Abstract
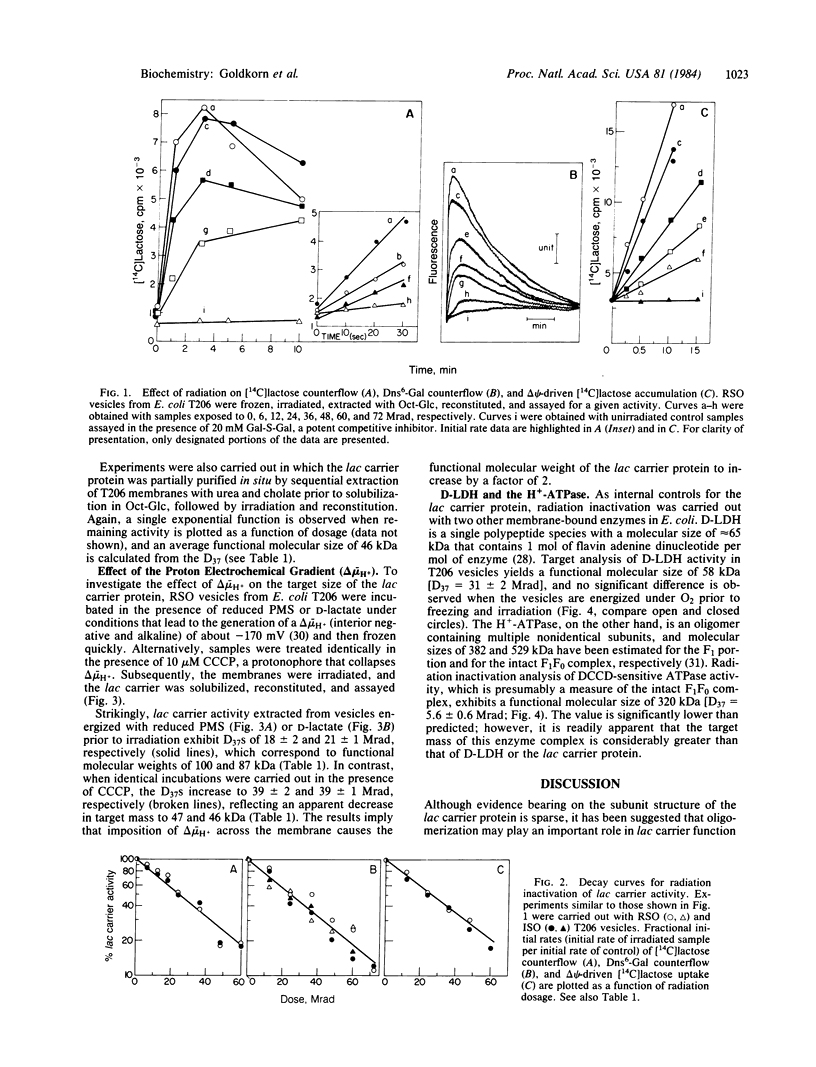
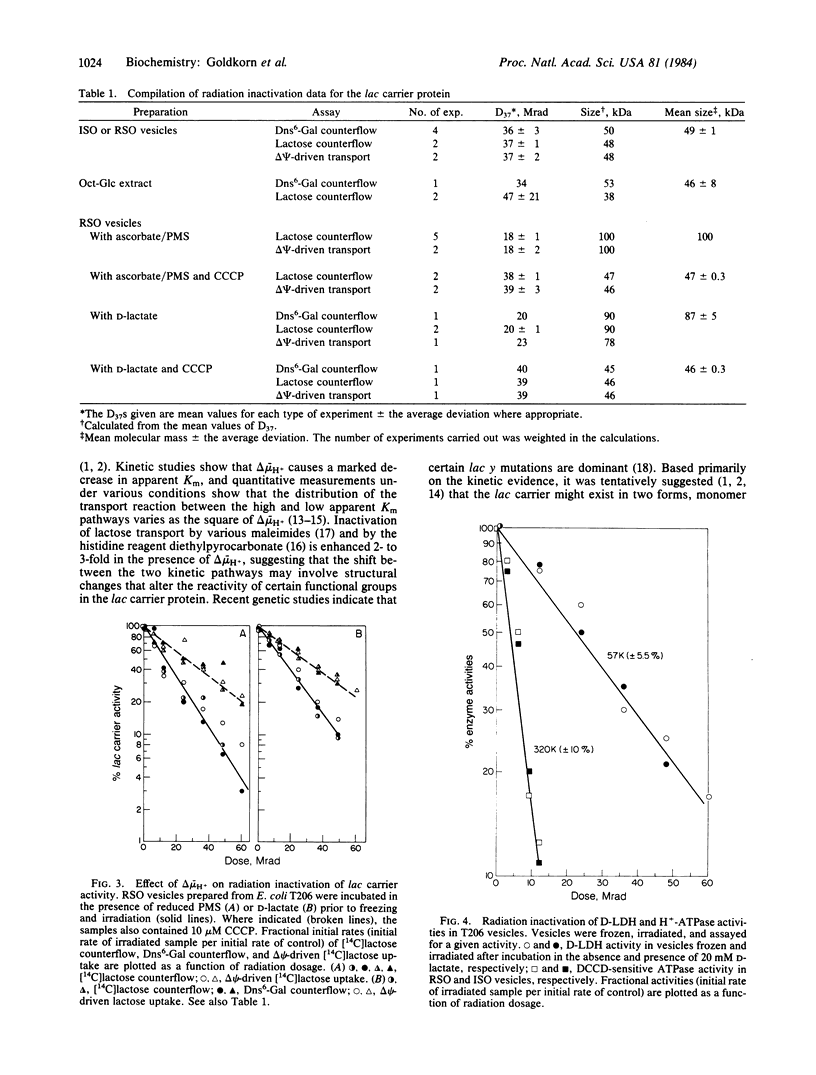
Cytoplasmic membrane vesicles prepared from Escherichia coli containing multiple copies of the lac y gene were frozen in liquid nitrogen before or after generation of a proton electrochemical gradient (interior negative and alkaline) and irradiated with a high-energy electron beam at -135 degrees C. Subsequently, the lac carrier protein was extracted into octyl beta-D-glucopyranoside, reconstituted into proteoliposomes, and assayed for transport activity. Under all conditions tested, activity decreased as a single exponential function of radiation dosage, allowing straightforward application of target theory for determination of functional molecular mass. When lac carrier activity solubilized from nonenergized vesicles was assayed, the results obtained were consistent with a functional molecular size of 45-50 kDa, a value similar to the size of the protein as determined by other means. Similar values were obtained when the octyl beta-D-glucopyranoside extract was irradiated, and the target size observed for D-lactate dehydrogenase was in good agreement with the molecular size of this enzyme. Strikingly, when the same procedures were carried out with vesicles that were energized with appropriate electron donors prior to freezing and irradiation, a functional molecular size of 85-100 kDa was obtained for the lac carrier with no change in the target size of D-lactate dehydrogenase. In contrast, when the vesicles were energized under conditions in which the proton electrochemical gradient was collapsed, the target mass of the lac carrier returned to 45-50 kDa. The results indicate that the functional mass of the lac carrier protein is no greater than a dimer and suggest that the proton electrochemical gradient may cause an alteration in subunit interactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Büchel D. E., Gronenborn B., Müller-Hill B. Sequence of the lactose permease gene. Nature. 1980 Feb 7;283(5747):541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- Carrasco N., Tahara S. M., Patel L., Goldkorn T., Kaback H. R. Preparation, characterization, and properties of monoclonal antibodies against the lac carrier protein from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6894–6898. doi: 10.1073/pnas.79.22.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn D. E., Kaczorowski G. J., Kaback H. R. Effect of the proton electrochemical gradient on maleimide inactivation of active transport in Escherichia coli membrane vesicles. Biochemistry. 1981 May 26;20(11):3308–3313. doi: 10.1021/bi00514a050. [DOI] [PubMed] [Google Scholar]
- Cuppoletti J., Jung C. Y., Green F. A. Glucose transport carrier of human erythrocytes. Radiation target size measurement based on flux inactivation. J Biol Chem. 1981 Feb 10;256(3):1305–1306. [PubMed] [Google Scholar]
- Foster D. L., Boublik M., Kaback H. R. Structure of the lac carrier protein of Escherichia coli. J Biol Chem. 1983 Jan 10;258(1):31–34. [PubMed] [Google Scholar]
- Foster D. L., Fillingame R. H. Stoichiometry of subunits in the H+-ATPase complex of Escherichia coli. J Biol Chem. 1982 Feb 25;257(4):2009–2015. [PubMed] [Google Scholar]
- Foster D. L., Garcia M. L., Newman M. J., Patel L., Kaback H. R. Lactose-proton symport by purified lac carrier protein. Biochemistry. 1982 Oct 26;21(22):5634–5638. doi: 10.1021/bi00265a038. [DOI] [PubMed] [Google Scholar]
- Garcia M. L., Viitanen P., Foster D. L., Kaback H. R. Mechanism of lactose translocation in proteoliposomes reconstituted with lac carrier protein purified from Escherichia coli. 1. Effect of pH and imposed membrane potential on efflux, exchange, and counterflow. Biochemistry. 1983 May 10;22(10):2524–2531. doi: 10.1021/bi00279a033. [DOI] [PubMed] [Google Scholar]
- Ghazi A., Shechter E. Lactose transport in Escherichia coli cells. Dependence of kinetic parameters on the transmembrane electrical potential difference. Biochim Biophys Acta. 1981 Jun 22;644(2):305–315. doi: 10.1016/0005-2736(81)90388-6. [DOI] [PubMed] [Google Scholar]
- Goldkorn T., Rimon G., Kaback H. R. Topology of the lac carrier protein in the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3322–3326. doi: 10.1073/pnas.80.11.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C. Y., Hsu T. L., Hah J. S., Cha C., Haas M. N. Glucose transport carrier of human erythrocytes. Radiation-target size of glucose-sensitive cytochalasin B binding protein. J Biol Chem. 1980 Jan 25;255(2):361–364. [PubMed] [Google Scholar]
- Kaback H. R. The lac carrier protein in Escherichia coli. J Membr Biol. 1983;76(2):95–112. doi: 10.1007/BF02000610. [DOI] [PubMed] [Google Scholar]
- Kaczorowski G. J., Robertson D. E., Kaback H. R. Mechanism of lactose translocation in membrane vesicles from Escherichia coli. 2. Effect of imposed delata psi, delta pH, and Delta mu H+. Biochemistry. 1979 Aug 21;18(17):3697–3704. doi: 10.1021/bi00584a010. [DOI] [PubMed] [Google Scholar]
- Kaczorowski G., Kohn L. D., Kaback H. R. Purification and properties of D-lactate dehydrogenase from Escherichia coli ML 308-225. Methods Enzymol. 1978;53:519–527. doi: 10.1016/s0076-6879(78)53054-1. [DOI] [PubMed] [Google Scholar]
- Kempner E. S., Haigler H. T. The influence of low temperature on the radiation sensitivity of enzymes. J Biol Chem. 1982 Nov 25;257(22):13297–13299. [PubMed] [Google Scholar]
- Kempner E. S., Miller J. H. Radiation inactivation of glutamate dehydrogenase hexamer: lack of energy transfer between subunits. Science. 1983 Nov 11;222(4624):586–589. doi: 10.1126/science.6635656. [DOI] [PubMed] [Google Scholar]
- Kempner E. S., Schlegel W. Size determination of enzymes by radiation inactivation. Anal Biochem. 1979 Jan 1;92(1):2–10. doi: 10.1016/0003-2697(79)90617-1. [DOI] [PubMed] [Google Scholar]
- LeBlanc G., Rimon G., Kaback H. R. Glucose 6-phosphate transport in membrane vesicles isolated from Escherichia coli: effect of imposed electrical potential and pH gradient. Biochemistry. 1980 May 27;19(11):2522–2528. doi: 10.1021/bi00552a034. [DOI] [PubMed] [Google Scholar]
- McIntyre J. O., Churchill P., Maurer A., Berenski C. J., Jung C. Y., Fleischer S. Target size of D-beta-hydroxybutyrate dehydrogenase. Functional and structural molecular weight based on radiation inactivation. J Biol Chem. 1983 Jan 25;258(2):953–959. [PubMed] [Google Scholar]
- Mieschendahl M., Büchel D., Bocklage H., Müller-Hill B. Mutations in the lacY gene of Escherichia coli define functional organization of lactose permease. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7652–7656. doi: 10.1073/pnas.78.12.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. J., Foster D. L., Wilson T. H., Kaback H. R. Purification and reconstitution of functional lactose carrier from Escherichia coli. J Biol Chem. 1981 Nov 25;256(22):11804–11808. [PubMed] [Google Scholar]
- Newman M. J., Wilson T. H. Solubilization and reconstitution of the lactose transport system from Escherichia coli. J Biol Chem. 1980 Nov 25;255(22):10583–10586. [PubMed] [Google Scholar]
- Padan E., Patel L., Kaback H. R. Effect of diethylpyrocarbonate on lactose/proton symport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6221–6225. doi: 10.1073/pnas.76.12.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. E., Kaczorowski G. J., Garcia M. L., Kaback H. R. Active transport in membrane vesicles from Escherichia coli: the electrochemical proton gradient alters the distribution of the lac carrier between two different kinetic states. Biochemistry. 1980 Dec 9;19(25):5692–5702. doi: 10.1021/bi00566a005. [DOI] [PubMed] [Google Scholar]
- Saccomani G., Sachs G., Cuppoletti J., Jung C. Y. Target molecular weight of the gastric (H+ + K+)-ATPase functional and structural molecular size. J Biol Chem. 1981 Aug 10;256(15):7727–7729. [PubMed] [Google Scholar]
- Schlegel W., Kempner E. S., Rodbell M. Activation of adenylate cyclase in hepatic membranes involves interactions of the catalytic unit with multimeric complexes of regulatory proteins. J Biol Chem. 1979 Jun 25;254(12):5168–5176. [PubMed] [Google Scholar]
- Schuldiner S., Spencer R. D., Weber G., Weil R., Kaback H. R. Lifetime and rotational relaxation time of dansylgalactoside bound to the lac carrier protein. J Biol Chem. 1975 Dec 10;250(23):8893–8896. [PubMed] [Google Scholar]
- Short S. A., Kaback H. R., Kohn L. D. Localization of D-lactate dehydrogenase in native and reconstituted Escherichia coli membrane vesicles. J Biol Chem. 1975 Jun 10;250(11):4291–4296. [PubMed] [Google Scholar]
- Teather R. M., Bramhall J., Riede I., Wright J. K., Fürst M., Aichele G., Wilhelm U., Overath P. Lactose carrier protein of Escherichia coli. Structure and expression of plasmids carrying the Y gene of the lac operon. Eur J Biochem. 1980;108(1):223–231. doi: 10.1111/j.1432-1033.1980.tb04715.x. [DOI] [PubMed] [Google Scholar]
- Viitanen P., Garcia M. L., Foster D. L., Kaczorowski G. J., Kaback H. R. Mechanism of lactose translocation in proteoliposomes reconstituted with lac carrier protein purified from Escherichia coli. 2. Deuterium solvent isotope effects. Biochemistry. 1983 May 10;22(10):2531–2536. doi: 10.1021/bi00279a034. [DOI] [PubMed] [Google Scholar]


