Abstract
We demonstrate a fiber-based, three-color femtosecond source for simultaneous imaging of three fluorescent proteins (FPs) using two-photon fluorescence microscopy (2PM). The three excitation wavelengths at 775 nm, 864 nm and 950 nm, are obtained through second harmonic generation (SHG) of the 1550-nm pump laser and the 1728-nm and 1900-nm solitons generated through soliton self-frequency shift (SSFS) in a large-mode-area (LMA) fiber. These energetic pulses are well matched to the two-photon excitation peaks of red, cyan and yellow fluorescent proteins (TagRFPs, TagCFPs, and TagYFPs) for efficient excitation. We demonstrate simultaneous 2PM of human melanoma cells expressing a “rainbow” combination of these three fluorescent proteins.
OCIS codes: (060.4370) Nonlinear optics, fibers; (180.2520) Fluorescence microscopy; (180.4315) Nonlinear microscopy; (190.2620) Harmonic generation and mixing
1. Introduction
Fluorescent proteins (FPs) are genetically encoded reporters that have become indispensable tools in biomedical research ranging from tissue imaging down to single molecule studies [1]. Simultaneous imaging of cells expressing multiple FPs is of particular interest in applications such as mapping neural circuits [2], tracking multiple immune cell populations [3], monitoring cancer growth and metastasis [4,5], and clonal analysis of stem cells [6]. To visualize both in vivo and ex vivo tissue morphology and physiology at a cellular level deep within scattering tissues, two-photon fluorescence microscopy (2PM) [7] is a powerful tool that has found wide applications. In 2PM, there are two requirements on the laser source for efficient fluorescence excitation: (1) energetic femtosecond pulses are needed, and (2) the laser wavelength should match the two-photon absorption peak of the fluorophores. To efficiently excite multiple FPs for 2PM, a third requirement arises: multi-color femtosecond pulses are needed because the two-photon absorption peaks of the FPs can be separated by hundreds of nanometers. For example, the absorption peaks of red fluorescent proteins (RFPs) and yellow fluorescent proteins (YFPs) are at 775 nm and 950 nm, respectively [8]. However, simultaneous imaging of multiple FPs with 2PM is greatly hampered by the lack of proper ultrafast lasers offering multi-color femtosecond pulses, each targeting the two-photon absorption peak of a different FP. Conventional femtosecond lasers, such as the mode-locked titanium:sapphire (Ti:S) lasers routinely used for 2PM, can only efficiently generate one-color femtosecond pulses, which makes simultaneous excitation of multiple FPs impossible. Using two femtosecond sources to generate two wavelengths has been demonstrated for 2PM [9]. However, the source consisting of two Ti:S lasers and an optical parametric oscillator (OPO) is complicated and costly. Additionally, limited by the spectral coverage of the source, the two FPs had to be chosen to have very close excitation and emission wavelengths, causing severe bleed-through on color mapping. It’s possible to excite multiple FPs with a very short pulse (<10 fs) that has a spectral bandwidth spans hundreds of nanometers. However, the excitation will be rather inefficient especially towards the edges of the spectrum due to the low spectral power density, and the excitation power within a majority of the spectrum will be wasted.
Here we demonstrate simultaneous two-photon fluorescence excitation of RFP, YFP, and CFP in human melanoma cells engineered to express a “rainbow” pallet of colors, using a novel fiber-based source offering energetic, three-color femtosecond pulses. The three-color pulses, centered at 775 nm, 864 nm and 950 nm, are obtained through second harmonic generation (SHG) of the 1550 nm pump laser and SHG of the solitons at 1728 nm and 1900 nm generated through soliton self-frequency shift (SSFS) in a large-mode-area (LMA) fiber. The resulting wavelengths are well matched to the two-photon absorption peaks of the three FPs for efficient excitation. The three FPs with well separated excitation and emission wavelengths reduce bleed-through. Our results demonstrate that multi-color femtosecond pulse generation using SSFS and a turn-key, fiber-based femtosecond laser can fulfill the requirements for simultaneous imaging of multiple FPs in 2PM, opening new opportunities for a wide range of biological applications where non-invasive, high-resolution imaging of multiple fluorescent indicators is required.
The phenomenon of SSFS in optical fibers [10], in which intrapulse stimulated Raman scattering continuously transfers energy from higher to lower frequencies, has been exploited to create widely frequency-tunable, femtosecond pulse sources with fiber delivery [11–15]. Recent experiments on SSFS in LMA fibers have demonstrated that widely tunable, energetic pulses with high pulse quality can be generated from 1580 nm to 2130 nm using a compact, fiber-based pump source at 1550 nm [15]. Furthermore, as the input power is increased, multiple solitons can be generated [15], indicating that multi-color femtosecond pulses can be generated from a single fiber-based source. In most previous experiments, the appearance of multiple solitons was undesirable, because most applications need only one soliton and the rest must be carefully filtered to avoid interference. In order to create a multi-color femtosecond source at the desired wavelengths, we take advantage of this interesting property of SSFS through optimization of the fiber length and launch pulse energy. The combination of the LMA fiber and multiple soliton generation provides a practical source with energetic multi-color femtosecond pulses for simultaneous 2PM of multiple FPs.
2. Experimental setup and system characterization
In our experiment, the pump source at 1550-nm is a compact, turnkey, fiber-based laser (Calmar, FLCPA-01C) with 1-MHz pulse repetition rate and 360-fs pulse width. The linearly polarized pulses are divided into two branches by the combination of a half-wave plate (HWP) and a polarizing beam splitter (PBS, Fig. 1 ). SSFS is performed in 143-cm LMA fiber (PMLMA35, NKT Photonics) with an effective mode area (Aeff) of 615 μm2. The LMA fiber is polarization maintained to enhance the stability of pulse propagation, so a HWP is inserted in front of the fiber to align the polarization of the incoming beam to the polarization axis of the fiber for most efficient SSFS. The coupling efficiency is 60% measured at low power. Wavelength tuning of the solitons can be easily realized through changing the input power [15]. After collimation, the two solitons at 1728 nm and 1900 nm are separated by an 1800-nm short-pass dichroic mirror (DC1) for SHG. In order to use one bismuth borate (BiBO) crystal (1.5-mm thick, cut at θ = 34.9°, φ = 0°, with broadband antireflection coating) for SHG of both solitons, the two soliton beams are offset vertically to separate the two SH beams. The incident angles of each beam are also optimized for perfect phase matching. After collimation, SH beams at 864 nm and 950 nm are combined with a 900-nm short-pass dichroic (DC2). The other branch (100 nJ) of the initial 1550-nm pulse is focused into a 0.5-mm beta barium borate crystal (BBO) for SHG. After collimation, the 775 nm pulse is combined with the 864 nm and 950 nm pulses by an 801-nm short-pass dichroic (DC3). The three collinear beams are sent into a multiphoton microscope (described in detail in [16]). Two-photon fluorescence from three FPs is excited and collected in the epi-direction with a water immersion objective lens (XLUMPlanFl, Olympus, NA = 0.95). Three band-pass filters at 630/92, 460/80 and 525/50 are inserted before three photomultiplier tubes (PMTs) to transmit fluorescence generated by RFP, CFP and YFP, respectively.
Fig. 1.
Experimental setup. HWP: half-wave plate, L1~L8: lenses, M: mirror, DC1: 1800 nm dichroic, DC2: 900 nm dichroic, DC3: 801 nm dichroic, LMA fiber: PM LMA35 fiber.
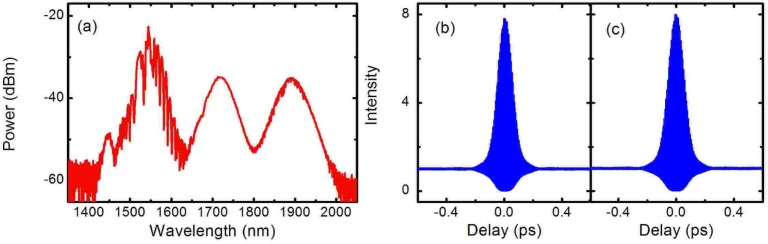
We first characterize the solitons. When the output power from the LMA fiber is 88 mW, the first and second solitons shift to 1728 nm and 1900 nm, respectively [Fig. 2(a) ]. There is no temporal overlap of the three pulses. To isolate the 1728-nm soliton from the residual for characterization, a 1600-nm long pass filter is used. The deconvolved pulse widths from the measured second-order interferometric autocorrelation traces [Figs. 2(b) and (c)] are 71 fs and 76 fs for the 1728-nm and 1900-nm solitons, respectively assuming sech2 intensity profile, with corresponding pulse energies of 16 nJ and 26 nJ. Due to the large mode area of the LMA fiber, coupling is very stable on both short-term and day-to-day basis. The maximum wavelength fluctuation (peak-to-peak) is less than 5 nm, which is well within the acceptance bandwidth of the BiBO crystal (38 nm calculated) and inconsequential for the two-photon excitation efficiency of the FPs.
Fig. 2.
(a) Soliton spectra (in log scale) after the LMA fiber. Second-order interferometric autocorrelation traces for the solitons at 1728 nm (b) and 1900 nm (c).
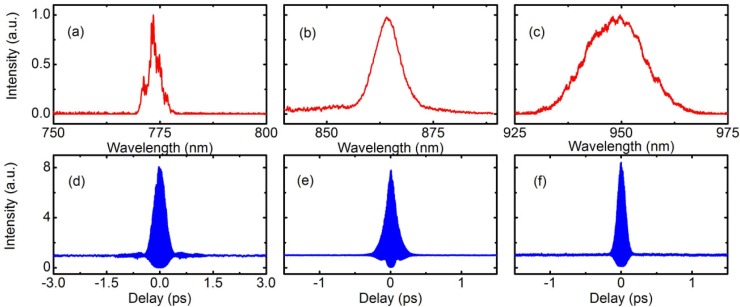
We then characterize the three second harmonic pulses for imaging. The deconvolved pulse widths from the measured second-order interferometric autocorrelation traces (Fig. 3 ) assuming Gaussian pulse are, respectively, 245 fs, 132 fs and 88 fs for 775 nm, 864 nm and 950 nm, with the corresponding pulse energies of 14 nJ, 7.2 nJ and 12 nJ. Pulse broadening compared to the solitons is likely due to group velocity mismatch. For SHG of the 1550 nm pulse, the efficiency (currently 14%) can be markedly increased by using an optimized SHG crystal. For example, with the same 1.5-mm BiBO crystal used for SHG of the shifted solitons, we obtained SHG efficiency of 30% (i.e., 30 nJ at 775 nm) for 100 nJ input at 1550 nm under the same focusing condition.
Fig. 3.
SH spectra at 775 nm (a), 864 nm (b) and 950 nm (c). The corresponding second-order interferometric autocorrelation traces for 775 nm (d), 864 nm (e) and 950 nm (f).
3. Two-photon fluorescence imaging of rainbow cells
With this three-color laser source, as a demonstration, we performed 2PM on human melanoma cells expressing a combination of RFP, YFP, and CFP such that each cell has a distinct hue. The human amelanotic melanoma cell line A375 was cultured in 10% FBS + DMEM. A375-Rainbow was created by incubating the cells with a combination of MISSION pLKO.1-puro-CMV-TagCFP/TagYFP/TagRFP positive control transduction particles, each at a multiplicity of infection (MOI) of 10, and 8 μg/ml hexadimethrine bromide. Cells expressing FPs were selected with 2 μg/ml puromycin, then sorted for the triple positive TagCFPhi, TagYFPhi and TagRFPhi population using flow cytometry. MISSION transduction particles, hexadimethrine bromide and puromycin were all purchased from Sigma Aldrich (St Louis, MO). These A375-Rainbow cells were cultured and attached on glass bottom petri dishes for 2PM on the inverted microscope. To avoid photo-damage and photo-bleaching of the FPs, the power level of the three colors was kept around 200 μW (0.2 nJ pulses) after the objective. Generated signals were detected by three PMTs for red fluorescence (584-676 nm, GaAsP), yellow fluorescence (500-550nm, ultra bialkali) and cyan fluorescence (420-500 nm, ultra bialkali), and sampled at a 16-bit pixel depth. Sampling vectors were reconstructed to form 512 × 512 images with 343 × 343 μm fields of view. The acquisition time for each 2PM image of A375-Rainbow cells was 2 sec per frame.
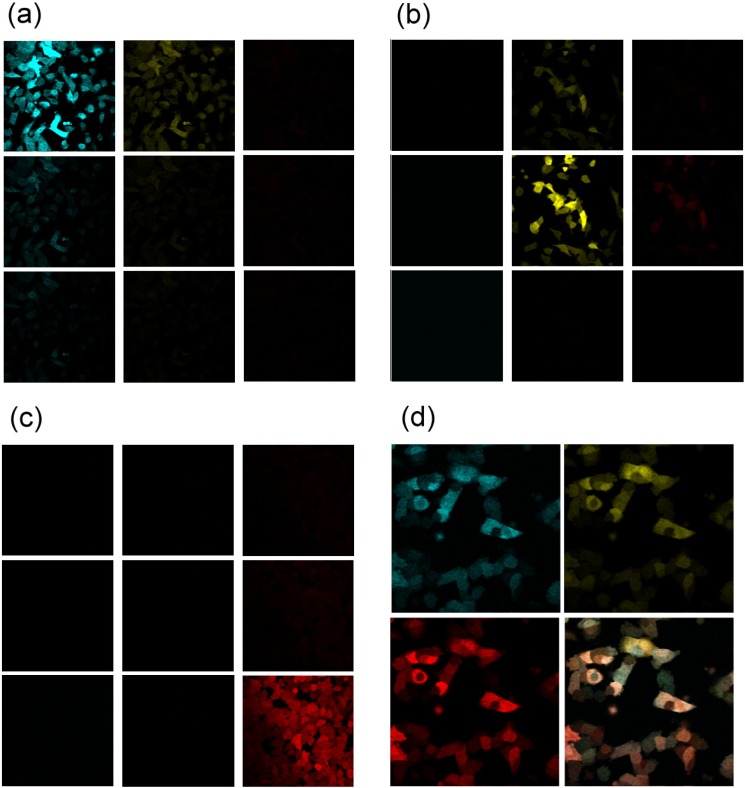
For calibration of bleed-through and noise background, we imaged cells labeled with one FP only and excited by one color at a time to get a matrix of images [Figs. 4(a) -4(c)]. Obviously, FPs only show the best contrast when excited at their two-photon peak absorption wavelength and detected by the corresponding channels. Images in the matrices [Figs. 4 (a)-4(c)] also show the co-excitation (vertical direction) and emission bleed-through (channel with longer detection wavelength) for the individual FPs. Because the bleed-through is mostly caused by the overlapping emission spectra of the FPs [i.e., within the rows in Figs. 4(a) and 4(b)], further optimization of the band pass filters can reduce the bleed-through. With this calibration, for each channel, the proportion of the fluorescence signals from individual FPs is obtained. Then we excited the rainbow labeled melanoma cells simultaneously with all three colors [Fig. 4(d)]. The signals from TagCFP (cyan), TagYFP (yellow), and TagRFP (red) can thus be combined to obtain the hue of each melanoma cell [bottom right image in Fig. 4(d)].
Fig. 4.
2PM fluorescence images of (a) TagCFP, (b) TagYFP, and (c) TagRFP cell lines excited separately at 864 nm (first rows), 950 nm (second rows), and 775 nm (third rows). Three detection channels have bandpass filters of 420-500nm (first columns), 500-550nm (second columns), and 584-676 nm (third columns). (d) Simultaneous 2PM images of A375-Rainbow cells excited by the three-color femtosecond lasers. Calibrated from (a-c), signals from TagCFP (cyan), TagYFP (yellow), and TagRFP (red) channels are combined into a color coded image [bottom right one in (d)], representing cells expressing different colors.
4. Conclusion
In conclusion, based-on SSFS in an LMA fiber and SHG, we developed a three-color near-infrared femtosecond laser source with ~10-nJ pulse energy. Their wavelengths, 775-nm, 864-nm, and 950-nm, match the maxima of the two-photon excitation spectra of three commonly used FPs: RFP, CFP and YFP. Applying this source to 2PM of rainbow-coded systems, signals from different FPs can be simultaneously excited, acquired, and reconstructed into combined images representing the color of each rainbow-coded cell. The demonstrated contrast and imaging speed is high, and can be further increased by using a higher repetition rate pump laser for future applications on tracking the rainbow-coded cells in vivo. By incorporating more fibers or using the polarization multiplexing technique [17], it is easy to scale the number of colors available to map more “colorful” rainbow-coded cells.
Acknowledgments
We acknowledge Hensin Tsao's Laboratory at the Wellman Center for Photomedicine for giving us the amelanotic melanoma cells. This work is supported in part by National Science Council, Taiwan, under Grant No. 101-2918-I-002-022 to T. Liu, NIH R21RR032392 to C. Xu, and NIH P50 CA086355 and U01HL100402 to C. P. Lin. N. G. Horton is supported by NSF Graduate Research Fellowship (Grant No. DGE-0707428).
References and links
- 1.Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C., “Green fluorescent protein as a marker for gene expression,” Science 263(5148), 802–805 (1994). 10.1126/science.8303295 [DOI] [PubMed] [Google Scholar]
- 2.Livet J., Weissman T. A., Kang H., Draft R. W., Lu J., Bennis R. A., Sanes J. R., Lichtman J. W., “Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system,” Nature 450(7166), 56–62 (2007). 10.1038/nature06293 [DOI] [PubMed] [Google Scholar]
- 3.Fan Z., Spencer J. A., Lu Y., Pitsillides C. M., Singh G., Kim P., Yun S. H., Toxavidis V., Strom T. B., Lin C. P., Koulmanda M., “In vivo tracking of ‘color-coded’ effector, natural and induced regulatory T cells in the allograft response,” Nat. Med. 16(6), 718–722 (2010). 10.1038/nm.2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamauchi K., Yang M., Jiang P., Xu M., Yamamoto N., Tsuchiya H., Tomita K., Moossa A. R., Bouvet M., Hoffman R. M., “Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification whole-mouse imaging system,” Cancer Res. 66(8), 4208–4214 (2006). 10.1158/0008-5472.CAN-05-3927 [DOI] [PubMed] [Google Scholar]
- 5.Weber K., Thomaschewski M., Warlich M., Volz T., Cornils K., Niebuhr B., Täger M., Lütgehetmann M., Pollok J. M., Stocking C., Dandri M., Benten D., Fehse B., “RGB marking facilitates multicolor clonal cell tracking,” Nat. Med. 17(4), 504–509 (2011). 10.1038/nm.2338 [DOI] [PubMed] [Google Scholar]
- 6.Hope K., Bhatia M., “Clonal interrogation of stem cells,” Nat. Methods 8(4Suppl), S36–S40 (2011). 10.1038/nmeth.1590 [DOI] [PubMed] [Google Scholar]
- 7.Denk W., Strickler J. H., Webb W. W., “Two-photon laser scanning fluorescence microscopy,” Science 248(4951), 73–76 (1990). 10.1126/science.2321027 [DOI] [PubMed] [Google Scholar]
- 8.Drobizhev M., Makarov N. S., Tillo S. E., Hughes T. E., Rebane A., “Two-photon absorption properties of fluorescent proteins,” Nat. Methods 8(5), 393–399 (2011). 10.1038/nmeth.1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Entenberg D., Wyckoff J. B., Gligorijevic B., Roussos E. T., Verkhusha V. V., Pollard J. W., Condeelis J., “Setup and use of a two-laser multiphoton microscope for multichannel intravital fluorescence imaging,” Nat. Protoc. 6(10), 1500–1520 (2011). 10.1038/nprot.2011.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon J. P., “Theory of the soliton self-frequency shift,” Opt. Lett. 11(10), 662–664 (1986). 10.1364/OL.11.000662 [DOI] [PubMed] [Google Scholar]
- 11.Nishizawa N., Goto T., “Compact system of wavelength-tunable femtosecond soliton pulse generation using optical fibers,” IEEE Photon. Technol. Lett. 11(3), 325–327 (1999). 10.1109/68.748223 [DOI] [Google Scholar]
- 12.Liu X., Xu C., Knox W. H., Chandalia J. K., Eggleton B. J., Kosinski S. G., Windeler R. S., “Soliton self-frequency shift in a short tapered air-silica microstructure fiber,” Opt. Lett. 26(6), 358–360 (2001). 10.1364/OL.26.000358 [DOI] [PubMed] [Google Scholar]
- 13.Skryabin D. V., Luan F., Knight J. C., Russell P. S., “Soliton self-frequency shift cancellation in photonic crystal fibers,” Science 301(5640), 1705–1708 (2003). 10.1126/science.1088516 [DOI] [PubMed] [Google Scholar]
- 14.Wang K., Xu C., “Wavelength-tunable high-energy soliton pulse generation from a large-mode-area fiber pumped by a time-lens source,” Opt. Lett. 36(6), 942–944 (2011). 10.1364/OL.36.000942 [DOI] [PubMed] [Google Scholar]
- 15.Wang K., Xu C., “Tunable high-energy soliton pulse generation from a large-mode-area fiber and its application to third harmonic generation microscopy,” Appl. Phys. Lett. 99(7), 071112 (2011). 10.1063/1.3628337 [DOI] [PubMed] [Google Scholar]
- 16.Kobat D., Durst M. E., Nishimura N., Wong A. W., Schaffer C. B., Xu C., “Deep tissue multiphoton microscopy using longer wavelength excitation,” Opt. Express 17(16), 13354–13364 (2009). 10.1364/OE.17.013354 [DOI] [PubMed] [Google Scholar]
- 17.Nishizawa N., Okamura R., Goto T., “Simultaneous generation of wavelength tunable two-colored femtosecond soliton pulses using optical fibers,” IEEE Photon. Technol. Lett. 11(4), 421–423 (1999). 10.1109/68.752535 [DOI] [Google Scholar]