Abstract
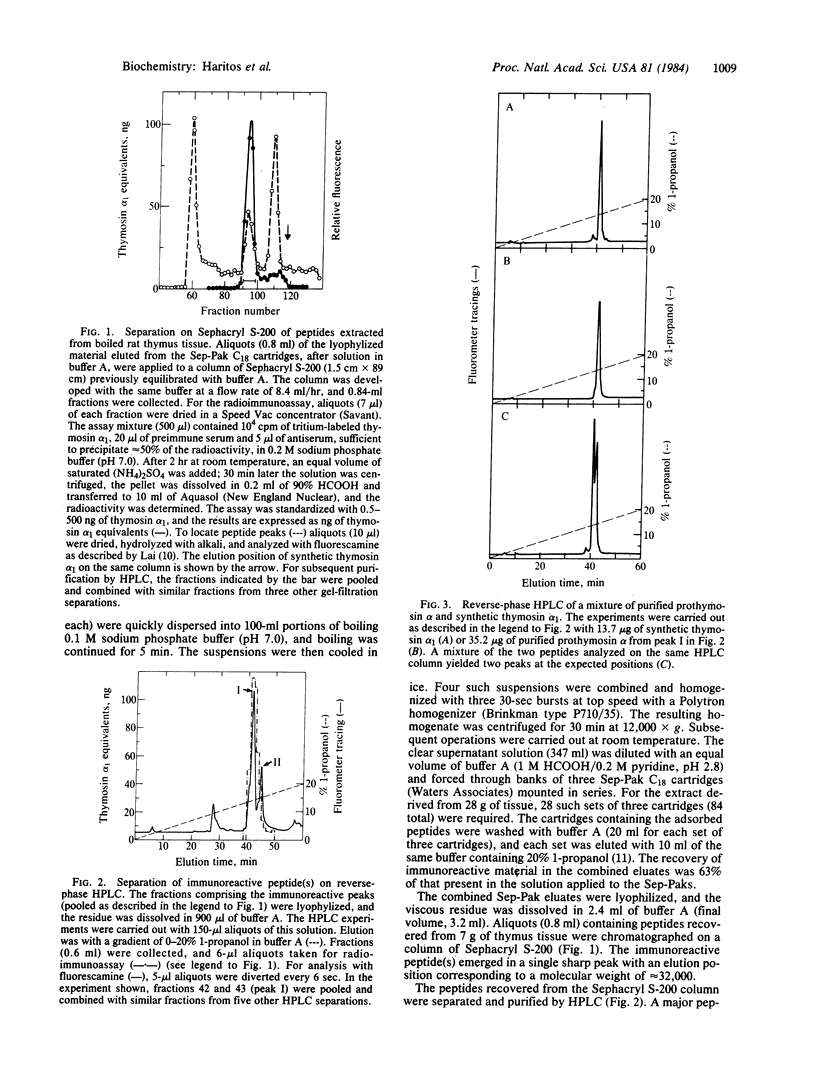
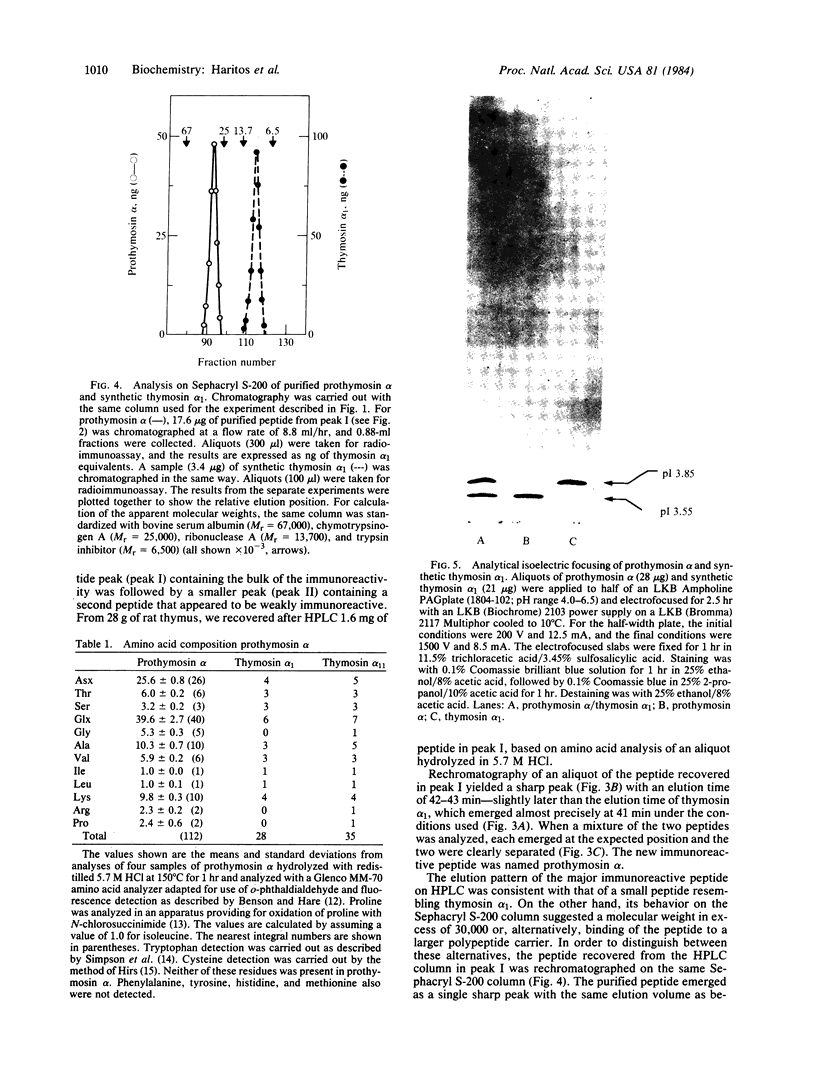
A polypeptide containing approximately equal to 112 amino acid residues, with the thymosin alpha 1 sequence at its NH2 terminus, has been isolated from rat thymus by using a radioimmunoassay with an antibody prepared against synthetic thymosin alpha 1. The new polypeptide, named "prothymosin alpha," was found to be the major substance crossreacting with thymosin alpha 1 antiserum in rat thymus extracts; peptides corresponding to thymosin alpha 1 or thymosin alpha 11 were not detected. In gel filtration at pH 2.8, prothymosin alpha emerged as a single symmetrical peak corresponding to an apparent molecular weight of 32,000, approximately 3 times larger than the minimum molecular weight calculated from its amino acid composition. On the same gel filtration columns, synthetic thymosin alpha 1 (calculated Mr = 3108) emerged at a position corresponding to a molecular weight of 10,000-11,000. Thus, both prothymosin alpha and thymosin alpha 1 appear to exist in solution as oligomers, possibly as trimers. Prothymosin alpha and synthetic thymosin alpha 1 also were separated readily in reverse-phase HPLC and in isoelectric focusing; the isoelectric point of prothymosin alpha determined by the latter procedure was found to be 3.55, consistent with an unusually high content of glutamic and aspartic acids based on amino acid analyses. Prothymosin alpha appears to represent the native polypeptide from which thymosin alpha 1 and other fragments are generated during the isolation of thymosin fraction 5.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson J. R., Hare P. E. O-phthalaldehyde: fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin. Proc Natl Acad Sci U S A. 1975 Feb;72(2):619–622. doi: 10.1073/pnas.72.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Marconi P., Frati L., Bonmassar E., Garaci E. Increase of mouse resistance to Candida albicans infection by thymosin alpha 1. Infect Immun. 1982 May;36(2):609–614. doi: 10.1128/iai.36.2.609-614.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarella J., Goodall G. J., Felix A. M., Heimer E. P., Salvin S. B., Horecker B. L. Thymosin alpha 11: a peptide related to thymosin alpha 1 isolated from calf thymosin fraction 5. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7424–7427. doi: 10.1073/pnas.80.24.7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau G. R. Cleavage at glutamic acid with staphylococcal protease. Methods Enzymol. 1977;47:189–191. doi: 10.1016/0076-6879(77)47023-x. [DOI] [PubMed] [Google Scholar]
- Goldstein A. L., Guha A., Zatz M. M., Hardy M. A., White A. Purification and biological activity of thymosin, a hormone of the thymus gland. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1800–1803. doi: 10.1073/pnas.69.7.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., Low T. L., McAdoo M., McClure J., Thurman G. B., Rossio J., Lai C. Y., Chang D., Wang S. S., Harvey C. Thymosin alpha1: isolation and sequence analysis of an immunologically active thymic polypeptide. Proc Natl Acad Sci U S A. 1977 Feb;74(2):725–729. doi: 10.1073/pnas.74.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannappel E., Davoust S., Horecker B. L. Isolation of peptides from calf thymus. Biochem Biophys Res Commun. 1982 Jan 15;104(1):266–271. doi: 10.1016/0006-291x(82)91969-6. [DOI] [PubMed] [Google Scholar]
- Hannappel E., Xu G. J., Morgan J., Hempstead J., Horecker B. L. Thymosin beta 4: a ubiquitous peptide in rat and mouse tissues. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2172–2175. doi: 10.1073/pnas.79.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper J. A., McDaniel M. C., Thurman G. B., Cohen G. H., Schulof R. S., Goldstein A. L. Purification and properties of bovine thymosin. Ann N Y Acad Sci. 1975 Feb 28;249:125–144. doi: 10.1111/j.1749-6632.1975.tb29063.x. [DOI] [PubMed] [Google Scholar]
- Ishioka N., Isobe T., Okuyama T., Numata Y., Wada H. A new acidic protein in porcine brain. Biochim Biophys Acta. 1980 Oct 21;625(2):281–290. doi: 10.1016/0005-2795(80)90292-5. [DOI] [PubMed] [Google Scholar]
- Ishitsuka H., Umeda Y., Nakamura J., Yagi Y. Protective activity of thymosin against opportunistic infections in animal models. Cancer Immunol Immunother. 1983;14(3):145–150. doi: 10.1007/BF00205352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Gibson K. D., Jones B. N. Is adrenal proenkephalin glycosylated? Arch Biochem Biophys. 1983 Jul 1;224(1):402–404. doi: 10.1016/0003-9861(83)90226-6. [DOI] [PubMed] [Google Scholar]
- Lai C. Y. Detection of peptides by fluorescence methods. Methods Enzymol. 1977;47:236–243. doi: 10.1016/0076-6879(77)47028-9. [DOI] [PubMed] [Google Scholar]
- Low T. L., Thurman G. B., McAdoo M., McClure J., Rossio J. L., Naylor P. H., Goldstein A. L. The chemistry and biology of thymosin. I. Isolation, characterization, and biological activities of thymosin alpha1 and polypeptide beta1 from calf thymus. J Biol Chem. 1979 Feb 10;254(3):981–986. [PubMed] [Google Scholar]
- Simpson R. J., Neuberger M. R., Liu T. Y. Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem. 1976 Apr 10;251(7):1936–1940. [PubMed] [Google Scholar]
- Stein S., Moschera J. High-performance liquid chromatography and picomole-level detection of peptides and proteins. Methods Enzymol. 1981;79(Pt B):7–16. doi: 10.1016/s0076-6879(81)79006-2. [DOI] [PubMed] [Google Scholar]
- Weigele M., DeBernardo S., Leimgruber W. Fluorometric assay of secondary amino acids. Biochem Biophys Res Commun. 1973 Jan 23;50(2):352–356. doi: 10.1016/0006-291x(73)90847-4. [DOI] [PubMed] [Google Scholar]




