Abstract
The objective of this study was to determine electroencephalographic and complementary physiologic changes in Xenopus leavis frogs after bath immersion in MS222. We also evaluated the addition of sodium pentobarbital injected intracoelomically 2 h after MS222 immersion to achieve euthanasia. Frogs (n = 9) weighing 105.5 ± 8.4 g (mean ± 1 SD) were immersed in MS222 at either 1 or 3 g/L until anesthesia was achieved; a conductive stainless steel screw then was implanted in the skull on top of the outer pial surface of the brain. Frogs were immersed again in MS222 at the same concentration as previously, and electroencephalograms, heart rate, oxygen saturation, and respiratory movements were recorded. Amplitude and mean frequency of the electroencephalographic signal were evaluated at 15-min intervals until a flat-line signal was achieved. At 2 h after induction, frogs were injected intracoelomically with sodium pentobarbital (0.5 mL; 240 mg/mL) to accelerate euthanasia. Immersion of frogs in 1 or 3 g/L of MS222 depressed cerebral activity within 30 min without a significant effect on cardiac function. Intracoelomic injection of sodium pentobarbital at 2 h after MS222 administration rapidly (3.2 ± 1.7 min) induced cardiac arrest. In conclusion, immersion in MS222 can be used for the collection of organs from X. laevis frogs, but the addition of pentobarbital is required to achieve euthanasia.
Abbreviation: MS222, tricaine methanesulfonate
Xenopus laevis frogs have been one of the most popular aquatic research models for developmental studies17 and genetic research.4 Despite the wide use of these animals, few studies have evaluated euthanasia methods in this species. Euthanasia, as defined by the AVMA guidelines, is an irreversible process by which death is given in a humane and ethical way to cause a rapid loss of consciousness, no distress, respiratory or cardiac arrest, and loss of brain function.2 Cardiac arrest is a well-accepted euthanasia criterion for mammals. However, in amphibians, cardiac function can persist for very long periods of time (that is, 3 to 5 h) after administration of chemical euthanasia agents.13 No information is available on the effects of anesthesia and euthanasia methods on brain activity in frogs.
Overdose of tricaine methanesulfonate (MS222), administered via bath immersion, is an accepted and commonly used method of euthanasia for frogs.2,13,25 However, with MS222 concentrations of 3 of 5 g/L and an immersion time of 1 h, complete cardiac arrest may not occur until 3 to 5 h after administration.23 Other common methods such as intracoelomic injection of sodium pentobarbital and sodium phenytoin or ventral application of 20% benzocaine gel also require 3 h or more for the cessation of heart function.23
Both pentobarbital and sodium phenytoin are antiepileptic drugs that depress the CNS either by opening GABAergic receptors (negatively charged chlorine ions entering the cell) or by blockade of sodium voltage-gated channels.1 However, benzocaine is a local analgesic that acts on sodium voltage-gated channels in the peripheral nervous system, and because MS222 is in the same family of drugs, similar effects are expected.21 MS222 is a widely used anesthetic in fish and amphibians, but its utility as a euthanasia agent is not well established.
The main goal of the current study was to determine electroencephalographic, cardiovascular, and respiratory changes during the euthanasia of X. leavis frogs by overdose of MS222 administered via an immersion bath. Our main hypothesis was that brain activity is depressed significantly after the administration of MS222, justifying its use for euthanasia as well as anesthesia in this species. Because the time to achieve euthanasia is extremely long when MS222 is used as a sole agent,23 a second objective was to evaluate whether intracoelomic injection of sodium pentobarbital hastened the onset of MS222-induced cardiac arrest.
Materials and Methods
Animals, housing, and husbandry.
A total of 9 nonbreeding female X. laevis frogs (Xenopus I, Dexter, MI) weighing 105.5 ± 8.4 g (mean ± 1 SD) were used. Frogs were housed in a modular recirculating system (XenoPlus Housing System, Techniplast, Philadelphia, PA) filled with water purified by reversed osmosis. The system renews 10% of its circulating water daily, and salts (Instant Ocean synthetic salts, Aquarium Systems, Mentor, OH) are added automatically to maintain appropriate pH (pH, 7.0 ± 0.5) and conductivity (1800 ± 100 µS). The ammonia concentration was tested weekly to verify 0 ppm. Room and water temperatures were kept at 18 °C, with a 12:12-h light:dark cycle. Each 27-L tank contained 3 to 5 frogs, which were fed a commercial pelletted diet (Xenopus Frog Brittle, Nasco, Madison, WI) at 10 to 15 pellets per frog twice weekly.
The experimental protocol was approved by the IACUC of the Sainte-Justine Hospital Research Center (University of Montreal) in accordance with the guidelines of the Canadian Council on Animal Care (CCAC).5
MS222 immersion and pentobarbital administration.
We first evaluated 6 frogs with the 3-g/L MS222 bath immersion. This dose was chosen for its ability to cause complete cardiac arrest in 5 h22 and therefore its potential to show electroencephalographic activity at the beginning of anesthetic state to compare with a flat-line signal. After obtaining the results with this concentration, we evaluated a second group of 3 frogs with the 1-g/L MS222 bath immersion because this concentration has been used for euthanasia.22 We mainly wanted to see whether MS222 at 1 g/L caused a similar CNS depression as that after 3 g/L. MS222 (ethyl 3-aminobenzoate methanesulfonate, Sigma–Aldrich, St Louis, MO) was weighed and added to reverse-osmosis–purified water. Sodium bicarbonate (Sigma–Aldrich) was added to bring the solution to a neutral pH (pH 7.2 for 1-g/L solutions and pH 6.9 for 3-g/L solutions) according to a pH meter (Accumet Basic AB15 pH meter, Fisher Scientific, Ottawa, Canada). Frogs were immersed in MS222 solution for 5 min and were then tested for the absence of withdrawal and righting reflexes prior to surgery. Frogs were returned into the MS222 solution after the surgical procedure, and the part of the animal in contact with air (to enable electroencephalography) was rinsed every 5 min with the MS222 solution until the end of the study. Care was taken to not immerse the electrode region. MS222 at 1 and 3 g/L is expected to stop heart activity after 5 h.23 Prior to the experiments, we decided that if electroencephalographic recordings and cardiovascular parameters were stable at 2 h after bath immersion, all frogs would be injected intracoelomically (near the heart) with 0.5 mL sodium pentobarbital (240 mg/L; Euthanyl, Vetoquinol, Lavaltrie, Quebec, Canada), with each frog receiving approximately 1100 mg/kg.22 All times reported in this study are from start of the immersion bath.
Implantation of electroencephalography electrodes.
When anesthesia was confirmed, a 0.5-cm skin incision was made laterally (0.5 to 1 cm) to the skull midline and caudally to the eye. The fascia under the skin was detached, exposing the muscles. Muscle tissues were cut, and periosteum was scraped with a scalpel blade to expose the skull. A hole (diameter, 0.5 mm) was drilled into the exposed skull, and the screw was placed firmly in the bone. The surgery consisted of putting an electricity-conducting stainless-steel blunt-tip screw in the skull of all frogs so that the tip of the screw made contact with the pial surface of the right cerebral hemisphere. This recording screw was attached to a connector by using stainless steel bipolar electrode outputs of 200 μm in diameter (Plastics 1, Roanoke, VA). We decided to use bipolar electrodes with this single output screw to have a more stable setup and the assurance that at least one electrode would record correctly, with the other one being used as a backup. Therefore, the monitoring configuration was single-ended, but bipolar electrodes outputs were used here for technical, stability, and monitoring consistency reasons. Similarly, a reference electrode was placed under the skin at the most caudal pole of the incision site near the midline and consisted of a copper crocodile clip (width, 2 mm; length, 1.25 cm) that was soldered to the wires of another bipolar connector output. The electroencephalography setup we used (Figure 1) is similar to one used previously, except that in the previous arrangement, the electrode was placed on the skull without direct contact to nervous tissue.3 Total time (mean ± 1 SD) of surgery was 9 ± 3 min. During recording, electrode placement was considered to be appropriate when signal peaks were approximately 50 to 60 µV.
Figure 1.
Electrode placement on a X. laevis frog for electroencephalographic recording, using a conducting screw (right) and a crocodile clip (left). The frog is partially immersed in the MS222 solution (1 or 3 g/L concentration).
Physiologic monitoring.
Once the screw electrode was placed in the skull, a crocodile clip electrode was attached on the skin of the frog to act as the reference electrode. The frog was replaced in the MS222 solution and placed in a Faraday cage to minimize electrical contamination (60 Hz) causing artifacts. Electroencephalographic signals and animal behaviors were recorded simultaneously by using a recording system (Stellate Harmonie, Natus Medical, San Carlos, CA) linked to a 32-channel Lamont amplifying unit and an infrared analogical video camera (Stellate Systems version 6.2e, Natus Medical) positioned 1.5 m in front of the Faraday cage. Data were acquired at 200 Hz, filtered at 0.1 to 35 Hz, and analyzed by using the Stellate Harmonie S program (Natus Medical). The experimenter was always present from the beginning of the procedures, and recordings were performed until complete cardiac arrest. Oxygen saturation and heart rate were measured by using a pulse oximeter (CANL 45SV, Med Associates, St Albans, VT). To collect data by using the pulse oximeter, the probe (model TDR-43, Transflectance Sensor, Med Associates) was positioned under the sternum of frogs placed in sternal recumbency.12,25 Respiratory rate was evaluated by direct observation of nare, gular, or abdominal movements.
Electroencephalographic analysis.
The amplitude and frequency of the electroencephalographic signal were collected at 15-min intervals. Care was taken to not include artifacts, such as the heartbeat of the frog or movements from the investigators, in the analysis window. A flat-line signal (no brain activity) was established at the end of each electroencephalographic recording (complete cardiac arrest for 10 to 15 min) to have a baseline electrical signal representative of the apparatus and environmental electrical contaminants. Consequently, baseline frequency was less than 2 Hz, and baseline amplitude was less than 3 µV; signals exceeding these thresholds were analyzed. Recordings were analyzed by 2 observers, who were blinded to the experimental conditions.
Histopathology.
At the end of the experiment, all frogs were weighed; the heads were removed, preserved in a buffered 10% formalin solution, and sent for analysis (Pathology Department, Faculty of Veterinary Medicine, University of Montreal, Canada). Heads were decalcified and stained with hematoxylin–eosin–saffron. Transverse sections (n = 3, that is, the middle and each end of the drill hole) of each head were observed under light microscopy (Dr Pierre Hélie, DMV, DACVP) to determine whether brains were intact during the recording and to confirm the location of the electrode.
Statistics.
Differences in heart rate, oxygen saturation, amplitude, and frequency values were analyzed for statistical significance (version 8.2, SAS Software, SAS Institute, Cary, NC). The repeated-measures linear model was used for analysis, and statistical significance was set at a P value of less than 0.05.
Results
Evaluation of the histology slides of the Xenopus skulls confirmed that the electrodes in all frogs were placed correctly for electroencephalographic recording. Figure 2 shows a frontal section the skull where a trepanation hole is seen, located just above the cerebral hemisphere, and Figure 3 shows a section that lacks a trepanation hole (continuous bone on the dorsal aspect of the skull). All frogs were free of brain lesions and signs of nervous tissue ischemia, according to microscopic observations.
Figure 2.
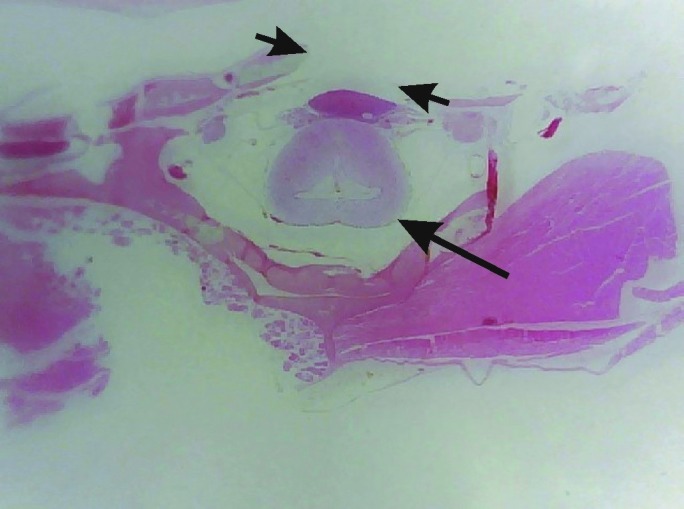
Photomicrograph of a histologic section of the head showing the trepanation hole (distance between the small arrow heads) and the undamaged brain (large arrow). Hematoxylin–eosin–saffron stain.
Figure 3.
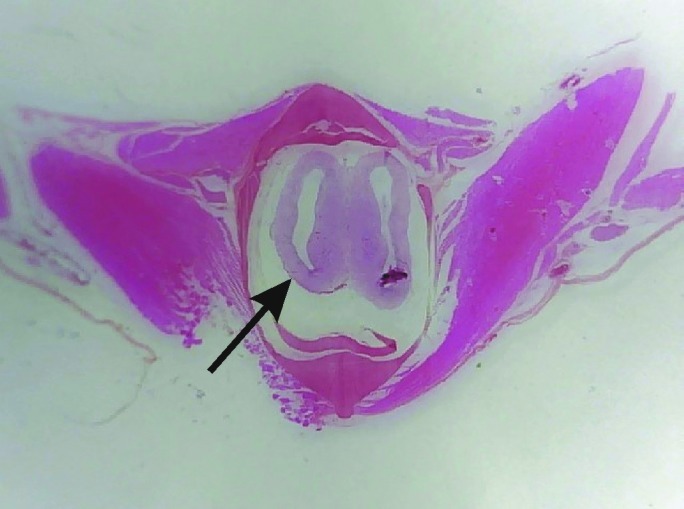
Photomicrograph of a histologic section anterior to a trepanation showing the undamaged brain (arrow) and surrounding bone of the skull. Hematoxylin–eosin–saffron stain.
Baseline values (mean ± SEM) prior to bath immersion were: respiratory rate, 12 ± 2. breaths per minute; heart rate, 51 ± 6 bpm; and oxygen saturation, 88.0% ± 6.0%. Withdrawal and righting reflexes were absent at approximately 6.7 ± 1.2 min (mean ± 1 SD) after immersion of frogs in 1 g/L MS222 and at 5 ± 0 min after immersion in 3 g/L MS222. When compared with the baseline value, remained unchanged after immersion in the 1-g/L MS222 solution but was significantly (P < 0.05) greater at the 30-, 45-, and 90-min time points after immersion in 3 g/L MS222 (Figure 4). The 3-g/L immersion significantly (P < 0.05) decreased oxygen saturation from 88% ± 6% at baseline to 62% ± 10% at 1 h after induction, with a progressive return to normal values over time (no significant difference at other time points; Figure 5). In all frogs, the respiratory frequency was 0 breaths per minute at 15 min after the start of the immersion bath and stayed depressed throughout the 2-h monitoring period.
Figure 4.
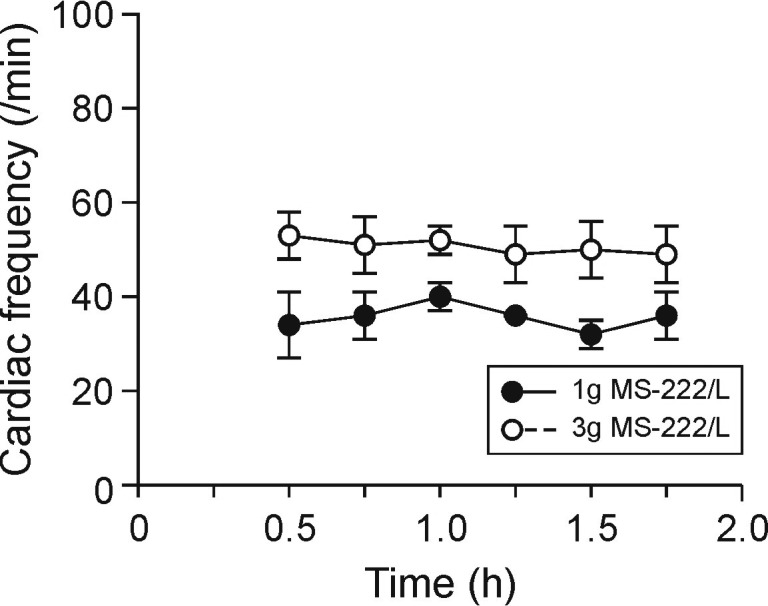
Heart rate (mean ± SEM) of X. laevis frogs after immersion in MS222 at 3 g/L (n = 6) or 1g/L (n = 3) for the duration of the study (2 h).
Figure 5.
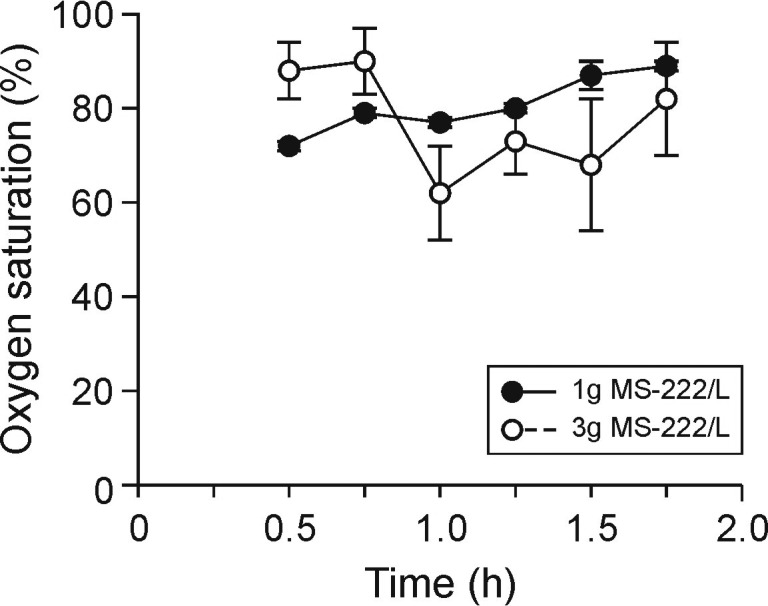
Oxygen saturation (mean ± SEM) of X. laevis frogs after immersion in MS222 at 3 g/L (n = 6) or 1g/L (n = 3) for the duration of the study (2 h).
After the MS222 immersion bath, electroencephalographic amplitudes were significantly (P < 0.05) lower at 30 min, with values ranging from 59.7 ± 12.3 µV at 15 min to 11.9 ± 5.8 µV at 30 min and stayed below this amplitude for the remainder of the study (Figure 6). Signal frequency varied between 3.2 ± 0.9 and 7.1 ± 2.5 for the entire electroencephalographic recording session (Figure 7). Data from all frogs, regardless of the concentration of the immersion bath, were pooled, given the lack of differences in the amplitude and frequency of the electroencephalographic signals. Representative electroencephalographic signals recorded at 15 min and the end of the study (prior to pentobarbital administration) are shown in Figure 8. Note that the terminal-phase electroencephalographic amplitude is very low and the electrocardiographic signal became clearly apparent.
Figure 6.
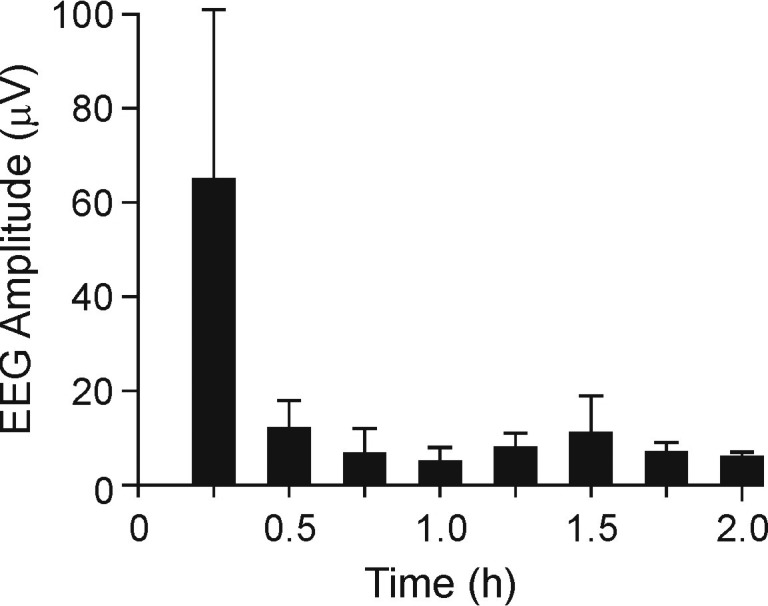
Electroencephalographic (EEG) amplitude (mean ± SEM) after immersion of X. laevis frogs in MS222 (n = 9; 6 frogs at 3g/L and 3 frogs at 1g/L) for the duration of the study (2 h).
Figure 7.
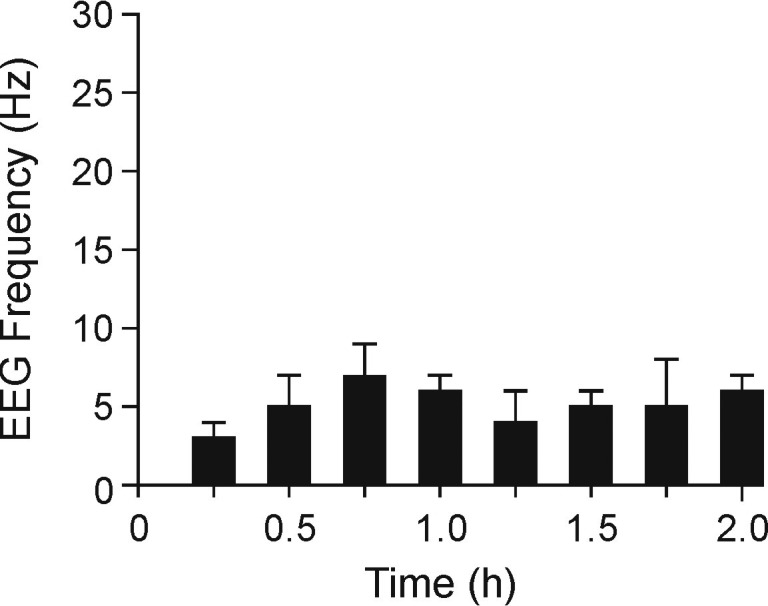
Electroencephalographic (EEG) frequency (mean ± SEM) after immersion of X. laevis frogs in MS222 (n = 9; 6 frogs at 3g/L and 3 frogs at 1g/L) for the duration of the study (2 h).
Figure 8.
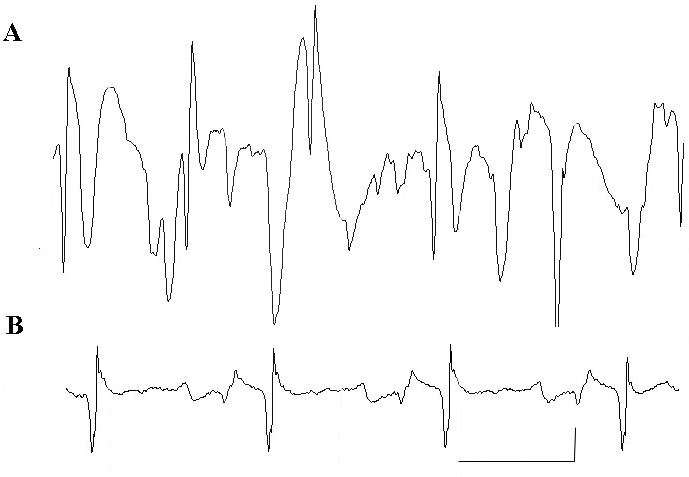
Representative electroencephalographic recording of a X. leavis frog at (A) 15 min after induction and (B) at the end of the recording session (prior to pentobarbital administration) after immersion in 3 g/L MS222. Note the high-amplitude low-frequency tracing at the early time point and the low-amplitude electroencephalographic recording at the late time point. Bars: x axis = 1 s; y axis = 10 µV.
Intraceolomic injection of 0.5 mL (240 mg/mL) sodium pentobarbital progressively decreased heart rate in all frogs, achieving complete arrest 3.2 ± 1.7 min after injection.
Discussion
Continuous bath immersion of X. laevis frogs in MS222 at a concentration of 1 or 3 g/L for euthanasia induces depression of CNS activity without any overt modification of cardiovascular parameters for 2 h after immersion. Cardiac arrest has been shown to occur at approximately 5 h after a 1-h immersion in MS222;23 our current study shows that brain activity is strongly reduced within 30 min of immersion. Recorded low amplitudes (less than 12 µV) and frequencies (3 to 7 Hz) are representative of a very deep level of anesthesia (θ and δ waves).19 Our study therefore clearly shows that MS222 causes profound CNS depression in frogs within the first 30 min of bath immersion.
Because some frogs in an earlier study recovered after a 1-h immersion in 1 g/L MS222,23 immersion of frogs in 1 g/L MS222 for a specified time period should be avoided as a method of euthanasia.7 However, the CNS depression after this treatment is sufficient for surgical procedures or organ collection, and intracoelomic pentobarbital can be added to ensure appropriate euthanasia of the frog. With the 3-g/L immersion bath, terminal tissue collection and nonsurvival surgery can be performed within 30 min, in light of the marked CNS depression.
Various electroencephalographic indicators of anesthesia depth have been identified in humans and other mammals.8,14,16,19,20,24 Frequency bands typically are categorized as δ (0 to 4 Hz), θ (4 to 8 Hz), α (8 to 13 Hz), and β (13 to 32 Hz).16,24 Deep anesthesia shows predominantly δ and θ bands, that is, frequencies of 0 to 8 Hz.19 In the current study, mean frequencies at 15-min intervals ranged from 2 to 8 Hz in all frogs throughout the 2-h recording session, suggesting a deep state of anesthesia. Electric activity from the heart prevented the recording of a complete electroencephalographic flat-line signal, but the regularity of the heart beat signal (shape and frequency) allowed us to differentiate it from cerebral activity. Baseline electroencephalographic recordings were not obtained from unanesthetized frogs because doing so was considered impractical in a freely moving aquatic animal.
Compared with the data gathered in the current study, other studies have found higher amplitude electroencephalography signals in awake and resting amphibians and high-frequency signals are mostly inhibited while the frogs are resting.9,18 Frogs and mammals have similar electroencephalographic frequencies and amplitudes.18 However, the electroencephalographic features of anesthesia have not yet been clarified in frogs. In fish, MS222 leads to severe depression (amplitude and frequency) of the electroencephalogram.11,26
We considered our frogs to be anesthetized after 5 to 8 min of immersion because they had lost the righting and withdrawal reflexes frogs.6,15,22 Interestingly, the 3-g/L MS222 solution decreased the oxygen saturation significantly after the 1-h immersion, and heart rate was significantly greater with the 3-g/L compared with the 1-g/L solution. However, heart rate did not significantly differ from baseline with either solution and remained stable throughout the recording period. A similar range for heart rate and oxygen saturation have previously been recorded in adult Xenopus frogs during anesthesia;15 however, depression can occur in young frogs.12 Therefore, in adult Xenopus frogs, MS222 appears to have little effect on the heart rate and oxygen saturation during the first hour after bath immersion.
Intracoelomic injection of sodium pentobarbital at 2 h after immersion caused the cessation of heart function and accomplished euthanasia. This combination of agents may be particularly appropriate if tissue samples need to be collected rapidly after MS222 immersion. However, pentobarbital can damage tissues submitted for histology,25 whereas MS222 does not appear to have this effect.21 Although lower doses of pentobarbital (for example, 100 mg/kg) are reported to be effective for euthanasia when used in the absence of anesthesia,25 a recent study showed that 1100 mg/kg sodium pentobarbital combined with sodium phenytoin (141 mg/kg) was necessary to achieve euthanasia of frogs within 1 h.22 More studies are required to identify the appropriate pentobarbital dose and time of the administration for the anesthesia or euthanasia of frogs in conjunction with MS222 deep anesthesia.
Although MS222 acts similarly as other local anesthetics (such as lidocaine and benzocaine) by blocking sodium currents,10 it has widely been used as a general anesthetic in fish and amphibians. Because high doses of MS222 lead to both sensory desensitization and motor block,21 the appropriateness of their use is questionable given the possibility of paresis or paralysis. MS222 therefore may inhibit the sensory and motor functions of the peripheral nervous system in frogs without having an effect on the CNS. Our study clearly demonstrates that MS222 leads to profound CNS depression, compatible with deep levels of anesthesia, in frogs. This study is the first to demonstrate that MS222 is an anesthetic—it is capable of causing loss of consciousness as characterized by the presence of δ and θ waves in the cerebral cortex.8,14,16,19,20,24
In conclusion, tricaine methanesulfonate (MS222) at a concentration of 1 or 3 g/L in an immersion bath for frogs leads to marked depression of cerebral activity that develops within 30 min after immersion and lasts for at least 2 h. The deep plane of anesthesia after 30 min enables tissue collection and surgical procedures. Beyond 1 h, oxygen saturation is low with the 3-g/L solution; the 1-g/L concentration should be used to ensure better tissue oxygenation. Euthanasia of MS222-anesthetized frogs can be accelerated by administering intracoelomic pentobarbital, which causes cardiac arrest in less than 5 min.
Acknowledgment
We thank Dr Lionel Carmant from the neurologic research laboratory at Ste Justine Hospital Research Center for the electroencephalographic material used in this study, Dr Gregor Andelfinger from the cardiology research laboratory at Ste Justine Hospital Research Center for giving us the frogs, and Marie-Thérèse Parent for preparing the figures.
References
- 1.Akula KK, Dhir A, Kulkami SK. 2009. Effects of various antiepileptic drugs in a pentylenetetrazol-induced seizure model in mice. Methods Find Exp Clin Pharmacol 31: 423–432 [DOI] [PubMed] [Google Scholar]
- 2.American Veterinary Medical Association [Internet]. 2007. AVMA guidelines on euthanasia, 2007 updated 1. [Cited July 2011]. Available at: http://www.avma.org/issues/animal_welfare/euthanasia.pdf
- 3.Balasandaram K, Ramalingam K, Selvarajan VR. 1997. Bioelectrical activity of brain in Rana tigrina (Daudin) in response to phosalone poisoning. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 118C:229–231 [DOI] [PubMed] [Google Scholar]
- 4.Beck CW, Slack JMW. 2001. An amphibian with ambition: a new role for Xenopus in the 21st century. Genome Biol 2:reviews1029.1–reviews1029.5. [DOI] [PMC free article] [PubMed]
- 5.Canadian Council on Animal Care. 1993. Guide to the care and use of experimental animals, vol 1, 2nd ed. Ottawa (Canada): Canadian Council on Animal Care.
- 6.Crawshaw GJ.1993. Amphibian medicine, p 131–139. In: Fowler M, Zoo and wild animal medicine: current therapy 3. Philadelphia (PA): WB Saunders.
- 7.Downes H. 1995. Tricaine methanesulfonate in amphibian: a review. Bull Assoc Rept Amph Vet 5:11–16 [Google Scholar]
- 8.Ekström PM, Short CE, Geimer TR. 1993. Electroencephalography of detomidine–ketamine–halothane and detomidine–ketamine–isoflurane anesthetized horses during orthopedic surgery. A comparison. Vet Surg 22:414–418 [DOI] [PubMed] [Google Scholar]
- 9.Fang G, Chen Q, Cui J, Tang Y. 2012. Electroencephalogram bands modulated by vigilance states in anuran species: a factor analytic approach. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 198:119–127 [DOI] [PubMed] [Google Scholar]
- 10.Frazier DT, Narahashi T. 1975. Tricaine (MS222): effects on ionic conductances of squid axon membranes. Eur J Pharmacol 33:313–317 [DOI] [PubMed] [Google Scholar]
- 11.Gentle MJ. 1971. Electrical activity in the optic tectum and color changes in the minnow (Phoxinus phoxinus L.). J Exp Biol 55:641–649 [DOI] [PubMed] [Google Scholar]
- 12.Goulet F, Hélie P, Vachon P. 2010. Eugenol anesthesia of Xenopus leavis frogs of different body weights. J Am Assoc Lab Anim Sci 49:460–463 [PMC free article] [PubMed] [Google Scholar]
- 13.Green SL.2010. The laboratory Xenopus sp. Boca Raton (FL): CRC Press.
- 14.Grocott HP, Davie S, Fedorow C. 2010. Monitoring of brain function in anesthesia and intensive care. Curr Opin Anaesthesiol 23:759–764 [DOI] [PubMed] [Google Scholar]
- 15.Guénette SA, Hélie P, Beaudry F, Vachon P. 2007. Eugenol for anesthesia of African clawed frogs (Xenopus laevis). Vet Anaesth Analg 34:164–170 [DOI] [PubMed] [Google Scholar]
- 16.Hudetz AG, Vizuete JA, Pillay S. 2011. Differential effects of isoflurane on high-frequency and low-frequency γ oscillations in the cerebral cortex and hippocampus in freely moving rats. Anesthesiology 114:588–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoka M, Wallingford J, Blitz I, Conlon FL, Harland R, Lau N, Miller A, Philpott A, Stukenberg T, Veenstra G, Zorn A. [Internet]. 2011. 2011 Xenopus Community White Paper. [Cited August 2011]. Available at: http://www.xenbase.org/community/static/xenopuswhitepaper/2011/XWP_xenbase.jsp.
- 18.Laming PR. 1982. Electroencephalic correlates of behavior in the anurans Bufo regularis and Rana temporaria. Behav Neural Biol 34:296–306 [DOI] [PubMed] [Google Scholar]
- 19.Otto K, Short CE. 1991. Electroencephalographic power spectrum analysis as a monitor of anesthetic depth in horses. Vet Surg 20:362–371 [DOI] [PubMed] [Google Scholar]
- 20.Otto KA, Cebotari S, Höffler HK, Tudorache I. 2012. Electroencephalographic narcotrend index, spectral edge frequency, and median power frequency as guide to anaesthetic depth for cardiac surgery in laboratory sheep. Vet J . [DOI] [PubMed] [Google Scholar]
- 21.Selden S.1989. Local anesthesia drugs and techniques, p 15–28. In: Muir WW, Hubbell JAE, Handbook of veterinary anesthesia. St Louis (MO): CV Mosby.
- 22.Stetter MD, Raphael B, Indiviglio F, Cook RA.1996. Isoflurane anesthesia in amphibians: comparison of 5 application methods, p 255–257. In: Proceedings American Association of Zoo Veterinarians 1996.Yulee (FL): AAZV.
- 23.Torreilles SL, McClure DE, Green SL. 2009. Evaluation and refinement of euthanasia methods for Xenopus laevis. J Am Assoc Lab Anim Sci 48:512–516 [PMC free article] [PubMed] [Google Scholar]
- 24.Vachon P, Dupras J, Prout R, Blais D. 1999. Electroencephalographic recordings in anesthetized rabbits: comparison of ketamine–midazolam and telazol with or without xylazine. Contemp Top Lab Anim Sci 38:57–61 [PubMed] [Google Scholar]
- 25.Wright KM.2001. Restraint techniques and euthanasia, p 111–122. In: Wright KM, Whitaker BR, Amphibian medicine and captive husbandry. Malabar (FL): Krieger Publishing.
- 26.Yoshikawa H, Yokoyama Y, Ueno S, Mitsuda H. 1991. Electroencephalographic spectral analysis in carp, Cyprinus carpio, anesthetized with high concentrations of carbon dioxide. Comp Biochem Physiol A Physiol 98:437–444 [Google Scholar]



