Priest BT. 2009. Future potential and status of selective sodium channel blockers for the treatment of pain. Curr Opin Drug Discov Devel 12:682–692 [PubMed] [Google Scholar]
- 40.Rogers M, Tang L, Madge DJ, Stevens EB. 2006. The role of sodium channels in neuropathic pain. Semin Cell Dev Biol 17:571–581 [DOI] [PubMed] [Google Scholar]
- 41.Sheets PL, Heers C, Stoehr T, Cummins TR. 2008. Differential block of sensory neuronal voltage-gated sodium channels by lacosamide, lidocaine, and carbamazepine. J Pharmacol Exp Ther 326:89–99 [DOI] [PubMed] [Google Scholar]
- 42.Sima AA, Sugimoto K. 1999. Experimental diabetic neuropathy: an update. Diabetologia 42:773–788 [DOI] [PubMed] [Google Scholar]
- 43.Suzuki Y, Sato J, Kawanishi M, Mizumra K. 2002. Lowered response threshold and increased responsiveness to mechanical stimulation of cutaneous nociceptive fibers in streptozotocin diabetic rat skin—in vitro correlates of mechanical allodynia and hyperalgesia observed in the early stage of diabetes. Neurosci Res 43:171–178 [DOI] [PubMed] [Google Scholar]
- 44.Wood JN, Boorman JP, Okuse K, Baker MD. 2004. Voltage-gated sodium channels and pain pathways. J Neurobiol 61:55–71 [DOI] [PubMed] [Google Scholar]
- 45.Zimmermann M. 1983. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110 [DOI] [PubMed] [Google Scholar]
- 46.Zuliani V, Rivara M, Fantini M, Costantino G. 2010. Sodium channel blockers for neuropathic pain. Expert Opin Ther Pat 20:755–779 [DOI] [PubMed] [Google Scholar]