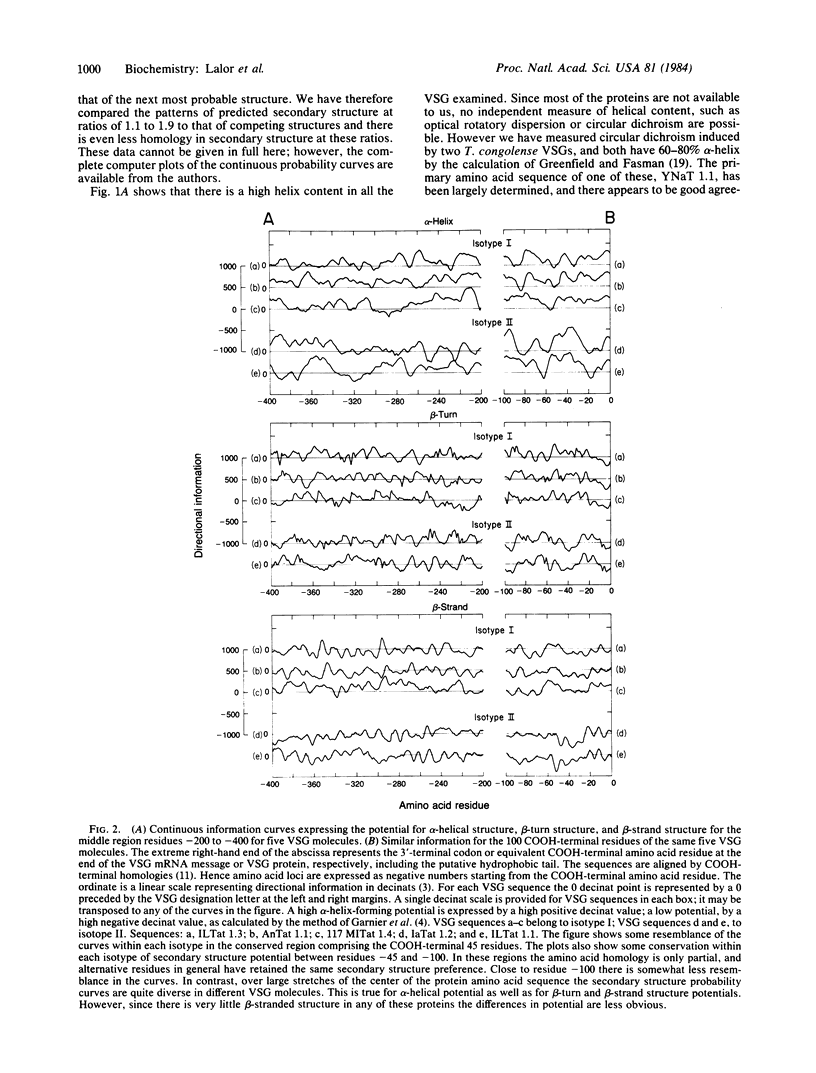
Abstract
Infection with the African trypanosomes gives rise to relapsing waves of parasitemia in the host. A predominant population of trypanosomes is present in each wave, and such predominant populations are usually serologically distinct from each other. Trypanosomes are covered by an extramembranous, highly antigenic, variant-specific glycoprotein coat that is 15 nm thick. The primary structure of a large portion of the glycoprotein molecule is different in the predominant trypanosome populations of each parasitemic wave. Analysis of the secondary structure potential of five full-length and five partial amino acid sequences of variant-specific glycoproteins from members of the Trypanosoma brucei complex has been carried out. The potentials for alpha-helix, beta-turns, and beta-strand structure have been calculated. A high degree of alpha-helical structure potential is present in all the full-length or partial sequences examined. There is conservation of secondary structure potential in the COOH-terminal 100 amino acids, where both partial and complete conservation of primary amino acid sequence exists. The NH2-terminal regions are rich in alpha-helix potential. However, over large stretches of the middle of the VSG molecules there is wide diversity of secondary structure potential. This suggests that tertiary folding structures may also be different in this region. If these predictions are true, different regions of the variant-specific glycoprotein could be exposed to the solvent in different variant-specific trypanosome serotypes. The implication is that antigenic variation is mediated by a polygene family of glycoproteins containing highly polymorphic regions. These could fold differently and expose different surface regions of the protein to the solvent. This device might reduce immune crossreactivity among members of the variant-specific glycoprotein family.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G., Gurnett L. P., Cross G. A. Complete amino acids sequence of a variant surface glycoprotein (VSG 117) from Trypanosoma brucei. J Mol Biol. 1982 May 25;157(3):527–546. doi: 10.1016/0022-2836(82)90474-0. [DOI] [PubMed] [Google Scholar]
- Benoist C. O., Mathis D. J., Kanter M. R., Williams V. E., 2nd, McDevitt H. O. Regions of allelic hypervariability in the murine A alpha immune response gene. Cell. 1983 Aug;34(1):169–177. doi: 10.1016/0092-8674(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Bogucki M. S., Onodera M., Rosen N. L., Lifter J., Hotez P. J., Konigsberg W. H., Richards F. F. Trypanosoma congolense: surface glycoproteins of two early bloodstream variants. III. Immunochemical characterization. Exp Parasitol. 1982 Feb;53(1):1–10. doi: 10.1016/0014-4894(82)90086-8. [DOI] [PubMed] [Google Scholar]
- Bogucki M. S., Onodera M., Rosen N. L., Lifter J., Hotez P. J., Konigsberg W. H., Richards F. F. Trypanosoma congolense: surface glycoproteins of two early bloodstream variants. III. Immunochemical characterization. Exp Parasitol. 1982 Feb;53(1):1–10. doi: 10.1016/0014-4894(82)90086-8. [DOI] [PubMed] [Google Scholar]
- Boothroyd J. C., Cross G. A. Transcripts coding for variant surface glycoproteins of Trypanosoma brucei have a short, identical exon at their 5' end. Gene. 1982 Dec;20(2):281–289. doi: 10.1016/0378-1119(82)90046-4. [DOI] [PubMed] [Google Scholar]
- Borst P., Cross G. A. Molecular basis for trypanosome antigenic variation. Cell. 1982 Jun;29(2):291–303. doi: 10.1016/0092-8674(82)90146-5. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Donelson J. E., Young J. R., Dorfman D., Majiwa P. A., Williams R. O. The ILtat 1.4 surface antigen gene family of Trypanosoma brucei. Nucleic Acids Res. 1982 Nov 11;10(21):6581–6595. doi: 10.1093/nar/10.21.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvillier G., Aubert J. P., Baltz T., Richet C., Degand P. Variant specific surface antigens from Trypanosoma equiperdum: chemical and physical studies. Biochem Biophys Res Commun. 1983 Jan 27;110(2):491–498. doi: 10.1016/0006-291x(83)91176-2. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Lifson S., Sander C. Antiparallel and parallel beta-strands differ in amino acid residue preferences. Nature. 1979 Nov 1;282(5734):109–111. doi: 10.1038/282109a0. [DOI] [PubMed] [Google Scholar]
- Matthyssens G., Michiels F., Hamers R., Pays E., Steinert M. Two variant surface glycoproteins of Trypanosoma brucei have a conserved C-terminus. Nature. 1981 Sep 17;293(5829):230–233. doi: 10.1038/293230a0. [DOI] [PubMed] [Google Scholar]
- Merritt S. C., Tschudi C., Konigsberg W. H., Richards F. F. Reverse transcription of trypanosome variable antigen mRNAs initiated by a specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1536–1540. doi: 10.1073/pnas.80.6.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels F., Matthyssens G., Kronenberger P., Pays E., Dero B., Van Assel S., Darville M., Carvador A., Steinert M., Hamers R. Gene activation and re-expression of a Trypanosoma brucei variant surface glycoprotein. EMBO J. 1983;2(7):1185–1192. doi: 10.1002/j.1460-2075.1983.tb01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice-Ficht A. C., Chen K. K., Donelson J. E. Point mutations during generation of expression-linked extra copy of trypanosome surface glycoprotein gene. Nature. 1982 Aug 12;298(5875):676–679. doi: 10.1038/298676a0. [DOI] [PubMed] [Google Scholar]
- Robson B., Pain R. H. Analysis of the code relating sequence to conformation in globular proteins. An informational analysis of the role of the residue in determining the conformation of its neighbours in the primary sequence. Biochem J. 1974 Sep;141(3):883–897. doi: 10.1042/bj1410883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B., Pain R. H. Analysis of the code relating sequence to conformation in globular proteins. Development of a stereochemical alphabet on the basis of intra-residue information. Biochem J. 1974 Sep;141(3):869–882. doi: 10.1042/bj1410869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B., Pain R. H. Analysis of the code relating sequence to conformation in globular proteins. The distribution of residue pairs in turns and kinks in the backbone chain. Biochem J. 1974 Sep;141(3):899–904. doi: 10.1042/bj1410899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B., Suzuki E. Conformational properties of amino acid residues in globular proteins. J Mol Biol. 1976 Nov 5;107(3):327–356. doi: 10.1016/s0022-2836(76)80008-3. [DOI] [PubMed] [Google Scholar]
- Strickler J. E., Patton C. L. Trypanosoma brucei: effect of inhibition of N-linked glycosylation of the nearest neighbor analysis of the major variable surface coat glycoprotein. Mol Biochem Parasitol. 1982 Feb;5(2):117–131. doi: 10.1016/0166-6851(82)90046-9. [DOI] [PubMed] [Google Scholar]
- Strickler J. E., Patton C. L. Trypanosoma brucei: nearest neighbor analysis on the major variable surface coat glycoprotein--crosslinking patterns with intact cells. Exp Parasitol. 1982 Feb;53(1):117–132. doi: 10.1016/0014-4894(82)90098-4. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Valerio D., De Lange T., Bernards A., Borst P., Grosveld F. G. An analysis of cosmid clones of nuclear DNA from Trypanosoma brucei shows that the genes for variant surface glycoproteins are clustered in the genome. Nucleic Acids Res. 1982 Oct 11;10(19):5905–5923. doi: 10.1093/nar/10.19.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]


