Abstract
Development of a detector case for complete co-registration of images in a non-fiber-based combined near-infrared spectral tomography and digital breast tomosynthesis, required analysis to find materials that could support a breast under full mammographic compression without affecting the x-ray images or the quality of the near infrared measurements. Several possible solutions were considered, and many types of plastics were tested in the development of the detector case. Light channeling within the detector case changed the data obtained in resin and agarose phantoms, lowering recovered absorption values. Additional developments focusing on blocking stray light were successful and permitted a normal subject imaging exam.
OCIS codes: (120.3890) Medical optics instrumentation, (170.0110) Imaging systems, (170.3830) Mammography
1. Introduction
Diffuse optical spectroscopy has shown substantial promise in distinguishing benign from malignant lesions in the breast based on estimation of tissue metabolic properties. Several stand-alone near-infrared (NIR) imagers have been developed for the breast in both academic and commercial settings [1–6]. Unfortunately, these systems offer low spatial resolution because of the high propensity for light scattering in tissue in the NIR. More recent approaches have overcome this problem by incorporating prior information from a second imaging modality such as x-ray tomosynthesis [7,8], MRI [9,10], or ultrasound [11]. Studies have indicated that including spatial priors on tissue structure improves NIR imaging resolution [12,13].
Although benefits are derived from combining multiple modalities for high-resolution functional and anatomical imaging, realizing the requisite system integration can be complex. For example, when performing NIR spectral tomography (NIRST) with MR guidance, physical space is limited inside the scanner bore and magnetic materials cannot be used [14]. Development of a combined NIRST and digital breast tomosynthesis (DBT) system at Dartmouth is also currently underway.
Challenges are posed when combining NIRST and DBT into a non-fiber-based fully-integrated system. Constraints include space and examination time limitations due to breast compression during the exam. In addition, the optical detector panel must be positioned under the breast during the NIRST exam but removed during the DBT acquisition. Additionally, a layer of material is needed to support the breast while under compression without excessive deformation or alteration of the x-ray or NIRST image quality. Management of stray light is another issue of particular importance because of the lack of fiber coupling of the light to the detectors, the larger distance between the light source and the tissue, and the attempt to obtain data from longer (10 cm) source detection distances.
In this paper, we describe the development and initial evaluation of a detector array cassette for achieving co-registration of the NIRST and DBT acquisitions. The design is particularly important because of the lack of fiber-coupling; yet, the need for mechanical support that does not degrade the NIRST and DBT images.
2. Materials and methods
2.1. NIRST/DBT system
Materials testing, phantom experiments, and normal subject imaging were performed on a combined NIRST/DBT system. The DBT component is a Genesis unit from Hologic, Inc. The NIRST instrumentation was developed at Dartmouth and is fully integrated with DBT. It includes eight wavelengths of light from 660 to 940 nm that are sequentially raster-scanned across the phantom or breast tissue from above at 2 cm spacing with a 1 cm spot size. The light is detected beneath the breast (caudal side) via a removable panel of 1 cm2 active-area silicon photodiodes comprised of 75 individual detectors. A more complete description of the system hardware can be found in a prior publication [15].
2.2. Detector case materials
To develop the detector case, seven (7) different plastics were studied along with a silica fiber plate and an NIR light-blocking material with openings over the detector locations. Comparisons of NIR light attenuation were performed with a resin phantom from INO [16]. The phantom was placed directly on top of the detector panel during data acquisition with each plastic material positioned between the phantom and the detector panel. Detector amplitudes from the five light source locations closest to the middle of the phantom and their nearest detector were averaged across all wavelengths and compared for each plastic against the case without the material in place. For polycarbonate, several samples with different surface properties were examined. In addition, different materials were imaged with the DBT system to assess x-ray absorption. These studies were performed at 26kVp and 35mAs. Average pixel intensities from a region of interest for each material were compared to the background to calculate the linear attenuation coefficient.
2.3. Phantom experiments
Phantoms were constructed from water, type 1 Agarose, whole porcine blood, and Intralipid, and mimicked the typical size and optical properties of the breast [17–19]. They were 6 cm in height with background values of 15 μM blood concentration and 1% Intralipid. Three (3) cm diameter, 2 cm high liquid inclusions with varying contrast levels (1:1, 2:1 and 3:1) of hemoglobin and Intralipid (2% in the first set, 1% for the second experiment) were embedded in the phantom. The first set of measurements was collected by placing the phantom on a thin piece of diffusive plastic, which was then placed on the detector panel. The second set of data was acquired with the phantom on top of the detector panel cover with the exterior surrounded by (optical) blackout material. The first experiment utilized all eight wavelengths, but 660nm was not functioning properly, and thus, was not included in the second case. More details on phantom creation can be found in an earlier work [20].
2.4. Normal subject imaging
NIRST imaging under mild compression was performed on a 25-year-old healthy volunteer using the integrated NIRST/DBT system. Scattering estimates were obtained from the DOSI imaging system from University of California at Irvine, currently housed at Dartmouth as part of a multicenter clinical trial [21]. The handheld probe was placed on the breast to obtain scattering values across an 8 x 8cm grid at 1cm spacing. Estimates near the areola were eliminated and the rest of the results were averaged and used in the reconstruction of the NIRST data. The NIRST reconstruction employed a finite element mesh consisting of 49,397 nodes and 280,187 elements. The breast measured 11 cm diameter at the chest wall and extended outwards by 5.5 cm.
3. Results
3.1. Detector case materials testing
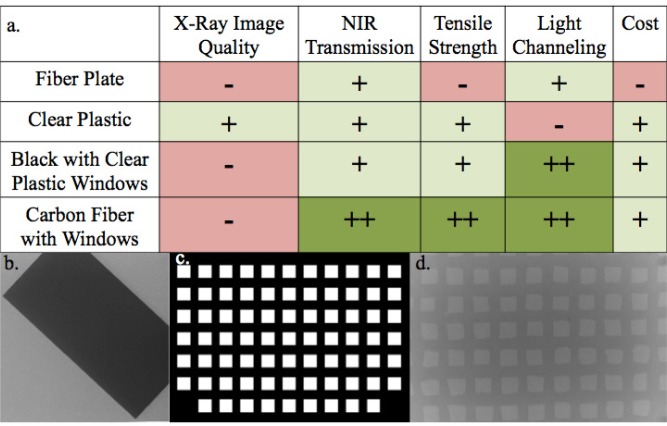
Figure 1 evaluates several potential detector case design options that would enable both DBT and NIRST imaging under the constraints imposed by this dual-modality system. Initial feasibility studies with DBT indicated a high level of x-ray attenuation with the silica fiber plates, and visible artifacts were generated by the light-blocking model with openings over the individual detector locations as shown in Fig. 1(b-d). Thus, a detector case constructed from fiber plates, a single material with openings, or a composite of two different types of plastics would all affect the DBT image.
Fig. 1.
(a) Attributes of possible detector case designs for the detector panel. (b) Single x-ray frame from a DBT scan showing attenuation from a thin fiber plate. (c) Depiction of a detector case model with windows over the locations of the detectors. (d) X-ray frame from DBT scan indicating attenuation of a panel with windows.
In seeking the optimal detector case material, several options were studied further. The most important material qualities include NIR and x-ray attenuation, tensile strength, and flex modulus. The tensile strength and flex modulus of the various materials were found in the literature [22], while the NIR and x-ray attenuation coefficients were experimentally derived (a picture of the materials is shown in Fig. 2 , and Table 1 exhibits comparisons). Polycarbonate plastic exhibited the highest level of NIR light penetration, second lowest x-ray attenuation, and sufficiently high tensile strength to withstand full breast compression during a DBT examination.
Fig. 2.
Photograph of the plastic materials tested in this study. Top row (from left to right) is polycarbonate, polypropylene, acrylic, high-density polyethylene, and polycarbonate with a diffusive sticker. Bottom row (from left to right) is acetal, acetal resin, polytetrafluoroethylene and sandblasted polycarbonate.
Table 1. Material qualities of plastics tested for detector case [22].
| Tensile Strength (MPa) | Flexural Modulus (GPa) | NIR Attenuation Coefficient (cm−1) | X-Ray Attenuation Coefficient (cm−1) | |
|---|---|---|---|---|
| Polycarbonate | 62 | 2.4 | 0.66 | 0.35 |
| Polypropylene | 35 | 1.4 | 0.91 | 0.29 |
| Acrylic | 60 | 3.0 | 1.08 | 0.68 |
| High Density Polyethylene | 35 | 0.3 | 1.56 | 0.32 |
| Acetal | 61 | 2.5 | 2.18 | 0.89 |
| Acetal Resin | 69 | 2.8 | 2.00 | 1.05 |
| Polytetrafluoroethylene | 25 | 0.6 | 3.31 | 2.26 |
3.2. Phantom experiments
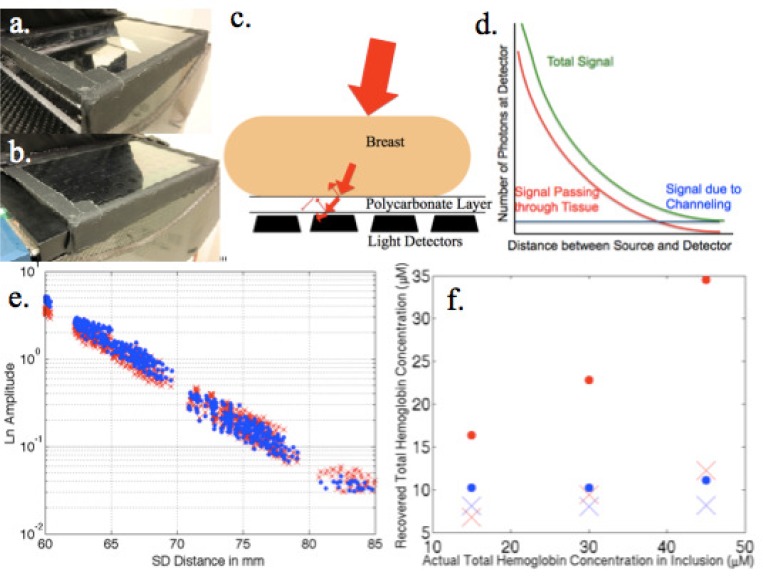
Polycarbonate appeared to be an ideal material; hence, a prototype detector case was created from clear 1/8” polycarbonate integrated into the carbon fiber imaging platform as shown in Fig. 3(a, b) . Unfortunately, significant artifacts were present due to light channeling within the plastic (see homogeneous phantom data represented by blue dots in Fig. 4(f) ). Alternatives involving physical alteration of the plastic surface through sandblasting or addition of a thin superficial diffusive material (sticker) did not eliminate the channeling effects. Placement of materials to block stray light entering from outside the phantom (or tissue) was needed.
Fig. 3.
(a) Photograph of the detector case developed for the combined NIRST/DBT system. (b) Same as (a) with the detector panel inserted. (c) Depiction of light channeling in the detector case. (d) Graph illustrating the effects of light channeling on the acquired NIRST data. (e) Raw 660 nm data acquired from a homogeneous resin phantom when placed directly on top of the detectors (in blue) relative to when imaged with the detector enclosed in the case (red). (f) Agar inclusion phantoms with increasing hemoglobin contrast (background in blue, inclusion in red) when imaged on a thin plastic film above the detectors (dots) and when imaged on the detector case (×).
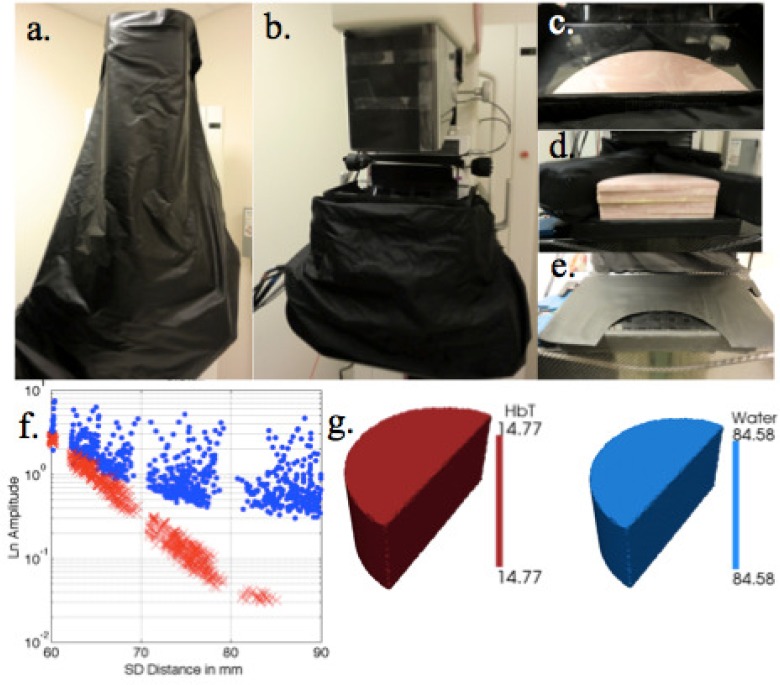
Fig. 4.
Light blocking materials used in patient exams. (a) Light blocking fabric draped over the DBT unit. (b) Light blocking skirt surrounding the breast compression paddle. (c) Compressible light blocking foam blocks surrounding a breast phantom. (d) Foam blocks without the compression paddle on top. (e) Light blocking paper on top of the detector case. (f) Raw data acquired from a homogeneous phantom imaged through the detector case with (in red) and without (in blue) the light blocking precautions. (g) Reconstructed total hemoglobin (μM) and water (%) from the first patient imaged using the NIRST imaging components of the NIRST/DBT system.
The data obtained from a homogeneous resin phantom in Fig. 3(e) has a slight slope difference between the measurements acquired with and without the polycarbonate detector panel in place. Reconstructed absorption coefficients at a wavelength with known scattering from these data resulted in a decrease in absorption of nearly 27% – dropping the value to 0.0059 mm−1 from 0.0081 mm−1 (which are 59% and 81% of the actual value, respectively) – when data was recorded through the detector case. Similar results were found in the more complicated inclusion phantom series shown in Fig. 3(f). Reconstructed background and inclusion hemoglobin levels were much lower when the detector case was in place. The average total hemoglobin recovery for the background region was 70% of the expected value without the detector case, but only 54% with it in place – a 23% decrease due to the detector case. Contrast in the inclusion regions showed linearity in both instances with R2 values of 0.9995 and 0.9727, respectively. Again, an average of 87% of the actual value was observed in the inclusion total hemoglobin without the detector (recovery was only 37% with the case).
3.3. Patient study
Initial patient results are limited but promising. Eliminating stray light was especially important, yet challenging, in this situation, and the methods employed during the exam are shown in Figs. 4(a)–(4e). Each included two layers of blackout material, compressible foam blocks situated next to the breast, and blackout paper covering the unused detectors. Quality data was obtained from six of eight wavelengths from the patient exam summarized in Fig. 4. One laser was not functioning properly and the other (940 nm) did not receive enough signal because the breast was not fully compressed. Total hemoglobin and water were estimated to be 14.5 μM and 85%, respectively, based on the data acquired during this imaging session.
4. Discussion
4.1. Detector case development
Multiple factors can influence image quality, and ultimately, sensitivity and specificity in a fully integrated multi-modality imaging system. For the detector case presented here, the material selected (plastic) needed to transmit both x-rays and NIR light. It also had to withstand the compressive forces applied during a DBT exam without excessive bending of the structure. Several options were considered including a fiber plate similar to one used in MR-guided NIR [23]. Others included a carbon fiber frame with openings over the detectors, and an absorbing polycarbonate frame with clear polycarbonate windows. All of these cases produced artifacts in the DBT images, which prevented their implementation. For a combined NIRST/DBT platform to gain clinical acceptance, the DBT portion of the exam needs to be the same quality as stand-alone systems at the same dose. The plastic cover was the material solution that did not extensively affect DBT images.
Optimizing the plastic cover to obtain the highest fidelity NIRST data involved the testing of several options. The plastics needed to possess the tensile strength to avoid breaking during a full compression DBT exam; this guided the selections of thickness of the materials tested [22,24,25]. Consideration of the flex modulus was also critical because significant differential deformation during the exam would vary the air gap between the detectors and the detector cover at different locations. Materials with higher flex moduli were more favorable in this regard. NIR light attenuation was evaluated and found to be highly variable when different materials were used. X-ray attenuation was lower overall than NIR attenuation, but also varied widely amongst the plastics examined. The material with the optimal combination of these properties was polycarbonate. It had the lowest NIR attenuation and second lowest X-ray attenuation as well as high flex modulus and tensile strength. In addition, its relative ease in machining made it an excellent choice for developing the prototype detector cover. Light channeling was an issue that significantly affected the performance of the polycarbonate material. Efforts to mitigate the light channeling effect by using a sandblasted polycarbonate sheet or a diffusive sticker applied to both sides were not successful.
4.2. Phantom imaging
Resin and agar phantom experiments using the detector case provided useful data. These results consistently underestimated absorption values, likely due to light channeling within the detector cover. Differences in indices of refraction at the polycarbonate-tissue boundary cause approximately 9% of the light to be reflected back into the tissue [26,27]. Similar reflections can also occur at the polycarbonate-air boundary leading to light channeling during which some photons travel far from where they exited the tissue as illustrated in Fig. 3(c). This effect alters the signal recorded at the detectors, most significantly at farther source-detector distances where the light signal has been most attenuated as indicated in Fig. 3(d), which changes the slope of the data, and hence, the absorption coefficient estimate. While the light channeling is similar for all source-detector positions, it most strongly influences data at far source-detector distances because the number of photons traveling through the tissue and reaching these locations is relatively low. The effect decreases the magnitude of the overall slope of the linear relationship between logarithm of the detected signal and source-detector distance, as a larger (than expected) signal is measured at far source-detector distances leading to lower (than expected) absorption coefficient estimates in both the single wavelength resin phantom as well as the more complicated agar inclusion phantoms with variable contrast, as seen in Figs. 3(e) and 3(f).
Specifically, the addition of the detector case led to a drop of 27% in the absorption coefficient estimate for a single wavelength in the resin phantom, and a 23% drop in total hemoglobin recovered in the background of the inclusion phantom, indicating that the accuracy of absorption quantification is significantly reduced when the case was present. Because most imaging systems based on diffuse light are unable to recover total hemoglobin values fully [28], contrast between regions is often examined as a measure of image quality. Efforts are also focused on improving calibration methods with homogenous phantom data in order to increase the overall accuracy of absorption property recovery [29].
4.3. Normal subject study
Adaptation of the new detector case facilitated the first healthy volunteer exam involving NIRST delivered on the DBT platform. After the initial challenge of finding an acceptable material for the detector case that did not compromise DBT image quality, the most important task was to block any stray light in the imaging field. Light hitting the detector cover directly without traveling through the tissue is many orders of magnitude stronger than light attenuated by the tissue; hence, the blocking of this light was of utmost importance and critical to obtaining accurate measurements in the breast. Direct light can reflect internally within the plastic cover before being transmitted to a detector far from the light source and producing a falsely high signal, thereby increasing the amount of channeled light impinging on the detectors.
Unique challenges need to be overcome to eliminate stray light in a non-fiber based NIRST imaging system. In the mirror-scanning source, most of the light reflects off the top compression paddle and disperses throughout the imaging cavity. As the DBT platform was not originally designed for optical imaging, many metallic and reflective surfaces exist that needed to be covered with blackout fabric and tape to reduce stray light propagation. These efforts are necessary because estimation of tissue optical properties relies on measurements at long source-detector distances, where channeling plays a large role. Scattering coefficient estimates applied in the image reconstruction of the NIR breast data were obtained from reflection measurements, and were 1.51 mm−1 for scattering amplitude and 0.72 mm−1 for scattering power. Bulk recoveries of total hemoglobin and water from the breast were within the normal range at 14.5 μM and 85%, respectively [1,6,10,21,30]. Further characterization of the breast was not possible for this subject as the DBT portion of the examination was not performed but will be included in a future series of subject studies. More efficient and universal solutions to eliminate stray light during breast exams are also being pursued.
5. Conclusions
NIRST/DBT imaging was optimized for initial patient exams while also overcoming several unique challenges regarding full integration of the two modalities and eliminating stray light contamination of the detected optical signals. Development of a detector case and effective light blocking materials enabled initial and successful phantom and human imaging studies. The goal of the NIRST/DBT approach is to improve sensitivity and specificity of breast cancer screening. To achieve this goal, realization of a clinically robust implementation is essential. Thus, the length of the exam, its cost, and the ease of system use and image interpretation as well as the ability to examine a wide range of breast sizes, shapes and optical properties must be carefully considered. The efforts presented here show first steps in optimizing an integrated, non-fiber based NIRST/DBT breast imaging platform.
Acknowledgments
The authors would like to acknowledge Adele Shenoy and Yesha Maniar for materials testing and construction of agarose phantoms. This work was funded by National Institutes of Health (NIH) Grant No. R01CA139449 with support and equipment provided by Hologic Inc.
References and links
- 1.Cerussi A. E., Jakubowski D., Shah N., Bevilacqua F., Lanning R., Berger A. J., Hsiang D., Butler J., Holcombe R. F., Tromberg B. J., “Spectroscopy enhances the information content of optical mammography,” J. Biomed. Opt. 7(1), 60–71 (2002). 10.1117/1.1427050 [DOI] [PubMed] [Google Scholar]
- 2.Kukreti S., Cerussi A. E., Tanamai W., Hsiang D., Tromberg B. J., Gratton E., “Characterization of metabolic differences between benign and malignant tumors: high-spectral-resolution diffuse optical spectroscopy,” Radiology 254(1), 277–284 (2010). 10.1148/radiol.09082134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poellinger A., Martin J. C., Ponder S. L., Freund T., Hamm B., Bick U., Diekmann F., “Near-infrared laser computed tomography of the breast first clinical experience,” Acad. Radiol. 15(12), 1545–1553 (2008). 10.1016/j.acra.2008.07.023 [DOI] [PubMed] [Google Scholar]
- 4.Chance B., Nioka S., Zhang J., Conant E. F., Hwang E., Briest S., Orel S. G., Schnall M. D., Czerniecki B. J., “Breast cancer detection based on incremental biochemical and physiological properties of breast cancers: a six-year, two-site study,” Acad. Radiol. 12(8), 925–933 (2005). 10.1016/j.acra.2005.04.016 [DOI] [PubMed] [Google Scholar]
- 5.Gu X., Zhang Q., Bartlett M., Schutz L., Fajardo L. L., Jiang H., “Differentiation of cysts from solid tumors in the breast with diffuse optical tomography,” Acad. Radiol. 11(1), 53–60 (2004). 10.1016/S1076-6332(03)00562-2 [DOI] [PubMed] [Google Scholar]
- 6.Brooksby B., Pogue B. W., Jiang S., Dehghani H., Srinivasan S., Kogel C., Tosteson T. D., Weaver J., Poplack S. P., Paulsen K. D., “Imaging breast adipose and fibroglandular tissue molecular signatures by using hybrid MRI-guided near-infrared spectral tomography,” Proc. Natl. Acad. Sci. U.S.A. 103(23), 8828–8833 (2006). 10.1073/pnas.0509636103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang Q., Selb J., Carp S. A., Boverman G., Miller E. L., Brooks D. H., Moore R. H., Kopans D. B., Boas D. A., “Combined optical and X-ray tomosynthesis breast imaging,” Radiology 258(1), 89–97 (2011). 10.1148/radiol.10082176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q., Brukilacchio T. J., Li A., Stott J. J., Chaves T., Hillman E., Wu T., Chorlton M., Rafferty E., Moore R. H., Kopans D. B., Boas D. A., “Coregistered tomographic x-ray and optical breast imaging: initial results,” J. Biomed. Opt. 10(2), 024033 (2005). 10.1117/1.1899183 [DOI] [PubMed] [Google Scholar]
- 9.Ntziachristos V., Yodh A. G., Schnall M. D., Chance B., “MRI-guided diffuse optical spectroscopy of malignant and benign breast lesions,” Neoplasia 4(4), 347–354 (2002). 10.1038/sj.neo.7900244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpenter C. M., Pogue B. W., Jiang S., Dehghani H., Wang X., Paulsen K. D., Wells W. A., Forero J., Kogel C., Weaver J. B., Poplack S. P., Kaufman P. A., “Image-guided optical spectroscopy provides molecular-specific information in vivo: MRI-guided spectroscopy of breast cancer hemoglobin, water, and scatterer size,” Opt. Lett. 32(8), 933–935 (2007). 10.1364/OL.32.000933 [DOI] [PubMed] [Google Scholar]
- 11.Zhu Q., Cronin E. B., Currier A. A., Vine H. S., Huang M., Chen N., Xu C., “Benign versus malignant breast masses: optical differentiation with US-guided optical imaging reconstruction,” Radiology 237(1), 57–66 (2005). 10.1148/radiol.2371041236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pogue B. W., Davis S. C., Leblond F., Mastanduno M. A., Dehghani H., Paulsen K. D., “Implicit and explicit prior information in near-infrared spectral imaging: accuracy, quantification and diagnostic value,” Philos. Transact. A Math. Phys. Eng. Sci. 369(1955), 4531–4557 (2011). 10.1098/rsta.2011.0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooksby B., Srinivasan S., Jiang S., Dehghani H., Pogue B. W., Paulsen K. D., Weaver J., Kogel C., Poplack S. P., “Spectral priors improve near-infrared diffuse tomography more than spatial priors,” Opt. Lett. 30(15), 1968–1970 (2005). 10.1364/OL.30.001968 [DOI] [PubMed] [Google Scholar]
- 14.Brooksby B., Jiang S., Dehghani H., Pogue B. W., Paulsen K. D., Kogel C., Doyley M., Weaver J. B., Poplack S. P., “Magnetic resonance-guided near-infrared tomography of the breast,” Rev. Sci. Instrum. 75(12), 5262–5270 (2004). 10.1063/1.1819634 [DOI] [Google Scholar]
- 15.Krishnaswamy V., Michaelsen K., Pogue B. W., Paulsen K. D., “A digital x-ray tomosynthesis coupled near infrared spectral tomography system for dual-modality breast imaging,” Opt. Express 20, 19125–19136 (2012). 10.1364/OE.20.019125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouchard J. P., Veilleux I., Jedidi R., Noiseux I., Fortin M., Mermut O., “Reference optical phantoms for diffuse optical spectroscopy. Part 1—Error analysis of a time resolved transmittance characterization method,” Opt. Express 18(11), 11495–11507 (2010). 10.1364/OE.18.011495 [DOI] [PubMed] [Google Scholar]
- 17.Robinson M., Kotre C. J., “Trends in compressed breast thickness and radiation dose in breast screening mammography,” Br. J. Radiol. 81(963), 214–218 (2008). 10.1259/bjr/90916004 [DOI] [PubMed] [Google Scholar]
- 18.Michels R., Foschum F., Kienle A., “Optical properties of fat emulsions,” Opt. Express 16(8), 5907–5925 (2008). 10.1364/OE.16.005907 [DOI] [PubMed] [Google Scholar]
- 19.Wang J., Jiang S., Li Z., diFlorio-Alexander R. M., Barth R. J., Kaufman P. A., Pogue B. W., Paulsen K. D., “In vivo quantitative imaging of normal and cancerous breast tissue using broadband diffuse optical tomography,” Med. Phys. 37(7), 3715–3724 (2010). 10.1118/1.3455702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michaelsen K., Krishnaswamy V., Pogue B. W., Poplack S. P., Paulsen K. D., “Near-infrared spectral tomography integrated with digital breast tomosynthesis: effects of tissue scattering on optical data acquisition design,” Med. Phys. 39(7), 4579–4587 (2012). 10.1118/1.4728228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerussi A., Shah N., Hsiang D., Durkin A., Butler J., Tromberg B. J., “In vivo absorption, scattering, and physiologic properties of 58 malignant breast tumors determined by broadband diffuse optical spectroscopy,” J. Biomed. Opt. 11(4), 044005 (2006). 10.1117/1.2337546 [DOI] [PubMed] [Google Scholar]
- 22.J. E. Mark, Polymer Data Handbook (Oxford University Press, Oxford, 2009). [Google Scholar]
- 23.Mastanduno M. A., Jiang S., DiFlorio-Alexander R., Pogue B. W., Paulsen K. D., “Remote positioning optical breast magnetic resonance coil for slice-selection during image-guided near-infrared spectroscopy of breast cancer,” J. Biomed. Opt. 16(6), 066001 (2011). 10.1117/1.3587631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendrick R. E., Pisano E. D., Averbukh A., Moran C., Berns E. A., Yaffe M. J., Herman B., Acharyya S., Gatsonis C., “Comparison of acquisition parameters and breast dose in digital mammography and screen-film mammography in the American College of Radiology Imaging Network digital mammographic imaging screening trial,” AJR Am. J. Roentgenol. 194(2), 362–369 (2010). 10.2214/AJR.08.2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kayar R., Civelek S., Cobanoglu M., Gungor O., Catal H., Emiroglu M., “Five methods of breast volume measurement: a comparative study of measurements of specimen volume in 30 mastectomy cases,” Breast Cancer (Auckl) 5, 43–52 (2011). 10.4137/BCBCR.S6128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolin F. P., Preuss L. E., Taylor R. C., Ference R. J., “Refractive index of some mammalian tissues using a fiber optic cladding method,” Appl. Opt. 28(12), 2297–2303 (1989). 10.1364/AO.28.002297 [DOI] [PubMed] [Google Scholar]
- 27.Kasarova S. N., Sultanova N. G., Ivanov C. D., Nikolov I. D., “Analysis of the dispersion of optical plastic materials,” Opt. Mater. 29(11), 1481–1490 (2007). 10.1016/j.optmat.2006.07.010 [DOI] [Google Scholar]
- 28.Pogue B. W., Davis S. C., Song X., Brooksby B. A., Dehghani H., Paulsen K. D., “Image analysis methods for diffuse optical tomography,” J. Biomed. Opt. 11(3), 033001 (2006). 10.1117/1.2209908 [DOI] [PubMed] [Google Scholar]
- 29.Poplack S. P., Tosteson T. D., Wells W. A., Pogue B. W., Meaney P. M., Hartov A., Kogel C. A., Soho S. K., Gibson J. J., Paulsen K. D., “Electromagnetic breast imaging: results of a pilot study in women with abnormal mammograms,” Radiology 243(2), 350–359 (2007). 10.1148/radiol.2432060286 [DOI] [PubMed] [Google Scholar]
- 30.Intes X., “Time-domain optical mammography SoftScan: initial results,” Acad. Radiol. 12(8), 934–947 (2005). 10.1016/j.acra.2005.05.006 [DOI] [PubMed] [Google Scholar]