Abstract
Radiotelemetry transmitters support tracking of physiologic variables in conscious animals, but the size of the transmitter may alter animal health and behavior. We hypothesized that the size of the device adversely affects body weight, food intake, water intake, circadian core temperature, activity, voluntary running patterns, and the health of internal organs and that these negative effects can be minimized with smaller transmitter devices. Male C57BL/6J mice (weight, 20 to 24 g) were implanted with small (1.1 g, 0.52 mL) or large (3.5 g, 1.75 mL) radiotransmitters. Recovery of presurgical body weight, food intake, and water intake occurred within 3 d in mice implanted with small transmitter and 9 d in those with large transmitters. Mice with small transmitters displayed robust circadian core body temperature and activity patterns within 1 d after surgery, whereas activity was depressed in mice with large transmitters throughout experimentation. The most robust effects of the large transmitter included significantly reduced voluntary running, which never recovered to baseline, and inflammation of the diaphragm, large intestine, and duodenum. These results demonstrate that the large transmitter delayed surgical recovery, disrupted normal growth, reduced voluntary running, and induced inflammatory reactions of the internal organs of mice. The choice of radiotelemetry transmitter can significantly affect the health and wellbeing of experimental mice as well as data quality, such that the smallest transmitter device available and appropriate to the situation should be chosen for experimentation.
A recent development in the field of exercise physiology has been the integration of voluntary running wheel systems with radiotelemetry technology. Implantable radiotelemetry transmitters allow remote sensing of physiologic variables (for example, core temperature, electrocardiography, electroencephalography, heart rate, blood pressure) in conscious, free-ranging animals throughout the circadian cycle. Radiotelemetry has been used successfully to study thermoregulatory and cardiovascular responses to several stressors, such as bacterial infection,4,14 environmental stressors,6,10 and exercise15 in small rodents. This method of physiologic monitoring is considered the most humane and scientifically rigid because it eliminates the need for stressful procedures (for example, handling, restraint) yet simultaneously records multiple physiologic and behavioral variables in conscious, free-ranging animals, thereby supporting the refinement and reduction principles of animal experimentation.8,16,18
A recent concern regarding the use of radiotelemetry in mice is the potential negative effect of the size of the radiotransmitter on normal physiologic and behavioral health. Balb/C and 129/Sv mice intraperitoneally implanted with a 3-g radiotelemetry device (approximately 12% body weight) showed transient suppression of grooming and climbing and protracted reductions (14 d) in body weight.2 In CD1 mice implanted with transmitter devices (approximately 11% of body weight), blood pressure, heart rate, and activity showed telemetry-associated inhibitory effects on normal growth and daily running activity.13 Whereas body weight recovered to baseline levels within approximately 2 wk after surgery, running wheel activity remained approximately 35% below preimplantation levels throughout the experimental observation period, suggesting a permanent inhibitory effect of the large transmitter device on normal running wheel activity in these mice.13 We recently showed that mice (approximately 24 g) implanted with a 3.7-g transmitter device (approximately 15% of body weight) required as long as 14 d to recover presurgical body weight, food intake, and water intake and approximately 1 wk to establish robust circadian core temperature and activity rhythms; in contrast, whereas rats (approximately 310 g) implanted with the same size transmitter device (approximately 1% of body weight) showed a maximal 1-wk suppression of these variables.11 The conclusion from our study was that a large body mass to transmitter ratio minimizes the adverse effects of radiotelemetry implantation in small rodents.11
Although smaller transmitter devices have recently been introduced for use in mice, whether reductions in transmitter size effectively reduce the negative effect of this type of instrumentation on mouse growth and behavior is unknown. We hypothesized that a larger body mass-to-transmitter ratio would minimize the effect of these devices on normal growth and running behavior in mice. To test this hypothesis, we examined changes in body weight, food and water intake, core body temperature, daily activity, and running wheel activity throughout 16 d of surgical recovery in mice implanted with large (approximately 15% of body weight) compared with small (approximately 6% of body weight) transmitter devices.
Materials and Methods
Animals.
Adult male C57BL/6J mice (n =59; Jackson Laboratories, Bar Harbor, ME) were used in the current study. Health reports provided by the vendor indicated that all mice were free of contagious ectoparasites, helminthic endoparasites, and antibodies to 17 murine viruses (www.jax.org/jaxmice). On arrival to our facility, mice were individually housed in polycarbonate cages (11.5 in. × 7.5 in. × 5 in.) fitted with HEPA-filter cage tops and woodchip bedding (Pro-Chip, PWI, Saint-Hyacinthe, Quebec, Canada). Rodent laboratory chow (LM-485, Harlan Teklad, Madison, WI) and water were provided ad libitum under standard laboratory conditions (25 ± 2 °C; 12:12-h light:dark cycle; lights on, 0700). Environmental enrichment was provided with a mouse house (Nalgene Nunc, Rochester, NY) and a maple wood enrichment product (no. W0002, Bio-Serv, Frenchtown, NJ) stuffed with a rodent Supreme Mini-Treat (Bio-Serve) to encourage climbing and foraging behaviors, respectively. Fresh cages, food, and water were provided on a weekly schedule. In conducting research using animals, we adhered to the Guide for the Care and Use of Laboratory Animals7 in an AAALAC-accredited facility. All procedures received IACUC approval before experimentation began.
Experimental groups.
Upon arrival, mice were randomly assigned to receive a large or small radiotelemetry transmitter. After the quarantine period (14 d), mice in each transmitter group were further divided into sedentary (locked exercise wheels) and voluntarily running (freely rotating running wheels; diameter, 11.5 cm; width, 5.5 cm) groups. Mice were provided ad libitum access to locked or freely rotating wheels for 1 wk prior to surgery to obtain a baseline measurement of normal running activity.
Surgical analgesia.
Indomethacin (no. I-8280, Sigma, St Louis, MO) was prepared fresh in 2-hydroxy-propyl-β-cyclodextrin (1.28 mg/mL; pH 7.2; no. H-5784; Sigma) and filter-sterilized (pore size, 0.22 µm; Millipore, Bedford, MA) prior to dosing. Oral dosing was achieved by pipetting less than 20 μL of indomethacin solution onto half (approximately 0.5 g) of a Piña-Colada–flavored Supreme Mini Treat (product no. F05475-1, Bio-Serv). Each medicated treat was prepared with the indomethacin solution approximately 18 h prior to oral dosing and placed onto the cage floor for voluntary consumption on the morning of surgery (0800, day 0) and at 0900 on days 1, 2, and 3 of recovery. This oral treatment protocol was designed to minimize the need to handle mice after surgery in an effort to prevent further irritation of the surgical wound. Consumption of each medicated treat was verified daily by visual inspection. The efficacy of this analgesic treatment in intraperitoneally implanted mice has previously been demonstrated.9
Surgical procedures.
Based on our previous experience with the large transmitter devices,10 we required that mice receiving large transmitter weigh at least 23 g on the day of surgery to minimize potential adverse effects of the device on the organs within the peritoneal cavity (Table 1; ANOVA, P < 0.001). Mice in the small-transmitter group weighed less (approximately 20 g) at time of implantation because we did not expect that the size of the small transmitter would significantly affect recovery. Mice were anesthetized with isoflurane (induction, 2.5% in 100% O2; maintenance, 1% in 100% O2; flow rate, 2.0 L/min) and implanted intraperitoneally with either a battery-operated transmitter (weight, 3.5 g; volume, 1.75 mL; n = 20; large transmitter group; model TA10TA-F20, Data Sciences International, St Paul, MN) or a battery-free transmitter (weight, 1.1 g; volume, 0.52 mL; n = 14; small transmitter group; model G2 Emitter, Mini Mitter, Bend, OR). Surgical preparation consisted of shaving the abdominal fur and scrubbing the shaved area with a 10% povidone–iodine solution (Betadine Solution, Purdue Frederick, Stamford, CT) followed by 70% isopropyl alcohol. An incision (length, approximately 1 cm) was made through the skin and abdominal muscle layer by using aseptic technique. Each transmitter was disinfected by presoaking for 5 h in cold sterilant (Actril, Minntech, Minneapolis, MN) followed by multiple rinses in 0.9% sterile saline solution prior to placement in the peritoneal cavity. Large transmitters freely floated in the peritoneal cavity, whereas small transmitters were manufactured with a silicone notch for suturing to the peritoneal wall by using a single stitch of nonabsorbable suture (6-0 Prolene, Ethicon, Somerville, NJ). The peritoneal muscle layer (5-0 Vicryl, Ethicon) and skin (5-0 plain gut, Ethicon) were closed with absorbable sutures in interrupted and continuous subcuticular patterns, respectively. Immediately after surgery, each mouse was placed into a clean cage with ad libitum food and water and returned to the animal room with minimal disturbance during 16 d of recovery. Recovery from surgery was defined by 3 criteria: (1) the return of body weight and food and water intakes to presurgical (baseline) levels; (2) the establishment of a robust circadian core temperature and activity rhythms; and (3) the return to presurgical levels of running activity (voluntarily running mice only).
Table 1.
Baseline characteristics (mean ± SE) of mice (n = 10 per group) implanted with large compared with small radiotelemetry devices
| Large transmitter |
Small transmitter |
|||
| Sedentary | Runners | Sedentary | Runners | |
| Age (d) | 76 | 76 | 50 | 50 |
| Transmitter weight (g) | 3.5 ± 0.0 | 3.4 ± 0.0 | 1.1 ± 0.0 | 1.1 ± 0.0 |
| Body weight (g) | 23.5 ± 0.6a,c | 23.1 ± 0.4b,d | 20.3 ± 0.3a,b | 20.0 ± 0.5c,d |
| Food intake (g) | 2.8 ± 0.1a | 4.2 ± 0.1a,b,c | 3.0 ± 0.1c | 2.6 ± 0.2b |
| Water intake (mL) | 4.2 ± 0.3a | 5.9 ± 0.3a,b,c | 4.0 ± 0.2c | 4.6 ± 0.2b |
Body weight represents the value measured immediately prior to transmitter implantation on day 0. Food and water intake represent the amount consumed during the 24 h prior to transmitter implantation (day −1). Samples sizes are indicated in parentheses at the top of the table. Similar letters designate significant (P < 0.001) differences between groups within a particular measurement.
Radiotelemetry measurements.
Core body temperature (± 0.1 °C) and activity (counts) were monitored continuously at 1-min intervals in conscious, free-ranging mice immediately after surgical implantation. Each transmitter emits a unique frequency (Hz) that is proportional to core body temperature. Transmitter frequencies were detected by an antenna under each animal's cage and transferred to a peripheral processor that converted frequencies to core temperature by using predetermined calibration values. Activity was determined by changes in signal strength as the mouse moved on the receiver board. Activity represented a general measure that did not distinguish between locomotor movements and large postural changes. Running wheel activity was recorded at 1-min intervals by the telemetry software, via a magnetic switch that was activated with each revolution of the wheel. Total running distance was determined by multiplying the number of wheel revolutions by the circumference of the wheel and is reported as 12-h averages (day, 0700 to 1859; night, 1900 to 0659). Mice were provided locked or freely rotating wheels for 1 wk prior to surgical implantation of the transmitter device to obtain a baseline measurement of normal running activity.
Body weight, food intake, and water intake.
Body weight along with food and water intakes were measured between 0900 and 1000 each day by using a top-loading balance accurate to 0.1 g. Changes in body weight were calculated by subtracting each day's value from the value measured immediately prior to surgery and were corrected for transmitter weight. Changes in food and water intakes were calculated relative to the amount consumed during the 24-h period prior to surgery. Care was taken to correct for food spillage in the bottom of the cage, but values may represent slight overestimation because fine food crumbs could not be weighed. Water intake was determined by daily weighing of water bottles. Inadvertent water spillage during the weighing procedure was determined by simulating the weighing procedure and calculating average water losses in bottles that had never been placed into a cage with an animal. According to this control measurement, inadvertent water loss from the weighing procedure was determined to be less than 0.1 mL per bottle. Food intake provided a sensitive measure of postsurgical recovery, because the food intake patterns of mice experiencing discomfort or pain after surgery are expected to differ from those observed at baseline.
Organ damage.
A different population of large-transmitter mice (n = 25) than for behavioral assessment was used for histologic examination of the main organs that contact the transmitter (large intestine, duodenum, and diaphragm) for tissue injury. Approximately 8 wk after surgery, tissues were rapidly excised from isoflurane-anesthetized mice and fixed in 10% neutral-buffered formalin (Carson Millonig Formulation, Fisher Scientific, Springfield, MA). Tissues were embedded in paraffin blocks, serial sections were stained with hematoxylin and eosin for microscopic evaluation (magnification, 200×), and the extent of tissue injury was scored by a veterinary pathologist (IDEXX Laboratories, Grafton, MA). A total of 25 mice were examined for tissue injury, with 3 diagnostic options for large intestine and duodenum and 5 options for the diaphragm (Table 2). Organ damage was not assessed in mice implanted with small transmitters.
Table 2.
Incidence of organ injury in mice implanted with a large radiotelemetry device (n = 25 total)
| Organ | Lesion | Incidence |
| Diaphragm | None | 4 (16%) |
| Reactive mesothelium without inflammation | 7 (28%) | |
| Reactive mesothelium with inflammation | 5 (20%) | |
| Reactive mesothelium with fibrosis | 1 (4%) | |
| Reactive mesothelium with fibrosis and inflammation | 8 (32%) | |
| Large intestine | None | 20 (80%) |
| Focal, subacute peritonitis of mesentery | 4 (16%) | |
| Multifocal, subacute peritonitis of mesentery and serosa | 1 (4%) | |
| Duodenum | None | 11 (44%) |
| Focal, subacute peritonitis of mesentery | 11 (44%) | |
| Multifocal, subacute peritonitis of mesentery and serosa | 3 (12%) |
Data are from mice implanted with large, free-floating transmitter devices only.
Data analysis.
Data are provided as mean ± SEM. Presurgical body weight and food and water intake values were analyzed by using 2-way ANOVA with the Holm–Sidak test for multiple comparisons. Group differences in changes in body weight, food intake, water intake, core temperature, activity, and running distance were analyzed by using repeated-measures 2-way ANOVA with the Holm–Sidak test for multiple comparisons (SigmaStat 11.0, Systat, San Jose, CA). A P value of less than 0.05 was considered significant.
Results
Transmitter characteristics.
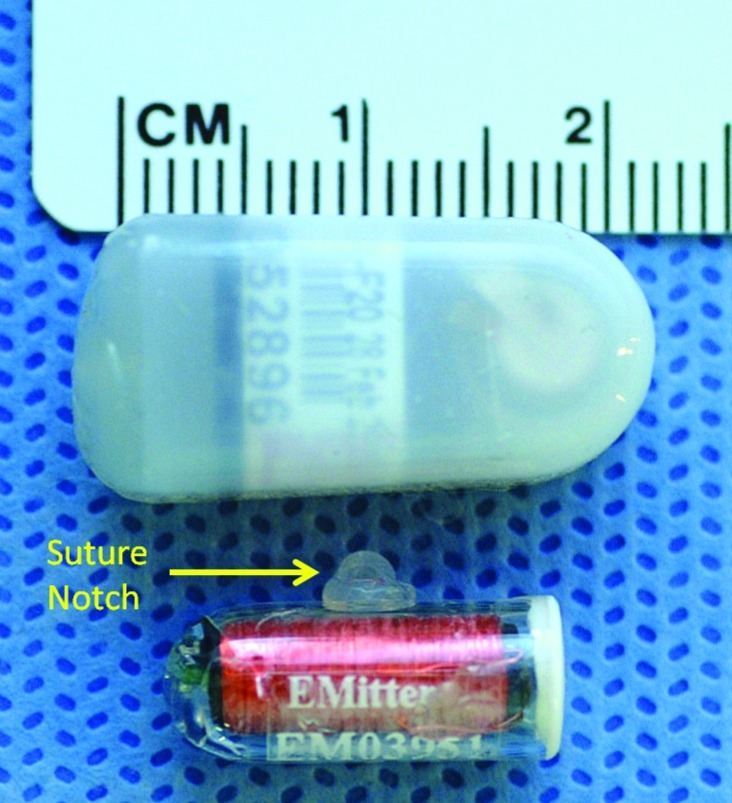
Figure 1 shows the relative sizes of the transmitter devices. The large transmitter (top) is coated with a silicone elastomer and freely floats in the peritoneal cavity. The small transmitter (bottom) is encased in glass and fitted with a silicone ‘suture notch’ for attachment to the wall of the peritoneal cavity of the mouse. The sizes of the large and small transmitters in the current study were 3.5 ± 0.1 (15.0% of body weight) and 1.1 ± 0.1 g (5.4% of body weight), respectively.
Figure 1.
Size comparison of the large transmitter (approximately 3.5 g; top) and small transmitter (approximately 1.1 g; bottom) devices implanted into male C57BL/6J mice. The large transmitter freely floats in the peritoneal cavity, whereas the small transmitter is attached to the peritoneal muscle layer via the suture notch. Both transmitter devices measure core temperature and activity in conscious, free-ranging animals and are designed specifically for use in small rodents (approximately 20 g).
Baseline characteristics.
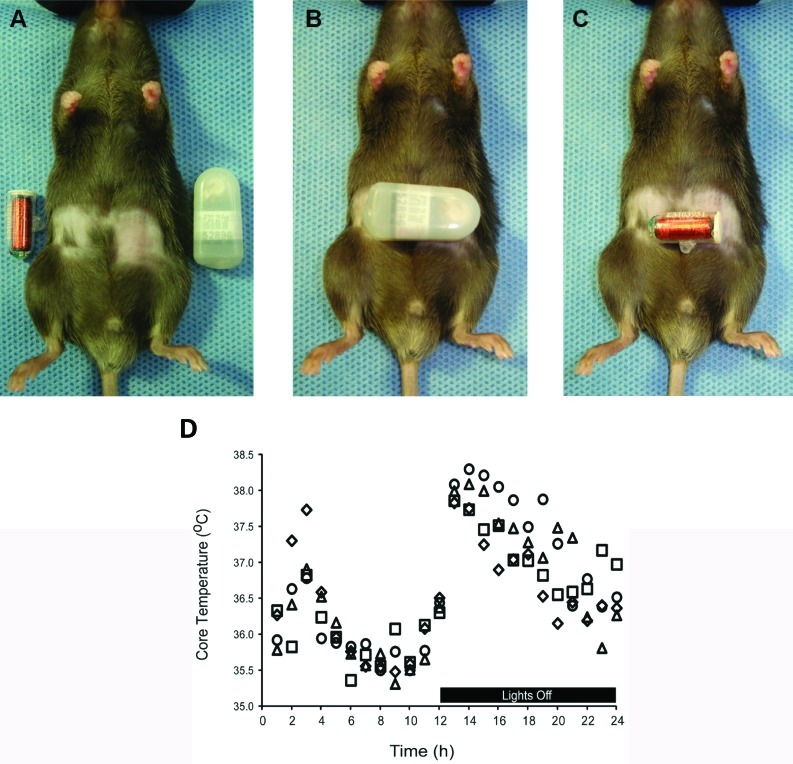
Body weight did not differ between groups. The relative sizes of the transmitter implants in comparison to an approximately 23-g mouse are shown in Figure 2 A. The large transmitter implant (1.75 mL; Figure 2 B) occupied considerably more of the abdominal region than did the small transmitter (0.52 mL, Figure 2 C). Demonstrating the consistency and accuracy of the systems, 24-h core temperature measurements (Figure 2 D) showed no differences between mice with small transmitters (36.61 ± 0.82 °C) compared with large transmitters (36.55 ± 0.79 °C). When assessed in the context of activity, the core body temperatures of sedentary and voluntary running groups were consistent regardless of the type of transmitter implanted.
Figure 2.
Comparison of (A) abdominal region, (B) large transmitter, and (C) small transmitter in C57BL/6J male mice. Both transmitters are designed for use in mice. (D) Core temperature during a 24-h period after recovery from surgery in sedentary mice with large transmitters (diamonds), sedentary mice with small transmitters (squares), voluntarily running mice with large transmitters (triangles), and voluntarily running mice with small transmitters (circles). The increased temperature during lights-off is a normal circadian response in rodent species.
We expected presurgical food intake to be greater in the voluntarily running groups compared with sedentary mice, owing to the increased energy requirements with exercise. Baseline food intake values in the large-transmitter runners were significantly (ANOVA, P < 0.001) greater than those in their sedentary counterparts and all other groups (Table 1). Voluntarily running mice with small transmitters showed similar baseline food intake values as their sedentary controls (Table 1).
Given our previous experience that food consumption typically is associated with water consumption, we expected large-transmitter running mice to show the greatest water intake before surgery. Water intake showed virtually identical trends as food intake, with the large-transmitter running mice consuming more water than all other groups (Table 1, P < 0.001). When compared with their sedentary controls, small-transmitter runners did not show a difference in water intake.
Recovery of body weight and food and water intakes.
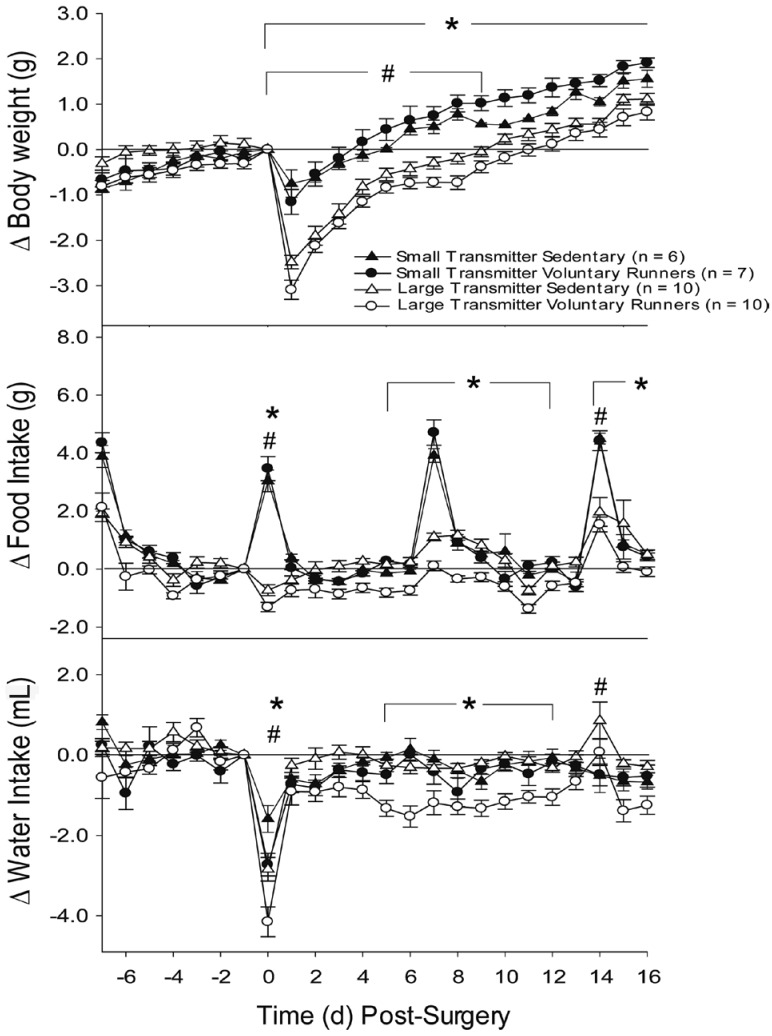
Prior to surgery, all groups showed similar growth rates (approximately 0.1 to 0.4 g/d) from the day of arrival (day −21, data not shown) until the day of surgery (day 0; Figure 3 A). Transmitter implantation induced body weight reductions that were significantly (P < 0.05) greater in mice with large compared with small transmitters (Figure 3 A). Within the small-transmitter group, sedentary and voluntarily running mice showed similar decreases in body weight on day 1 (approximately 1.0 g), which returned to presurgical levels by days 3 and 2 after surgery, respectively. The large-transmitter groups showed larger (ANOVA, P < 0.001) reductions in body weight (−2.8 ± 0.2 g) than did the small-transmitter groups on day 1. Within the large-transmitter group, the voluntarily running mice showed a larger (ANOVA, P < 0.001) reduction in body weight than did their sedentary controls on days 1 and 8. The return to presurgical body weight occurred on days 6 and 9 in the large-transmitter sedentary and voluntarily running mice, respectively (Figure 3 A). Despite differences in postsurgical body weight losses between the small-transmitter and large-transmitter mice, all groups showed a similar growth rate (approximately 0.2 g/d) from day 1 to day 16 of recovery. However, because the small-transmitter groups showed a smaller initial reduction in body weight after surgery, they attained presurgical body weight sooner than did the large-transmitter groups. Despite the slower recovery of large-transmitter mice, these animals did not show outward signs of stress or discomfort (that is, lack of grooming, breathing impairments, hunched position, and so on) at any time during the study.
Figure 3.
Change in (A) body weight, (B) food intake, and (C) water intake in C57BL/6J male mice implanted with radiotelemetry transmitters. All transmitters were implanted on day 0. Change in body weight is relative to the value obtained immediately prior to implantation. Changes in food and water intake are relative to the amount consumed during the 24 h prior to implantation. Sample sizes are indicated in parentheses. *, Significant (P < 0.05) difference between voluntary runners with small transmitters compared with large transmitters; #, significant (P < 0.05) difference between sedentary mice with large transmitters compared with voluntary runners with large transmitters.
One week prior to surgery, mice showed a robust increase in food intake that occurred in response to provision of fresh food into a new cage (that is, cage change day; day −7; Figure 3 B). Although this increase in food intake did not differ between the sedentary and voluntarily running mice in either transmitter group, the small-transmitter groups showed a significantly (ANOVA, P < 0.001) larger increase in food intake than did mice in the large transmitter groups (4.2 ± 0.4 compared with 2.0 ± 0.3 g). Approximately 2 to 3 d were required for food intake to return to baseline levels after the provision of new cages or fresh food (Figure 3 B). In this study, mice received new cages and fresh food on days −7, 0 (immediately after surgery), 7, and 14. On the day of surgery, mice in the small-transmitter groups showed the typical robust increase in food intake in response to the fresh food and new cage, but this response was attenuated in the large-transmitter groups (Figure 3 B). Although the large transmitter groups showed a progressive increase in food intake in response to fresh food and cages on days 7 and 14, it was less (P < 0.05) than those in the small transmitter groups on these days. Therefore, the response to an external stimulus remained depressed in the large-transmitter groups on all days of postsurgical recovery.
In response to transmitter implantation on day 0, the large-transmitter runners were the only mice that showed a significant (P < 0.05) reduction in food intake below baseline (−1.3 ± 0.2 g), which returned to presurgical levels on day 1. On subsequent days of recovery, the large-transmitter sedentary group showed progressive increases in food intake from days 2 to 11 and 14 to 16 that were not similarly manifest in the large transmitter runners. Conversely, mice in the small-transmitter groups did not differ from one another on any day of postsurgical recovery and showed the typical increase in food intake in response to new cages and fresh food at each weekly interval.
Our previous experience with surgically implanted mice led us to expect that water intake patterns would closely track food intake patterns. Because decreases in food intake occurred only in large-transmitter voluntarily running mice the day after surgery, we did not expect water intake to show robust decreases in response to transmitter implantation (Figure 3 C). All groups showed a significant (P < 0.05) decrease in water intake on day 0; although the small-transmitter groups did not differ from one another in this measure (approximately 1.4 ± 0.3 mL), voluntarily running mice in the large-transmitter group showed a significantly (ANOVA, P < 0.001) greater decrease in water intake than that in their sedentary controls (−4.2 ± 0.4 compared with −2.8 ± 0.3 mL). All groups had recovered presurgical water intake levels by day 1, except for the large-transmitter voluntarily running mice, whose water intake levels remained significantly (P < 0.05) decreased below presurgical levels on all days of recovery, except days 3 and 14, large-transmitter mice did not show outward signs of stress or discomfort (that is, lack of grooming, breathing impairments, hunched position, and so on) at any time during the study.
Circadian core temperature and activity rhythms.
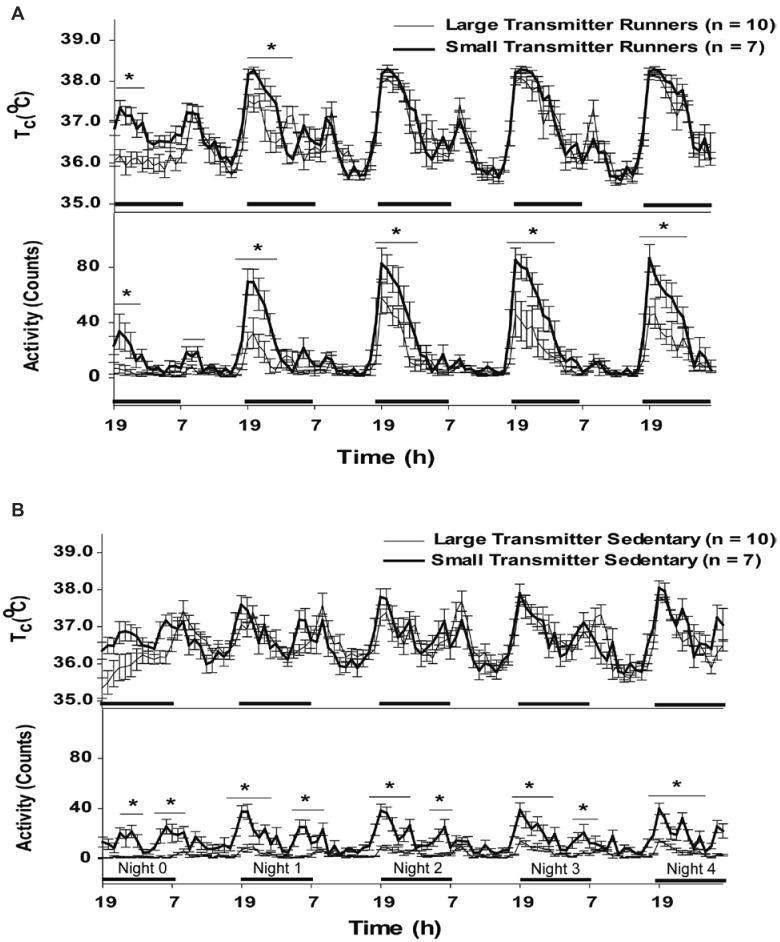
Circadian core temperature and activity rhythms typically closely match one another, showing a pattern of low values during the daytime (lights-on; inactive period) and elevated values during the night (lights-off; active period).10 Circadian core temperature and activity rhythms are presented graphically as 1-h averages in Figure 4, starting at 1900 on night 0 (first night after surgery) and are shown through night 4. From 1900 to 2400 during the first and second nights of recovery, small-transmitter runners showed significantly (ANOVA, P < 0.001) higher core temperature and activity levels than did large-transmitter runners (Figure 4 A). Although this trend did not continue during subsequent lights-off periods of observation, the activity levels of the voluntarily running mice with small transmitters remained significantly (ANOVA, P < 0.001) higher than those of the large-transmitter runners during all lights-on periods of observation (Figure 4 A). Core temperature and activity rhythms were tightly coupled in both the small- and large-transmitter runners; this association is a consequence of the influence of nighttime running activity (indicated by the high general-locomotor activities in Figure 4 A) on core temperature rhythms in the voluntarily running groups. This coupling is less evident in the sedentary groups, although core temperature and activity rhythms remained coupled to one another (Figure 4 B). The core temperature values of the sedentary groups did not differ on any day of recovery. However, small-transmitter sedentary mice showed 2 peaks of nighttime activity (1800 to 2400 and 0500 to 0800) that were significantly (ANOVA, P < 0.001) higher than those in large-transmitter sedentary mice on all days of recovery. Overall, large-transmitter mice showed significantly (P < 0.05) less activity than did mice in the small-transmitter groups, indicating an inhibitory effect of the large transmitter devices on normal activity levels.
Figure 4.
One-hour averages of core temperature (by radiotelemetry; ± 0.1 °C) and activity (counts) in male C57Bl/6J mice in the (A) voluntarily running and (B) sedentary groups implanted with small or large transmitter devices. Black horizontal bars represent lights-off (active) period during the 12:12-h light:dark cycle (lights on, 0700). Night 0 represents the first night after implantation (starting at 1900). *indicates significant difference between groups at P < 0.05. Sample sizes are indicated in parentheses.
Running distance.
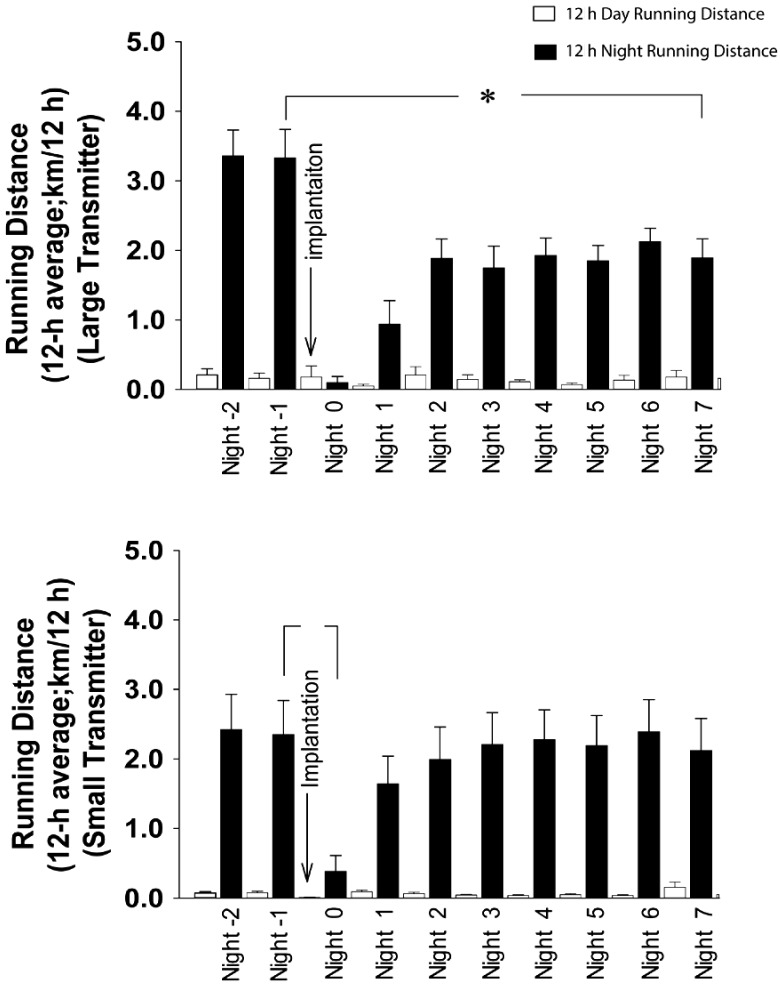
Large-transmitter mice ran significantly (ANOVA, P < 0.001) greater distances than did small-transmitter mice during the 12-h nighttime (lights-off, active period) prior to surgery (Figure 5, 12-h average: 3.3 ± 0.4 km compared with 2.3 ± 0.50 km, respectively). During the first night of surgical recovery (night 0), both transmitter groups showed significantly less (P < 0.05) running activity, with levels similar to those observed during the daylight hours (approximately 0.1 to 0.2 km/12 h; Figure 5). During the second night of recovery, the running activity of the small-transmitter mice recovered to presurgical levels (1.7 ± 0.4 km/12 h) and remained at this level throughout the remaining observation days. In contrast, large-transmitter mice did not reestablish presurgical running distances on any day of postsurgical recovery, indicating long-term reduction in voluntary running in this group (Figure 5).
Figure 5.
Distance run (12-h average; km/12 h) in male C57Bl/6J voluntary runners at night (black bars) and during the day (white bars); mice were implanted with (A) large transmitters or (B) small transmitters. Transmitters were implanted on day 0 (arrow). *, Significant (P < 0.05) difference from presurgical values.
Tissue injury.
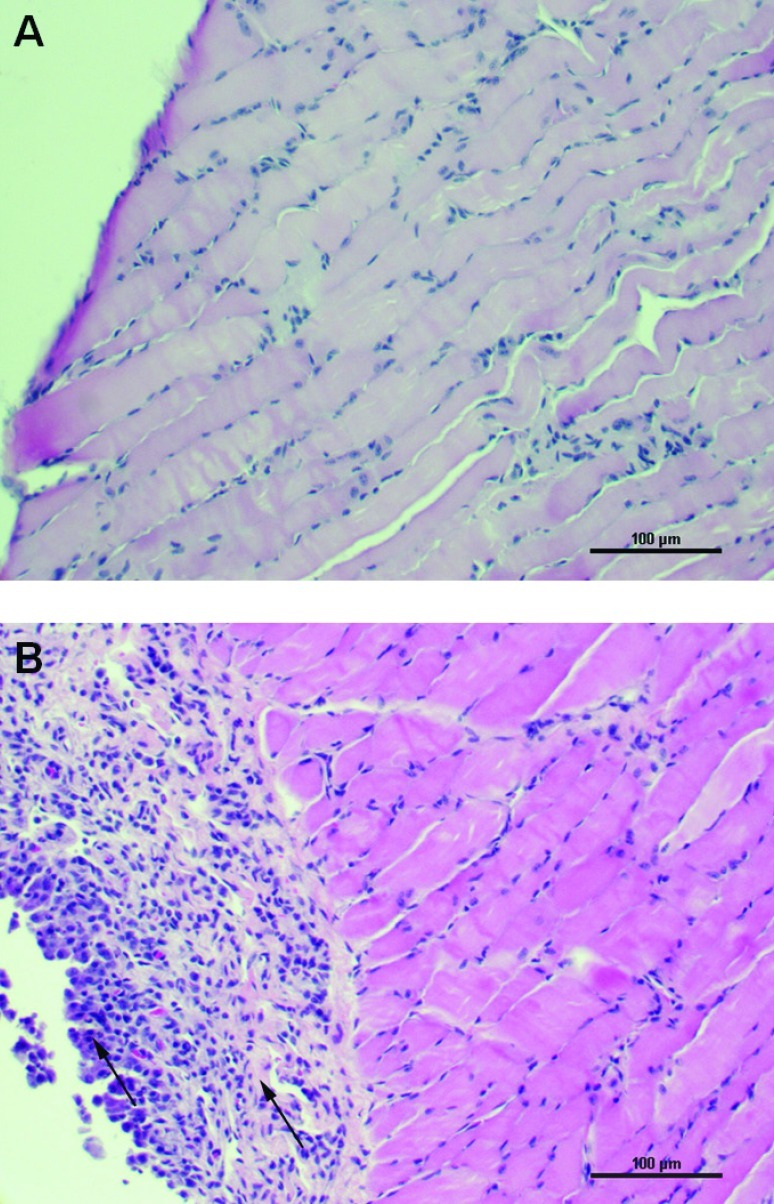
Of the 25 mice tested, 21 (84%) showed reactive mesothelium with different degrees of inflammation and fibrosis in the diaphragm (Table 2). The group differences in the degree of diaphragmatic inflammation or fibrosis did not relate to differences in body weight or relative transmitter size, because these factors were fairly evenly distributed among the various lesion groups (Table 2). Representative micrographs of diaphragms from mice with no lesions compared with the most severe condition of reactive mesothelium with inflammation and fibrosis are shown in Figure 6. The large intestine and duodenum showed evidence of peritonitis in 20% and 56% of large-transmitter animals, respectively (Table 2). We did not examine histopathologic changes to the diaphragm, large intestine, or duodenum of small-transmitter mice, because the telemetry implant was sutured to the peritoneal wall and was not able to float freely in the peritoneal cavity or contact the diaphragm in this group of mice.
Figure 6.
Representative photomicrographs of diaphragms from male C57BL/6J mice showing no lesions (A) or injury as indicated by reactive mesothelium with inflammation and fibrosis (B; arrows indicate sites of injury). Tissues were stained with hematoxylin and eosin for microscopic evaluation at magnification ×200. The numbers of mice detected with different degrees of diaphragmatic injury are shown in Table 2.
Discussion
This study is the first to demonstrate that implantation of a large transmitter alters exercise and activity patterns in mice throughout a 16-d postsurgical recovery period. In contrast, a small transmitter has minimal effect on these behaviors. Furthermore the current results reveal that mice implanted with large transmitters (3.5 g, 1.75 mL) have a 6-d delay in surgical recovery as compared with mice implanted with small transmitters (1.1 g, 0.52 mL).
We chose the C57BL/6J mouse strain because of their preferred use in animal research and tendencies to partake in voluntary exercise when provided a wheel. Specifically, C57BL/6J mice have a propensity to voluntarily run more than 4 km daily1,5 while outperforming most inbred strains in terms of voluntary running distance, duration, and speed, although the strain performs poorly during forced exercise.12,17 Baseline data taken 1 wk prior to implantation revealed that C57BL/6J mice randomly assigned to the large-transmitter group ran, on average, 1 km further per night than did small-transmitter animals. This finding is intriguing because prior to implantation, the only difference between running groups was that large-transmitter mice were approximately 3 g heavier; in our experience, these larger mice are better suited to receive large transmitters.
During the 7 nights after surgery, running distances were reduced compared with baseline levels in mice implanted with large transmitters. These results are similar to findings obtained in another study, in which a 1.9-mL mouse blood pressure catheter weighing 3.4 g and implanted in Swiss–Webster mice (approximately 28 g each) resulted in a 35% decrease in running distance during the 20-d experimental period when compared with preimplantation values.13 Similarly, the implantation of heart-rate telemetry units has been associated with a 33% decrease in running distance during the 2 wk after surgery in mice.3 The reduction in voluntary running in the current study was accompanied by decreases in normal daily activity in both sedentary and voluntarily running large-transmitter mice, demonstrating that the geometry of the large transmitter implant interferes with normal activity patterns in C57BL/6J mice. Transmitters were tested to ensure that measurements of activity were similar, because differences in activity between large-transmitter and small-transmitter systems could be related to discrepancies in the manner in which the 2 systems derive the activity count. When distance covered and elapsed time during movement were normalized, the recorded activity was not different between small-transmitter and large-transmitter implants. However, both transmitters recorded a greater change in activity when moved in the z plane when compared with the same distance moved in the x-y plane. This finding is not surprising, because activity counts are a function of change in signal strength, and a movement away from the board is likely associated with a greater change in signal strength. However, this variation does highlight that although activity counts between systems appear to be comparable, the counts obtained provide no insight into the type or time of activity (that is climbing, running, walking, and so on).
Although both large-transmitter mouse groups showed decreases in locomotion after implantation, only large-transmitter voluntarily running mice demonstrated consistent reductions in food and water intake during the 16-d postsurgery period when compared with the remaining 3 groups. The increased water intake of large-transmitter mice when compared with small-transmitter mice was likely a consequence of the longer distance run by large-transmitter mice during the night before surgery. An interesting response was the increased food consumption in response to cage change of small-transmitter mice compared with large-transmitter mice. Although the reason for this differential food-intake response to cage change in the absence of implanted transmitter devices is unknown, we postulate that it may be a consequence of differences in age and growth between the groups. Provision of new cages in the current study might have cofounded food intake measurements, but new cages (and fresh food) are always provided on a surgical day as a preventive measure against potential contamination or infection of the surgical wound.
Recovery of body weight and food and water intakes in the current study indicates that implantation of a large transmitter is associated with a 6-d delay in recovery as compared with small transmitters, and the amount of running remains depressed.3,13 In contrast, in small-transmitter mice, normal activity normalized to preimplantation levels after one day of recovery and running distance normalized by the second night in small-transmitter voluntarily running mice. These results demonstrate that the use of a large transmitter in C57BL/6J mice is associated with disruptions in normal growth, general motor activity, and exercise patterns after implantation. Although the body weight at the time of implantation was approximately 3 g less in small-transmitter compared with large-transmitter mice, weight-matching the groups at time of surgery likely would have had little effect on the data collected and likely would further highlight differences between the groups, because the small-transmitter group would be characterized by an increased body-to-transmitter weight ratio.
The association of decrements in activity and exercise with peritoneal organ irritation and damage was indicated by the histopathologic findings in large-transmitter mice. Histopathology revealed inflammation of the diaphragm in more than 80% of the animals implanted with large transmitters. Damage to the large and small intestines was present to lesser extents and likely was related to the mobility of these organs within the abdominal cavity in comparison to the more rigid nature of the diaphragm muscle. The large transmitter takes up considerable volume in the peritoneal cavity (Figure 2) and lacks a suture notch to secure it in place (although this option is now available from some vendors). Therefore organ damage associated with the large implant could result from multiple factors. First the large size and weight of the transmitter could have caused irritation of the organs in the abdominal cavity. Second, normal animal movements may have caused the transmitter to shift, thereby increasing the likelihood of implant-associated irritation of the diaphragm during normal activity patterns. Regardless, large-transmitter mice did not exhibit any signs of pain or discomfort at any time post-implant suggesting any shifting of the transmitter had minimal impact on the mouse. Furthermore, daily assessments of body weight, food and water intake, core body temperature, and activity patterns on day 16 or later were not indicative of these histopathologic changes. In addition, the damage observed in the current study was only detectable by histopathologic analysis and not by gross examination, a trend seen previously in mice implanted with a similarly sized transmitter.13 Organs from small-transmitter mice were not examined, because the small transmitter is anchored to the wall of the peritoneal cavity thereby minimizing the likelihood of transmitter-induced irritation. The small body-to-transmitter weight ratio suggests that when using a large transmitter, a heavier mouse may alleviate some of the issues demonstrated in the current study. However, even large-transmitter mice that weighed 28 g showed reduced running, suggesting that a mouse weighing more than 30 g may be ideal for receiving large transmitter implants.13 However, the use of larger mice may introduce an influence of animal age and body size on experimental outcomes. In the current study, we used male mice, which spend less time voluntarily exercising, run more slowly, and cover approximately 40% less total distance than do female mice.5 Because of this sex-associated difference, female mice are often the sex of choice in exercise studies, but female mice are often smaller in body size than are male mice of the same age, a factor that must be accounted for when designing experiments that use transmitter implants.
The transmitter devices used in the current study differed quite dramatically in size due to the inclusion of a battery in the large transmitter device. The small transmitter in this study lacked an internal battery and was powered by the receiver board—an added advantage of supporting exercise protocols throughout the life of the animal. At the time of this study, other small transmitter devices were not available, but the makers of the large transmitter device we used (TA-F20, Data Sciences International) recently introduced a smaller version of the implant (TA-F10) that may alleviate many of the problems that we encountered. The newly introduced implant has been reduced by approximately 1.5 mL (from 3.5 to 2.0 mL) and 1.9 g (from 3.5 to 1.6 g) and includes an optional suture rib, with the overall design being characterized by a reduced thickness of the transmitter. Therefore, rapid advances in radiotelemetry technology are occurring and will only serve to improve the quality of experimental data as the adverse effects of these devices are minimized as their size decreases. Despite these advances, the current results are relevant given that the price of the devices and the pace of technologic advances likely will prevent laboratories from consistently upgrading radiotelemetry systems. In light of these caveats, the current manuscript is not intended to endorse a single transmitter or company or suggest that users need to upgrade systems. Rather our purpose was to fill the knowledge gap regarding surgical recovery and behavioral responses associated with telemetry implantation.
Collectively, the results of the current study demonstrate that a large body-to-transmitter weight ratio is crucial for normal growth patterns and running behavior in mice after telemetry transmitter implantation. C57BL/6J mice implanted with small transmitters displayed rapid recovery and reattainment of presurgical body weight, food intake, water consumption, body weight, general activity, and exercise patterns after transmitter implantation. In contrast, the large transmitter was characterized by delayed recovery, inflammation of peritoneal organs, and the failure to recover presurgery levels of activity and voluntary exercise throughout the experimental paradigm. Therefore, as implantable radiotelemetry implants continue to gain popularity, investigators must balance the parameters to be captured and hardware design with species selection such that the transmitter size does not interfere with normal growth, exercise, or general activity patterns after implantation.
Acknowledgments
Research funded by US Army Medical Research and Materiel Command. Approved for public release, distribution is unlimited. The opinions or assertions contained herein are the private views of the author(s) and are not to be construed as official or reflecting the views of the Army or the Department of Defense. Any citations of commercial organizations and trade names in this report do not constitute an official Department of the Army endorsement of approval of the products or services of these organizations.
References
- 1.Avula CP, Muthukumar AR, Zaman K, McCarter R, Fernandes G. 2001. Inhibitory effects of voluntary wheel exercise on apoptosis in splenic lymphocyte subsets of C57BL/6 mice. J Appl Physiol 91:2546–2552 [DOI] [PubMed] [Google Scholar]
- 2.Baumans V, Bouwknecht JA, Boere H, Kramer K, Van Lith HA, van de Weerd HA, Van Herck H. 2001. Intraabdominal transmitter implantation in mice: effects on behavior and body weight. Anim Welf 10:291–302 [Google Scholar]
- 3.Bernstein D. 2003. Exercise assessment of transgenic models of human cardiovascular disease. Physiol Genomics 13:217–226 [DOI] [PubMed] [Google Scholar]
- 4.Conn CA, Kozak WE, Tooten PC, Gruys E, Borer KT, Kluger MJ. 1995. Effect of voluntary exercise and food restriction in response to lipopolysaccharide in hamsters. J Appl Physiol 78:466–477 [DOI] [PubMed] [Google Scholar]
- 5.De Bono JP, Adlam D, Paterson DJ, Channon KM. 2006. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol 290:R926–R934 [DOI] [PubMed] [Google Scholar]
- 6.Gordon CJ, Mack CM. 2003. Influence of gender on thermoregulation and cholinesterase inhibition in the Long–Evans rat exposed to diazinon. J Toxicol Environ Health A 66:291–304 [DOI] [PubMed] [Google Scholar]
- 7.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press.
- 8.Kramer K, Kinter L, Brockway BP, Voss HP, Remie R, Van Zutphen BL. 2001. The use of radiotelemetry in small laboratory animals: recent advances. Contemp Top Lab Anim Sci 40:8–16 [PubMed] [Google Scholar]
- 9.Leon LR, Blaha MD. 2008. Effects of indomethacin and buprenorphine analgesia on the postoperative recovery of mice. J Am Assoc Lab Anim Sci 47:8–19 [PMC free article] [PubMed] [Google Scholar]
- 10.Leon LR, DuBose DA, Mason CW. 2005. Heat stress induces a biphasic thermoregulatory response in mice. Am J Physiol Regul Integr Comp Physiol 288:R197–R204 [DOI] [PubMed] [Google Scholar]
- 11.Leon LR, Walker LD, DuBose DA, Stephenson LA. 2004. Biotelemetry transmitter implantation in rodents: impact on growth and circadian rhythms. Am J Physiol Regul Integr Comp Physiol 286:R967–R974 [DOI] [PubMed] [Google Scholar]
- 12.Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, Leinwand LA. 2002. Genetic variability in forced and voluntary endurance exercise performance in 7 inbred mouse strains. J Appl Physiol 92:2245–2255 [DOI] [PubMed] [Google Scholar]
- 13.Mills PA, Huetteman DA, Brockway BP, Zwiers LM, Gelsema AJ, Schwartz RS, Kramer K. 2000. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. J Appl Physiol 88:1537–1544 [DOI] [PubMed] [Google Scholar]
- 14.Rowsey PJ, Metzger BL, Carlson J, Gordon CJ. 2006. Effects of chronic exercise conditioning on thermal responses to lipopolysaccharide and turpentine abscess in female rats. Arch Toxicol 80:81–87 [DOI] [PubMed] [Google Scholar]
- 15.Rowsey PJ, Metzger BL, Gordon CJ. 2001. Effects of exercise conditioning on thermoregulatory response to anticholinesterase insecticide toxicity. Biol Res Nurs 2:267–276 [DOI] [PubMed] [Google Scholar]
- 16.Russell WM, Burch RL.1992. The principles of humane experimental technique. Wheathampstead (UK): Universities Federation for Animal Welfare.
- 17.Turner MJ, Kleeberger SR, Lightfoot JT. 2005. Influence of genetic background on daily running-wheel activity differs with aging. Physiol Genomics 22:76–85 [DOI] [PubMed] [Google Scholar]
- 18.van Acker SABE, Kramer K, Voest EE, Grimbergen JA, Zhang J, van der Vijgh WG, Bast A. 1996. Doxorubicin-induced cardiotoxicity monitored by ECG in freely moving mice. A new model to test potential protectors. Cancer Chemother Pharmacol 38:95–101 [DOI] [PubMed] [Google Scholar]