Abstract
Ulcerative dermatitis (UD) is a common, spontaneous condition in mice with a C57BL/6 background. Although initial lesions may be mild, UD is a progressive disease that often results in ulcerations or debilitating fibrotic contractures. In addition, lesions typically are unresponsive to treatment. Euthanasia is often warranted in severe cases, thereby affecting study outcomes through the loss of research subjects. Because the clinical assessment of UD can be subjective, a quantitative scoring method and documentation of the likely time-frame of progression may be helpful in predicting when animals that develop dermatitis should be removed from a study. Such a system may also be helpful in quantitatively assessing success of various treatment strategies and be valuable to clinical laboratory animal veterinarians. In this 1.5-y, prospective cohort study, we followed 200 mice to monitor the development and course of UD. Mice were examined every 2 wk. A clinical sign (alopecia, pruritus, or peripheral lymphadenopathy) was not identified that predicted development of UD lesions in the subsequent 2-wk period. Once UD developed, pruritus, the character of the lesion (single or multiple crust, coalescing crust, erosion, or ulceration), and the size of the lesion were the only parameters that changed (increased) over the course of the disease. Pruritus was a factor in the rapid progression of UD lesions. We used these findings to develop a quantitative scoring system for the severity of UD. This enhanced understanding of the progression of UD and the quantitative scoring system will enhance the monitoring of UD.
Abbreviation: UD, ulcerative dermatitis; S number, scratching number; COL, character of lesions
Ulcerative dermatitis (UD) is an idiopathic, spontaneous, debilitating syndrome of laboratory mice that is typically a disease of aged1,19,43,46 C57BL/6 mice or genetically engineered mice on a C57BL/6 background.1,19,43,44 Some reports discuss a similar condition in young, weanling mice that presents initially as alopecia.24,42,44,45 Prevalence rates of UD between 4.1% to 21% have been reported.1,6,19 Although no etiology has been identified, environmental factors,6,19,41,42,44 diet,5,29,41,42,46 season,19,41,43,44 age at weaning,42 alopecia,24,42,44,45 sex,19,39,41,43 immune complex vasculitis,1 follicular dysplasia,44 lesion location,20 and deficiencies in vitamin A metabolism44 have all been implicated as predisposing factors for disease development. In addition, oronasal pain and chronic inflammation may lead to self-mutilation as a result of, initiating an “itch” response.10 UD is diagnosed by ruling out other causes of dermatitis in laboratory mice, such as fur mites,9 infections, fight wounds,17 strain phenotype,15,35,40,49 and experimentally induced dermatitis.4,50 Other diagnostic criteria are based on professional judgment and may include strain (C57BL/6 background),1,19,44 lesion location (head and dorsal thorax),1,19,43,44 intense pruritus,1,19,44 peripheral lymphadenopathy,6,19,39 and failure to respond to treatment.19 The rapid progression of UD lesions results in significant morbidity in laboratory mice.6,19,44 Typically, the lesions progress to large, irregularly shaped, confluent ulcerations on the dorsal cervical and thoracic region.1,19,39,44 As the lesions heal, contracted scar tissue forms, which can impair species-typical behaviors and mobility.39,41,43,44 The presence of large dermal ulcerations or debilitating contractures affect animal welfare and typically necessitate euthanasia of affected mice. Although reports on the later stages of UD have been consistent,1,19,39,41,43,44 information on the initiation and progression of UD lesions is conflicting. Pruritus,1,19,44 pain,10 and genetic predisposition1,19,43,44 have been implicated as initiators of the disease. Alopecia, pruritus, erythema, and single or multiple(s) crust have all been reported as early signs of the disease.1,19,39,42-44 However, the majority of this information has been collected retrospectively, at timed necropsies, or based on anecdotal reports.
Scoring systems are useful tools to evaluate clinical diseases in laboratory animals. For example, scoring systems have been published for tumors,14,28 body condition,14,28,47 and neurologic phenotype13 in mice to aid in assessment of clinical disease severity. Even though the progressive and severe nature of UD typically warrants eventual euthanasia, determining the severity of disease has typically been based on professional judgment,1,39 subjective scoring,12,15,19,40,43,48,49 or postmortem histology.40,44 A quantitative scoring system for UD in live mice has not been described and could greatly aid laboratory animal veterinarians and researchers in determining the severity of the disease and response to treatment.
The purpose of the current study is to investigate clinical parameters that reflect the progression of UD to facilitate management and veterinary care of mice with UD. We followed 200 mice from 3 wk of age until the development of UD to determine the initial signs and progression of UD lesions. We hypothesized that mice will first develop signs of pruritus prior to any clinical lesion. From there, we predicted that clinical lesions will progress stepwise though the following stages of severity, with or without alopecia: (1) excoriations; (2) a single, small punctuate crust; (3) multiple, small punctuate crusts; (4) coalescing crust; (5) erosion; and (6) ulceration. In addition, we hypothesized that a quantitative, validated, and reliable UD scoring system can be created that is based on physical examination parameters that do, in fact, predict development and progression of UD. Having a more thorough understanding of the initiation and progression of ulcerative lesions likely will enhance our ability to predict the outcome for a given mouse and develop earlier end-points for that mouse. Furthermore, use of this scoring system will enable accurate monitoring of UD lesions.
Materials and Methods
Animals.
Male (n = 100) and female (n = 100) C57BL/6Crl mice (age 3 wk; Charles River Laboratories, Portage, MI) were used. These mice were part of a long-term study examining the chronic effects of diet and a nutritional supplement on multiple organ systems.2,3 For the collaborative study, mice were divided randomly into 4 diet groups consisting of 25 male and 25 female mice per diet. The diet groups were as follows: (1) AIN76A rodent chow diet; (2) AIN76A supplemented with the mineral-rich nutritional supplement; (3) a high-fat, Western-style diet; and (4) a high-fat, Western-style diet with the mineral-rich supplement. AIN76A is a routinely used, certified, low-fat rodent diet. The analysis of the AIN76A diet has been published elsewhere.31-34 The high-fat, Western-style diet is derived from AIN76A; however, it has several modifications designed to mimic the diet consumed by many persons in Western society.22,30,38 The high-fat Western-style diet was formulated as described previously.26,27 Diets were formulated and provided by Research Diets (New Brunswick, NJ).
In light of our collaborators’ experimental requirements, 2 time points were established. Mice were euthanized at 13 mo of age (40 male and 40 female) and 19 mo of age (60 male and 60 female). This study was ideal to look at the development of UD over time because 120 C57BL/6Crl mice would have a chronic time-point of 19 mo of age (1.6 y), thus allowing longitudinal examination of study mice from 3 wk of age until 1.6 y of age or death.
Mice were housed in same-sex groups (5 mice per cage) in static polycarbonate shoebox-type cages with filter tops (Allentown, Allentown, NJ) and 1/8-in. corn cob bedding (Bed O'Cobs, The Andersons, Maumee, OH). Cages were changed weekly in a laminar-flow changing station (NuAire, Plymouth, MN). Animal caretakers wore gloves, sprayed the laminar-flow hood with Clidox (Pharmacal Research Laboratories Naugatuck, CT), and dipped their gloves in Spore-Klenz (Steris Corporation Life Sciences Group Mentor, OH) between cage changes. Soiled cages were sanitized in a mechanical tunnel washer with a final rinse temperature of 180 °F. Cages and bedding were not sterilized. The room was kept on a 12:12-h light:dark cycle. The room temperature and humidity were maintained at 72 ± 4 °F (22.2 ± 2.2 °C) and 30% to 70%, respectively. Food and 5-µm-filtered tap water from a glass water bottle was provided ad libidum. Mice were housed in an AAALAC-accredited facility, in compliance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals25 and the Guide for the Care and Use of Laboratory Animals.16 All procedures were approved by the IACUC.
Sentinel mice were used to monitor the health status of our experimental animals. Quarterly, three 5-wk-old CD1 female mice were directly exposed to dirty bedding collected from all colony animals during each weekly cage change. Sentinel mice were exposed to dirty bedding for 6 wk and held for an additional 2 wk after the last dirty bedding was added to their cage. Sentinel mice were euthanized by using CO2, and blood was collected. Serologic analysis for viral antibodies was performed via inhouse ELISA. Direct cecal evaluations were performed to identify any potential pinworm infections. The maximal number of cages per sentinel animal was 19; however, this number decreased throughout the experiment. At the time of this study, all mice were free from pinworms, minute virus of mice, mouse parvovirus, epizootic diarrhea of infant mice, Ectromelia virus, sendai virus, pneumonia virus of mice, mouse poliovirus (Theiler meningoencephalitis virus), reovirus 3, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, murine adenovirus, and polyoma virus. Sentinel animals at this facility are not routinely screened for Helicobacter spp. and murine norovirus. All sentinel mice and a single colony mouse per box (20%), chosen at random, were examined for fur mites prior to scheduled euthanasia. A small amount of hair was plucked from 5 different locations (interscapular, sacral, abdomen, and both axillary regions). The hair was placed in mineral oil, and a cover slip was applied. Slides were examined by light microscopy for the presence of fur mites or eggs. All fur mite examinations were negative. Mouse hepatitis virus was identified in one cage of sentinel animals once during month 8. One colony mouse per cage (20%) was serologically tested. Only 1 of the 40 cages was seropositive by inhouse ELISA and confirmed on IFA and MFIA (Charles River Animal Diagnostic Service, Wilmington, MO). The positive cage was immediately placed in a separate, quarantined room. These mice did not develop dermatitis and were not included in our data set. Mouse hepatitis virus was not detected on subsequent quarterly evaluations in any other mice.
Progression.
Antemortem monitoring and physical examinations.
All mice were given a physical examination 1 d after arrival and every 2 wk until euthanasia. In addition, all mice, except for one that died spontaneously, were given a physical examination within 24 h of euthanasia. Data collected during examination included careful evaluation of skin for lesions and palpation of submandibular, brachial, and axillary lymph nodes. In addition, mice were evaluated for pruritus by visual examination. For this, the number of scratching bouts over a 2-min period was recorded. A scratching bout consisted of rapid movement of the forelimb, hind limb, or teeth with skin contact. A scratching bout ended when the limb made contact with the ground or the head was extended away from the skin. Another scratching bout started when the limb or mouth again contacted the skin. Pruritus exams were conducted with the filter top removed, under the laminar flow hood after a brief initial examination to wake the mouse up. A scratching bout in any location was counted as one scratch. The final number of scratching bouts was recorded. Normal grooming behaviors were not recorded as scratching.
UD was diagnosed based on lesion location (thorax, neck, and head) and characteristic crusting or ulcerated appearance. All study mice and sentinel mice were negative for fur mites, and all mice remained with cagemates after weaning throughout the experiment to minimize fighting. When a UD lesion was identified, the region of the lesion (Figure 1), character of lesion, size of the lesion (estimated body surface area7 affected by the lesion), presence of discharge and character, peripheral lymphadenopathy, and presence of alopecia at the lesion or elsewhere on the mouse were recorded and analyzed. To help quantify size and avoid ‘over-scoring’ of mice that had multiple small focal lesions, we chose 3 region categories that were easy to distinguish (Figure 1). The regions were: (1) the head cranial to the medial pinna attachment and lesions affecting the mandible cranial to the sternum; (2) the inner and outer pinna, dorsal cervical region caudal to the medial pinna attachment, dorsal and ventral thorax, and thoracic limbs; (3) any region caudal to the ribcage. The character of the lesion was categorized according to increasing severity, as follows: (1) excoriations only; (2) a single, small (diameter, 2 mm or less) punctuate crust with or without excoriations and with the epithelial layer visually intact; (3) multiple, small punctuate crusts with or without excoriations and with the epithelial layer visually intact; (4) coalescing crust (diameter, greater than 2 mm) with or without excoriation and the epithelial layer visually intact; (5) erosion with the first layer of skin gone but the dermis visually intact; (6) ulceration with the dermal layer affected but not extending into subcutis. Topical treatment was not used so that the natural progression of the disease could be monitored. Mice with skin lesions or other clinical issues were examined by a clinical veterinarian, who recommended euthanasia based on his or her professional judgment. Mice were euthanized by CO2 inhalation.
Figure 1.
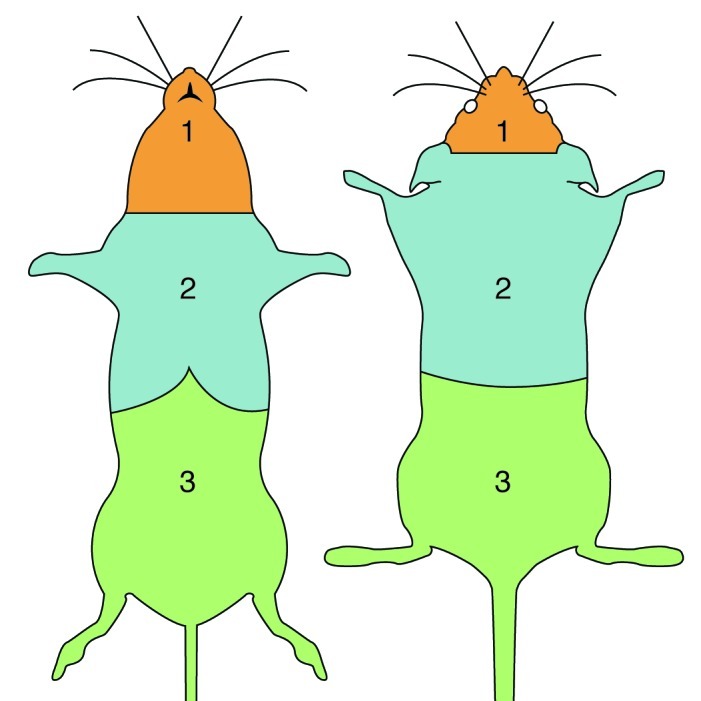
Lesion regions. Region 1: the head cranial to the medial pinna attachment and/or lesions affecting the mandible cranial to the sternum. Region 2: inner and outer pinna, dorsal cervical region caudal to the medial pinna attachment, dorsal and ventral thorax, and thoracic limbs. Region 3: any region caudal to the ribcage.
Postmortem examination.
Mice that did not die spontaneously were euthanized with CO2, and all mice were examined under a focal light for dermal lesions. When a lesion was present, its location, character, size (estimated surface area and direct measurements of the lesion), and other attributes were recorded. All peripheral lymph nodes (submandibular, axillary, and brachial) associated with draining of the UD lesions were examined grossly. Skin sections from all mice were collected for histologic examination, with sections taken from the lesions and adjacent skin in affected mice and from comparable skin areas (interscapular and dorsal neck) in unaffected mice. Skin sections were immersion-fixed in 10% neutral buffered formalin, routinely processed, and stained with hematoxylin and eosin. Slides were reviewed by a board-certified veterinary pathologist, who was blinded regarding tissue source, for confirmation of ulcerative dermatitis. Dermatitis was assessed as mild, moderate, severe, or marked, and the presence or absence of ulceration was noted. Inflammatory cells were categorized descriptively. The presence or absence of bone fide ulceration (full thickness through the epidermis) was noted. Follicles in affected and unaffected skin were surveyed for abnormal morphology. UD severity criteria were as follows: mild, focal or few foci with inflammation confined to the superficial dermis; moderate, multiple foci with inflammation extending to the deep dermis; severe, regionally extensive foci with inflammation occasionally extending to the subcutis; marked, same as for severe but with inflammation extending to the subcutis through a large portion of the lesion.
The UD scoring system.
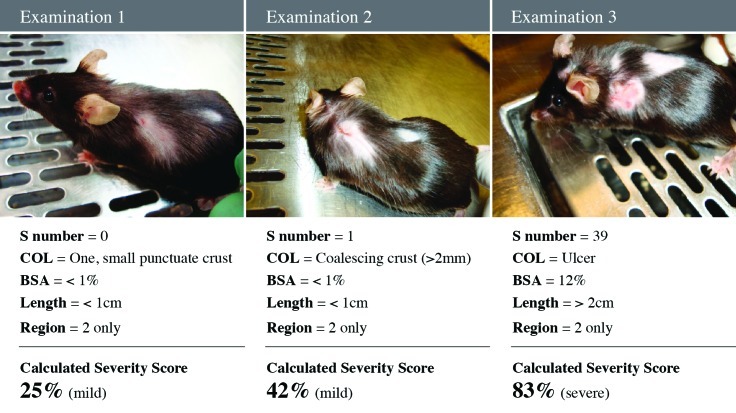
The scoring system we developed is a modification of established systems for scoring atopic dermatitis in humans,21,37 thus generating an objective, easy to interpret, scoring system that quantifies the severity of UD lesions by using parameters that increase with disease severity (number of scratching bouts, lesion character, and lesion size). In addition, number of scratching bouts, lesion character, and lesion size represent the symptomatic, intensity, and extent parameters of UD, respectively (Figures 2 A through D).37 During the biweekly examinations to assess disease progression, lesion size was determined by estimating the surface area affected. This estimate was performed by using a gridded diagram. However, during testing of the scoring system, interindividual variations revealed that estimates of surface area affected were unreliable. To objectively quantify size in the scoring system, maximal lesion length (in one dimension only) and region(s) affected (Figure 1) were used. Due to the rapidly progressive nature of UD, a lesion involving the head is given a higher score than a comparable one on another region of the body, because of the increased potential for interference with eating and drinking or involvement of the eye. The UD scoring system is on a 0 to 100 scale, with 100 being the highest (most severe) score (Figure 2 E). All 43 mice that developed UD were given a UD score within 24 h of euthanasia or at the first examination when a lesion was present (examination 1), if the lesion resolved. A Kaplan–Meier survival analysis of death due to the severity of UD lesions was performed, to learn whether mice above a threshold UD score would have a statistically significant likelihood of euthanasia. The test was based on the log-rank test (Figure 3).18
Figure 2.
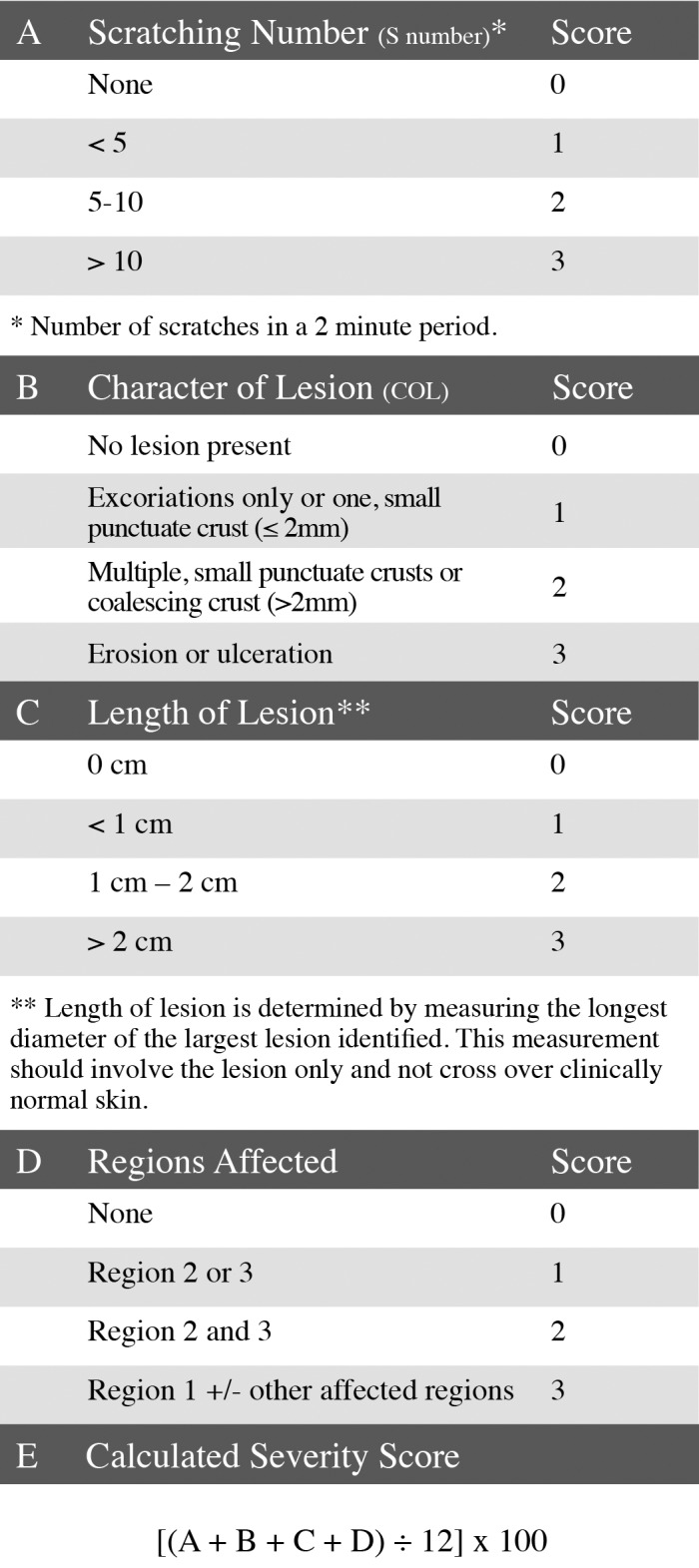
Ulcerative dermatitis scoring system. Simplified description for (A) scratching number, (B) character of lesion, (C) length of lesion, and (D) regions affected. Scores from A, B, C, and D are used in the (E) UD scoring system formula to generate the calculated severity score.
Figure 3.
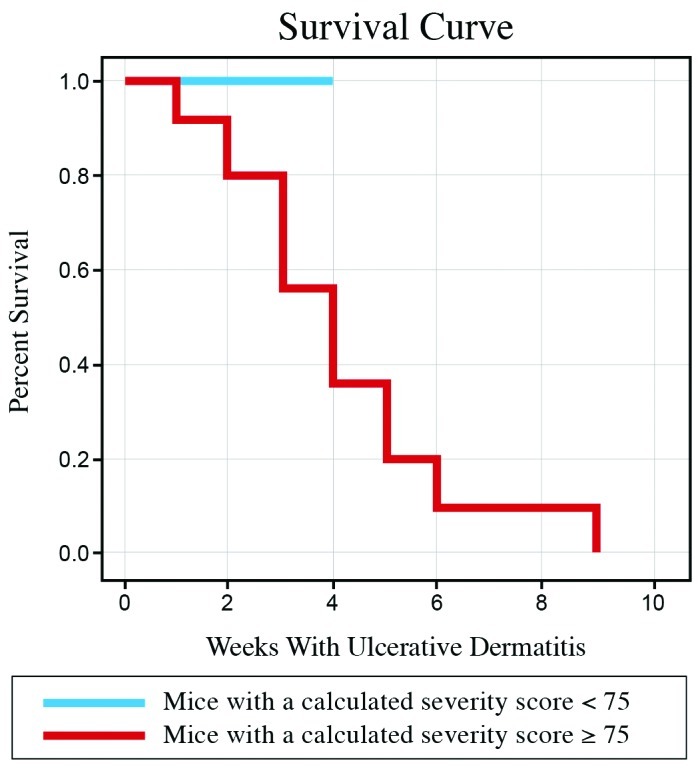
Kaplan–Meier survival analysis. Mice with a calculated severity score of 75 or greater eventually developed end-stage UD, necessitating euthanasia (red line). No mouse with a calculated severity score less than 75 necessitated early euthanasia due to UD (blue line).
To access the validity of the UD scoring system, the correlation of the calculated severity score with ranking of lesion severity (visual assessment and number of scratching bouts) by laboratory animal veterinary professionals was evaluated. During the course of this study, 43 of 200 mice developed UD lesions. Each of 25 laboratory animal veterinary professionals ranked the severity of UD lesions of all 43 affected mice on a mild, moderate, or severe (end-stage) scale.21 This panel consisted of 8 ACLAM board-certified laboratory animal veterinarians, 10 residents in our laboratory animal medicine training program (each with greater than 1 y of laboratory animal clinical experience), and 7 full-time laboratory animal veterinary technicians. Participants were told the number of scratching bouts and shown photographs of mice taken at the time their UD score was calculated. The average severity assigned by these laboratory animal veterinary professionals was used to assess the calculated UD score.
To assess the reproducibility of our UD scoring system, 10 laboratory animal veterinary professionals scored 10 live mice with clinical UD. Every participant received a 15-min tutorial on how to score the number of scratching bouts, lesion character, and lesion size (length and region involved).
Statistical analysis.
Statistical analysis of lesion progression and the UD scoring system was performed by using SSPS 19 (IBM, Armonk, NY). Statistical analysis of the UD score was performed by using Pearson product-moment correlation coefficient and Spearman rank correlation, to determine the correlation between the UD score and professional ranking of severity (mild, moderate, severe). Agreement was determined via ANOVA. In addition, a Kaplan–Meier survival analysis was conducted to examine the validity of our scoring system. A log-rank test was conducted to determine the cut-off point of the UD score.18 The statistical analysis of scores assigned by 10 laboratory animal veterinary professionals for 10 mice with clinical UD that were not associated with the primary study-set was based on Cronbach α.
Results
Mice.
Of the 200 mice in this study, 43 developed clinical UD during the course of the study, most (37 of 43 mice) of which were in the 19-mo cohort. The overall incidence in the 200 mice in the 13-mo time point was 3 of 50 (6%) male and 3 of 50 (6%) female mice. The overall incidence in the 120 mice in the 19-mo time point was 16 of 60 (26.7%) male and 21 of 60 (35%) female mice. The mean time of onset, when all mice in the study were considered, was 65.7 wk for male mice and 66.8 wk for female mice; these times were not statistically different between the sexes (65.7 ± 3.9 wk compared with 66.8 ± 2.8 wk; P = 0.8079, Student t test). The lesions of 9 of 37 (7.5%) mice with a clinical history of UD in the 19-mo cohort had resolved completely, with no evidence of disease at necropsy, representing disease resolution in 6 of 16 male mice (37.5%) and 3 of 21 (14.3%) female mice. Mice with lesions that resolved completely were significantly younger than were mice that had UD at euthanasia (57.9 ± 8.6 wk compared with 72.5 ± 1.2 wk; P = 0.0077, Student t test). Lesions in female mice were less likely to resolve (logistic regression). Due to the severity of their UD lesions, 23 mice (8 male, 15 female) were euthanized prior to their predetermined endpoint; 22 of these 23 mice were from the 19-mo cohort, and their mean age of onset did not statistically differ from the overall mean (71.9 ± 1.4 wk compared with 65.7 ± 3.9 wk [P = 0.3205] for male mice and 69.8 ± 2.3 compared with 66.8 ± 2.8 [P = 0.4680] for female mice; Student t test).
Mice with UD and clinically unaffected mice were evaluated histologically. Particular attention was paid to detecting subtle signs of inflammation, follicular abnormalities (for example, follicular dysplasia), and vascular lesions in unaffected skin, because these lesions have been implicated in previous studies as early indications of ulcerative dermatitis.1,44 Clinically unaffected mice had no histologically evident follicular or vascular alterations and no increase in dermal inflammatory cells at later time points. UD lesions were histologically confirmed and graded as mild, moderate, severe, or marked (Figure 4). Histologic assessment of UD correlated well with the UD score (P < 0.001; Pearson product-moment correlation coefficient). UD lesions consisted of variably sized foci of ulceration or serocellular neutrophilic crusting or both. The adjacent epidermis was hyperplastic, with parakeratotic to orthokeratotic hyperkeratosis. Superficial coccoid bacteria consistent with Staphylococccus spp. were often present in areas of ulceration. Interstitial and perivascular inflammation within the dermis varied. Inflammation consistently included numerous neutrophils and mast cells, with varying numbers of macrophages and eosinophils. Lymphocytes and plasma cells were comparatively rare. Occasional mice had increased plasma cells at the periphery of lesions. In severely affected mice, inflammation extended into the underlying subcutis. Some mice had varying degrees of fibrosis and granulation tissue, but these signs were always present concurrently with adjacent areas of neutrophilic and mastocytic inflammation. Follicles were sometimes dilated and cystic in areas of inflammation, but follicles in adjacent dermis were normal, without evidence of dysplasia. A perifollicular inflammatory distribution was not apparent. Vascular lesions were not present.
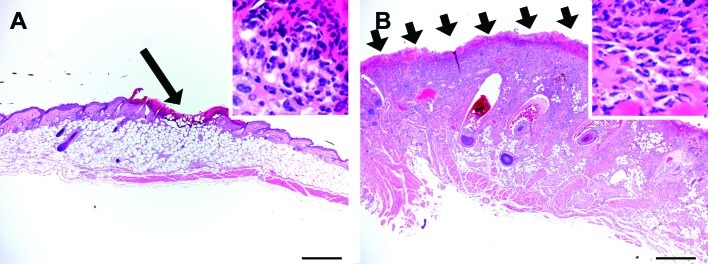
Figure 4.
The typical histopathologic appearance of a (A) mild and (B) severe UD lesion in this study. (A) Mild ulcerative dermatitis consisted of relatively limited areas of ulceration (arrow) and inflammation predominantly confined to the epidermis and superficial dermis. (B) Severe ulcerative dermatitis consisted of extensive areas of ulceration (arrows) and inflammation extending into the deep dermis or subcutis or both. In both cases, although inflammation differed by extent and intensity, it was qualitatively similar (insets) consisting predominantly of neutrophils within a serocellular crust (insets) and neutrophils, mast cells, and macrophages within the dermis. Hematoxylin and eosin stain; scale bars, 500 µm.
Progression.
Of the 43 mice with UD, 17 had lesions for only one examination time point, 16 for 2 examination time points, 7 for 3 examination time points, 2 for 4 examination time points, and one for 5 examination time points. For statistical analysis, only data from scheduled examination time points 1, 2, and 3 were used (n = 16 for comparison of exam 1 to 2, n = 7 for comparison of exam 2 to 3). Scheduled examination time points were 14 d apart. Adjustment for multiple tests was made by using Bonferroni adjustment. Overall, lesion character, number of scratching bouts, and lesion size (surface area) were the only examination parameters to change significantly over time. These 3 parameters showed a significant positive correlation; as one increased, the other 2 also increased. Figure 5 shows lesion progression in one mouse. The number of scratching bouts tended to increase between examinations 1 and 2 (7.6 to 9.5, P = 0.119, standardized t test) and increased significantly between examinations 2 and 3 (5.8 to 15.1; P = 0.021, standardized t test). Lesion character (scale of 0 to 6) significantly increased between time points 1 and 2 (4.1 to 4.6, P = 0.039, standardized t test). The lesion character tended to increase between examinations 2 and 3 (5.1 to 5.4; P = 0.135, standardized t test). Although lesion character increased from the initial score over the course of the disease, the initial lesion character varied at first detection (examination 1). At examination 1, of the 43 mice with clinical UD, 2 (4.7%) had a lesion score of 1, 11 (25.6%) had a lesion score of 2, 8 (18.6%) had a lesion score of 3, 1 (2.3%) had a lesion score of 4, 10 (23.3%) had a lesion score of 5, and 11 (25.6%) had a lesion score of 6. In addition, the affected surface area (size) increased significantly between examinations 1 and 2 (3.3% to 7.4%, P < 0.001) and examinations 2 and 3 (7.6% to 15.3%, P = 0.0065). Of the 43 mice with clinical UD, 24 (55.8%) had lesions affecting regions 1 and 2, 16 (37.2%) had lesions in region 2 only, 2 (4.7%) had lesions affecting region 1 only, and 1 (2.3%) had lesions in regions 1, 2, and 3. No mice had lesions on their limbs. Region 2 (inner and outer pinna, dorsal cervical region caudal to the medial pinna attachment, and dorsal and ventral thorax) was affected in 95% of the mice with UD.
Figure 5.
One mouse over three 2-wk examination time points. Photograph of the mouse with the examination findings and calculated severity score as determined by formula in Figure 2E.
Clinical evidence of palpable lymphadenopathy, discharge at the lesion, and alopecia did not significantly correlate with dermatitis and did not significantly change over time. Only one mouse had serous discharge at the site of the lesion. Peripheral lymphadenopathy was not detected by external palpation at examination 0 (that is, 2 wk prior to the development of UD), 1, or 2 in any affected mouse. The palpated peripheral lymphadenopathy and actual lymphadenopathy at necropsy differed significantly, resulting in a significant (P < 0.001, McNemar χ2 test) chance that lymphadenopathy could not be detected via external palpation. Alopecia did not change significantly between examination 0 to 1 or examination 1 to 2. In addition, although 79 (39.5%) of the 200 mice had alopecia at some time point, only 18 (22.8%) of these 79 mice developed UD, only 9 (11.4%) had alopecia in the area of UD, and only 3 (3.8%) had alopecia in this area prior to the development of UD.
Scoring system for UD.
The panel of 25 laboratory animal veterinary professionals gave 16 of the 43 mice evaluated an average ranked severity of mild (37.2%), 15 (34.9%) an average ranked severity of moderate, and 12 (27.9%) an average ranked severity of severe (end-stage). The UD score and the average ranked severity were strongly correlated: as one score increased, the other also increased. The log-rank test of survival showed that mice with a UD score of 75 or greater had a significant (P < 0.01) chance of being euthanized due to the severity of their UD lesions. No mice with a score less than 75 required early euthanasia due to UD lesions (Figure 3).
In a separate study set, 10 laboratory animal veterinary professionals scored 10 mice with clinical UD by using the UD scoring system. Cronbach α analysis showed that the average correlation between the 10 individual UD scores for each mouse was high (0.96), reflecting a high average correlation between each pair of raters and therefore a reliable measure of severity by using the UD scoring system. Although some variability between scores assigned by individual raters was seen (maximum, 7 points), score agreement for 92% of the raters was within 2 points.
Discussion
Our first objective in this study was to identify the early clinical signs of UD and define the progression of this disease. We did not identify a nondermatitis parameter (sex, alopecia, pruritus, or peripheral lymphadenopathy) that predicted onset of UD lesions within a 2-wk period after the assessment. Although female mice have been reported as more prone to UD development,19,39,41,43 our findings are consistent with those of another previous study.20 However, if dermatitis was grossly evident at the time of exam, the number of scratching bouts and lesion character and size (surface area) rapidly increased with the disease time course, illustrating that disease onset was sudden and the time course was rapid. The initial character of the lesion (at the first examination during which UD was identified) was highly variable. Although the rate of increase in lesion character was not uniform, this feature did not fluctuate but instead steadily increased in clinical severity unless the lesion healed (Figure 5). As lesion character increased, lesion size also increased.
Self-trauma appeared to play a role in the progression of UD. Pruritus could not be demonstrated as a precursor (that is, pruritus did not occur in the absence of dermatitis), perhaps reflecting the rapid onset of disease, such that the 2-wk interval between examinations may have been too long to detect any correlation that might exist. Pruritus did correlate with severity, in that some of the more severely affected mice scratched almost 50 times in a 2-min period. In combination with the histologic appearance, which typically included surface epithelial alterations, this behavior supports self-trauma as an important factor in disease progression. Scratching is likely to increase wound severity and delay wound healing. Further support of this theory comes from the clinical observations that trimming the nails of rodents with dermatitis promotes lesion resolution.11,23,36
We did not find an association between alopecia and UD; UD was neither preceded by nor consistently accompanied by alopecia. Some publications have reported alopecia as an early manifestation or predictive indicator of UD,8,24,42 with UD occurring as a consequence of a foreign-body reaction to broken hair follicles.44,45 In our study, only 3 of 43 mice with UD had evidence of alopecia at the lesion site prior to lesion development. In addition, folliculitis, perifolliculitis, and follicular dysplasia were not evident histologically in unaffected mice or in skin adjacent to UD lesions. One explanation for this discrepancy is that these may actually be different dermatoses. Reports showing a strong correlation of alopecia with UD also report a young age of onset and a perifollicular distribution, sometimes with follicular dysplasia.8,24,42,44,45 Studies investigating UD in aged mice did not report a direct association with alopecia and UD development.1,19,43 Although both conditions may clinically manifest as dermal ulceration, the discrepancy in age and differing histologic pictures suggest that different pathogenic mechanisms underlie these disorders. Genetic factors in substrains of C57BL/6 mice may play a role. A previous publication citing alopecia and follicular dysplasia in young (age, 6 to 13 wk) female mice actually used C57BL/6J mice.44 We used C57BL/6Crl mice, and none of the mice younger than 13 mo of age developed lesions, with the majority of lesions occurring between 13 and 19 mo. In our facility, the majority of spontaneous clinical cases of UD, apart from those associated with known fur mite infestation, occur in aged mice with a C57BL/6 background, and our scoring system is intended to be applied to this clinical situation.
Our second objective was to create a quantitative scoring system to assess the severity of UD. This scoring system is based on 3 parameters identified during physical examination: the number of scratching bouts, lesion character, and lesion size (lesion length and region(s) affected) affected. Validation of the scoring system was achieved through the strong correlation of the numerical value attained through our scoring system and the average clinical severity (mild, moderate, severe) as ranked by 25 laboratory animal veterinary professionals. A subset of 10 laboratory animal veterinary professionals tested the scoring system described. These veterinary professionals scored 10 live mice with UD. This test showed high reliability and high correlation between the persons using our UD scoring system. Due to variability between scorers, this high reliability and correlation does not necessarily attest to agreement of scores above or below a specified threshold.
The scoring system we developed might be used to monitor the progression of UD, quantify the therapeutic benefits of treatments, and more accurately determine end-stage disease. Assessment of end-stage disease in research animals is a common issue addressed by animal care and use personnel and IACUC. With regard to UD, some end-stage parameters necessitating early euthanasia are clearly defined, such as any lesions involving the ocular globe, lesions or contractures which result in a movement deficit or disorder, and lesions that impede normal eating, drinking, defecation, or urination. However, when the distinction is unclear, the use of a standard scoring system might provide a useful indicator of disease severity. In our study, we found a particular threshold based on the calculated severity score was predictive of end-stage UD. Based on the survival analysis findings, mice with a calculated severity score of 75 or greater eventually developed end-stage UD, necessitating euthanasia (Figure 3). Although, in our study, a UD score of 75 or greater was predictive of end-stage disease; this value may vary depending on the scorer or the facility's goal in monitoring UD. The mice in our study received no treatment for UD, perhaps altering the threshold value. In addition, despite inherent interindividual variability among scorers, the calculated severity score threshold of 75 can be used as adjunct indicator of end-stage disease.
In conclusion, with the ever-increasing number of genetically engineered mice that have a C57BL/6 background and the idiopathic and progressive nature of UD, this disease will continue to be an ongoing concern for laboratory animal veterinary professionals. We have identified no clinical signs that predict the onset of UD; however, pruritus is a factor in the rapid progression of UD lesions. The findings of the current study supported the creation of a validated UD scoring system that will enhance monitoring of UD and improve animal welfare with its use as an indicator of end-stage disease.
Acknowledgments
We thank Taryn Hetrick, LVT, LATG (University of Michigan, Unit for Laboratory Animal Medicine) for providing excellent clinical support of our research animals.
This study was supported in part by grant CA140760 from the NIH and by grant 11-0577 from the Agency for International Cancer Research (St Andrews, Fife, Scotland).
References
- 1.Andrews AG, Dysko RC, Spilman SC, Kunkel RG, Brammer DW, Johnson KJ. 1994. Immune complex vasculitis with secondary ulcerative dermatitis in aged C57BL/6NNia mice. Vet Pathol 31:293–300 [DOI] [PubMed] [Google Scholar]
- 2.Aslam MN, Kreider JM, Paruchuri T, Bhagavathula N, DaSilva M, Zernicke RF, Goldstein SA, Varani J. 2010. A mineral-rich extract from the red marine algae Lithothamnion calcareum preserves bone structure and function in female mice on a Western-style diet. Calcif Tissue Int 86:313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aslam MN, Paruchuri T, Bhagavathula N, Varani J. 2010. A mineral-rich red algae extract inhibits polyp formation and inflammation in the gastrointestinal tract of mice on a high-fat diet. Integr Cancer Ther 9:93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bae CJ, Shim SB, Jee SW, Lee SH, Kim MR, Lee JW, Lee CK, Hwang DY. 2010. IL6, VEGF, KC, and RANTES are a major cause of a high-irritant dermatitis to phthalic anhydride in C57BL/6 inbred mice. Allergol Int 59:389–397 [DOI] [PubMed] [Google Scholar]
- 5.Blackwell BN, Bucci TJ, Hart RW, Turturro A. 1995. Longevity, body weight, and neoplasia in ad-libitum-fed and diet-restricted C57BL6 mice fed NIH31 open-formula diet. Toxicol Pathol 23:570–582 [DOI] [PubMed] [Google Scholar]
- 6.Brayton C.2007. Spontaneous disease in commonly used mouse strains, p 648–649. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL, editors. The mouse in biomedical research, 2nd ed. Burlington (MA): Academic Press.
- 7.Cheung MC, Spalding PB, Gutierrez JC, Balkan W, Namias N, Koniaris LG, Zimmers TA. 2009. Body surface area prediction in normal, hypermuscular, and obese mice. J Surg Res 153:326–331 [DOI] [PubMed] [Google Scholar]
- 8.Crawley JN.2000. What's wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. New York (NY): Wiley–Liss.
- 9.Dawson DV, Whitmore SP, Bresnahan JF. 1986. Genetic control of susceptibility to mite-associated ulcerative dermatitis. Lab Anim Sci 36:262–267 [PubMed] [Google Scholar]
- 10.Duarte-Vogel SM, Lawson GW. 2011. Association between hair-induced oronasal inflammation and ulcerative dermatitis in C57BL/6 mice. Comp Med 61:13–19 [PMC free article] [PubMed] [Google Scholar]
- 11.Fox JG, Niemi SM, Murphy JC, Quimby FW. 1977. Ulcerative dermatitis in the rat. Lab Anim Sci 27:671–678 [PubMed] [Google Scholar]
- 12.Goto K, Iwasawa D, Kamimura Y, Yasuda M, Matsumura M, Shimada T. 2011. Clinical and histopathological evaluation of Dermatophagoides farinae-induced dermatitis in NC/Nga mice orally administered Bacillus subtilis. J Vet Med Sci 73:649–654 [DOI] [PubMed] [Google Scholar]
- 13.Guyenet SJ, Furrer SA, Damian VM, Baughan TD, La Spada AR, Garden GA. 2010. A simple composite phenotype scoring system for evaluating mouse models of cerebellar ataxia. J Vis Exp (39):1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickman DL. 2007. Use of body condition scoring as an adjunct endpoint for tumor growth studies. J Am Assoc Lab Anim Sci 46:111 [Google Scholar]
- 15.Hida S, Ogasawara K, Sato K, Abe M, Takayanagi H, Yokochi T, Sato T, Hirose S, Shirai T, Taki S, Taniguchi T. 2000. CD8+ T-cell-mediated skin disease in mice lacking IRF2, the transcriptional attenuator of interferon α/β signaling. Immunity 13:643–655 [DOI] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research. 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press.
- 17.Jacoby RO, Fox JG, Davisson M.2002. Biology and diseases of mice, p 35–113. In: Fox JG, Anderson LC, Loew FM, Quimby FW, editors. Laboratory animal medicine, 2nd ed. San Diego (CA): Academic Press.
- 18.Kalbfleisch JD, Prentice RL.2002. The statistical analysis of failure time data. Hoboken (NJ): Wiley–Interscience.
- 19.Kastenmayer RJ, Fain MA, Perdue KA. 2006. A retrospective study of idiopathic ulcerative dermatitis in mice with a C57BL/6 background. J Am Assoc Lab Anim Sci 45:8–12 [PubMed] [Google Scholar]
- 20.Lawson GW, Sato A, Fairbanks LA, Lawson PT. 2005. Vitamin E as a treatment for ulcerative dermatitis in C57BL/6 mice and strains with a C57BL/6 background. Contemp Top Lab Anim Sci 44:18–21 [PubMed] [Google Scholar]
- 21.Leung DY, Hirsch RL, Schneider L, Moody C, Takaoka R, Li SH, Meyerson LA, Mariam SG, Goldstein G, Hanifin JM. 1990. Thymopentin therapy reduces the clinical severity of atopic dermatitis. J Allergy Clin Immunol 85:927–933 [DOI] [PubMed] [Google Scholar]
- 22.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, Mayer RJ, Nelson H, Whittom R, Hantel A, Thomas J, Fuchs CS. 2007. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA 298:754–764 [DOI] [PubMed] [Google Scholar]
- 23.Mufford T, Richardson L. 2009. Nail trims versus the previous standard of care for treatment of mice with ulcerative dermatitis. J Am Assoc Lab Anim Sci 48:546 [Google Scholar]
- 24.Myers DD. [Internet]. 1997. Notice to users of JAX mice, 14 August 1997, C57BL/6J skin lesion problem eliminated. Animal Health Archives. The Jackson Laboratory. [Cited 19 June 2012]. Available at: http://web.archive.org/web/20020615091041/jaxmice.jax.org/html/archives/c57update.shtml.
- 25.Office of Laboratory Animal Welfare. 2002. Public Health Service policy on humane care and use of laboratory animals. Bethesda (MD): Department of Health and Human Services.
- 26.Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. 2009. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis 30:88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H. 2001. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis 22:1871–1875 [DOI] [PubMed] [Google Scholar]
- 28.Paster EV, Villines KA, Hickman DL. 2009. Endpoints for mouse abdominal tumor models: refinement of current criteria. Comp Med 59:234–241 [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins SN, Hursting SD, Phang JM, Haines DC. 1998. Calorie restriction reduces ulcerative dermatitis and infection-related mortality in p53-deficient and wild-type mice. J Invest Dermatol 111:292–296 [DOI] [PubMed] [Google Scholar]
- 30.Potter JD. 1995. Risk factors for colon neoplasia—epidemiology and biology. Eur J Cancer 31A:1033–1038 [DOI] [PubMed] [Google Scholar]
- 31. Report of the American Institute of Nutrition ad hoc Committee on Standards for Nutritional Studies. 1977. J Nutr 107: 1340–1348. [DOI] [PubMed]
- 32.Research Diets I. [Internet] 2004. Product data D10001: AIN76A. Open source diets. [Cited 22 March 2012]. Available at: http://www.researchdiets.com/pdf/Data%20Sheets/D10001.pdf.
- 33.Research Diets I. [Internet] Mineral mix S10001: mineral mix for AIN76A rodent diet. Open source diets. [Cited 22 March 2012]. Available at: http://www.researchdiets.com/pdf/Data%20Sheets/S10001.pdf.
- 34.Research Diets I. [Internet] Vitamin mix V10001: vitamin mix for AIN76A rodent diet. Open source diets. [Cited 22 March 2012]. Available at: http://www.researchdiets.com/pdf/Data%20Sheets/V10001.pdf.
- 35.Saikkonen B, Pareek TK, Agarwal N, Molinolo A, Kriete M, Kulkarni AB. 2008. Conditional deletion of cyclin-dependent kinase 5 in primary sensory neurons leads to atypical skin lesions. Cell Cycle 7:750–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seta S. 2009. A simplified method for the treatment of mouse dermatitis. J Am Assoc Lab Anim Sci 48:548 [Google Scholar]
- 37. Severity scoring of atopic dermatitis: the SCORAD index. Consensus report of the European Task Force on Atopic Dermatitis. 1993. Dermatology 186: 23–31. [DOI] [PubMed]
- 38.Slattery ML, Boucher KM, Caan BJ, Potter JD, Ma KN. 1998. Eating patterns and risk of colon cancer. Am J Epidemiol 148:4–16 [DOI] [PubMed] [Google Scholar]
- 39.Stowe HD, Wagner JL, Pick JR. 1971. A debilitating fatal murine dermatitis. Lab Anim Sci 21:892–897 [PubMed] [Google Scholar]
- 40.Sugiyama A, Hata S, Suzuki K, Yoshida E, Nakano R, Mitra S, Arashida R, Asayama Y, Yabuta Y, Takeuchi T. 2010. Oral administration of paramylon, a β-1,3-d-glucan isolated from Euglena gracilis Z inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. J Vet Med Sci 72:755–763 [DOI] [PubMed] [Google Scholar]
- 41.Sundberg J, Brown K, McMahon W.1994. Chronic ulcerative dermatitis in black mice, p 485–492. In: Sundberg JP, editor. Handbook of mouse mutations with skin and hair abnormalities: animal models and biomedical tools. Boca Raton (FL): CRC Press.
- 42.Sundberg JP, King LE.2000. Skin and its appendages: normal anatomy and pathology of spontaneous, transgenic, and targeted mouse mutations, p 183–216. In: Ward JM, Mahler JF, Maronpot RR, Sundberg JP, editors. Pathology of genetically engineered mice. Ames (IA): Iowa State University Press.
- 43.Sundberg JP, Sundberg BA, King LE.1996. Cutaneous changes in commonly used inbred mouse strains and mutant stocks, p 325–337. In: Mohr U, editor. Pathobiology of the aging mouse. Washington (DC): ILSI Press.
- 44.Sundberg JP, Taylor D, Lorch G, Miller J, Silva KA, Sundberg BA, Roopenian D, Sperling L, Ong D, King LE, Everts H. 2011. Primary follicular dystrophy with scarring dermatitis in C57BL/6 mouse substrains resembles central centrifugal cicatricial alopecia in humans. Vet Pathol 48:513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor DK, Lorch G, Silva KA, Miller J, Nicholson A, Vonder Harr R, Sperling L, King LE, Sundberg JP. 2005. Study of the etiology of spontaneous alopecia and ulcerative dermatitis in C57BL/6 laboratory mice. Contemp Top Lab Anim Sci 44:86 [Google Scholar]
- 46.Turturro A, Duffy P, Hass B, Kodell R, Hart R. 2002. Survival characteristics and age-adjusted disease incidences in C57BL/6 mice fed a commonly used cereal-based diet modulated by dietary restriction. J Gerontol A Biol Sci Med Sci 57:B379–B389 [DOI] [PubMed] [Google Scholar]
- 47.Ullman-Cullere MH, Foltz CJ. 1999. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci 49:319–323 [PubMed] [Google Scholar]
- 48.Williams-Fritze MJ, Carlson Scholz JA, Zeiss C, Deng Y, Wilson SR, Franklin R, Smith PC. 2011. Maropitant citrate for treatment of ulcerative dermatitis in mice with a C57BL/6 background. J Am Assoc Lab Anim Sci 50:221–226 [PMC free article] [PubMed] [Google Scholar]
- 49.Yagi R, Nagai H, Iigo Y, Akimoto T, Arai T, Kubo M. 2002. Development of atopic dermatitis-like skin lesions in STAT6-deficient NC/Nga mice. J Immunol 168:2020–2027 [DOI] [PubMed] [Google Scholar]
- 50.Zhao L, Jin H, She R, Hu Y, Xiao C, Yu Y, Wang J, Sun F, Ng T, Chu S, Wang B. 2006. A rodent model for allergic dermatitis induced by flea antigens. Vet Immunol Immunopathol 114:285–296 [DOI] [PubMed] [Google Scholar]