Abstract
A new series of pyrazolo[3,4-d]pyrimidines has been synthesized. The new compounds were tested for their antitumor activity on 60 different cell lines, and some of the compounds were found to have potent antitumor activity. In particular, 2-hydroxybenzaldehyde [1-(4-chlorophenyl)-3-methyl-1H-pyrazolo-[3,4-d]pyrimidin-4-yl]hydrazone (VIIa) was found to be the most effective among the other derivatives, showing IC50 values of 0.326 to 4.31 μM on 57 different cell lines.
Keywords: Pyrazolopyrimidines, Antitumor Activity, Cytotoxic Activity, Synthesis
Introduction
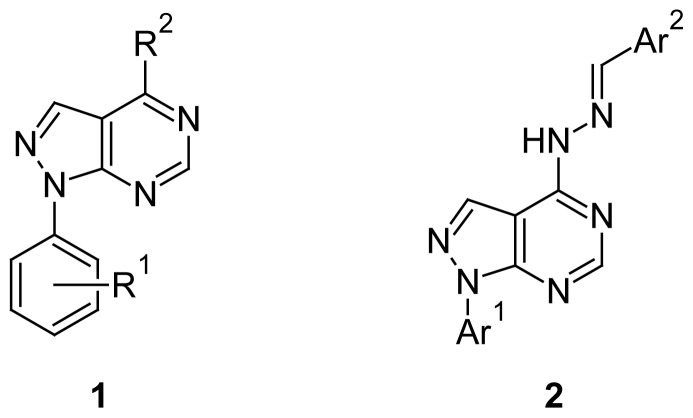
Increasing interest in biological studies of pyrazolo[3,4-d]pyrimidines in the last decade is a consequence of their wide usage as a pharmaceutically important class of compounds [1]. Pyrazolopyrimidine derivatives have considerable potential in the field of chemotherapy, as they were found to exhibit their antitumor activity by inhibiting different types of enzymes such as cyclin-dependent kinase [2–4], Src and Abl tyrosine kinase [5], glycogen synthase kinase-3 [6–8], adenosine deaminase [9], and epidermal growth factor receptor protein tyrosine kinase [10]. The derivatives of pyrazolo[3,4-d]pyrimidine have already been discovered as antitumor agents by the NCI (National Cancer Institute, USA) on HCT116 and other cell lines. The potency of these compounds is enhanced in anilide derivatives, and this translates into tumor growth inhibition in a mouse xenograft model [2]. Some pyrazolo[3,4-d]pyrimidines (1, Figure 1) structurally related with allopurinol, have also been reported as potent inhibitors of xanthine oxidase and the growth of several human tumor cell lines [11]. In addition, several substituted pyrazolo[3,4-d]pyrimidines (2) were reported as potent antitumor agents [12].
Fig. 1.
Structures of some reported antitumor pyrazolo[3,4-d]pyrimidines
Both the above findings and 4-substituted-1H-pyrazolo[3,4-d]-pyrimidines were reported to be cytotoxic and antitumor agents [1, 13–15]. In order to explore this possibility, compounds were prepared that had diverse groups at position 4 of the pyrazolopyrimidine core, and their antitumor activity was tested.
Results and Discussion
Chemistry
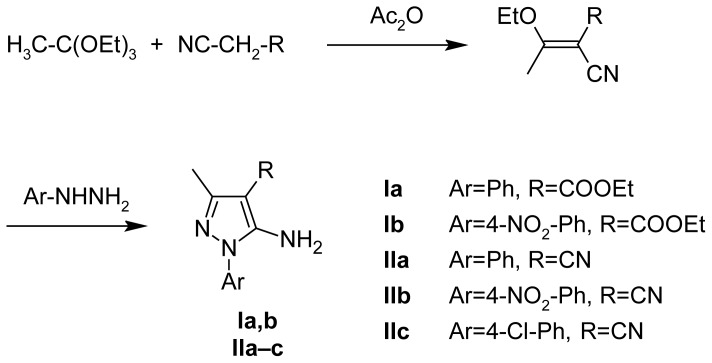
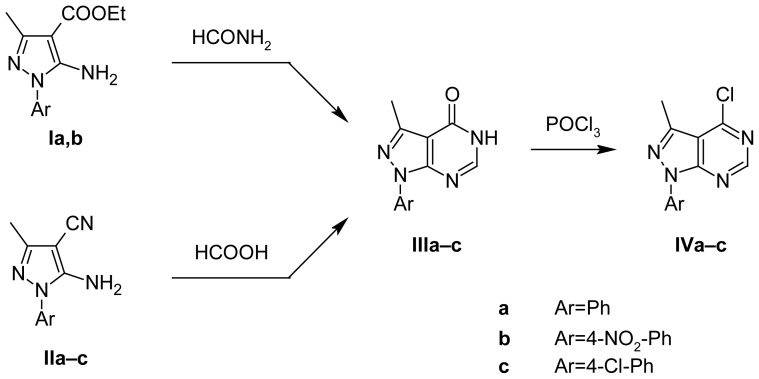
The synthesis of the designed compounds is outlined in schemes 1, 2, and 3. The main precursors for the synthesis of target derivatives, i.e. Ia,b and IIa–c, were prepared by a previously published synthetic method presented in Scheme 1. Compounds IIIa,b were prepared according to the synthetic methods presented in Scheme 2, either by heating compounds Ia,b in formamide or by heating compounds IIa,b in formic acid. Both procedures had high yields, but the second one had an even higher yield. Compound IIIc was prepared only by using one synthetic method from derivative IIc in formic acid, as it had the better yield. Structures of newly prepared compounds were confirmed by 1H NMR, IR, mass spectroscopy, and microanalyses.
Sch. 1.
Synthesis of pyrazole intermediates
Sch. 2.
Synthesis of pyrazolo[3,4-d]pyrimidines
Sch. 3.
Synthesis of new pyrazolo[3,4-d]pyrimidine derivatives
On the other hand, synthesis of IVa–c presented in scheme 2 was performed by the reflux of IIIa–c in phosphorus oxychloride. The structures of the new compounds were confirmed by 1H NMR, which revealed disappearance of the singlet D2O exchangeable signal corresponding to NH, and showed an increased deshielding of H at position 6, due to the inductive effect of the chlorine atom. It was also confirmed by mass spectra which gave fragments showing the isotopic pattern of the chlorine atom.
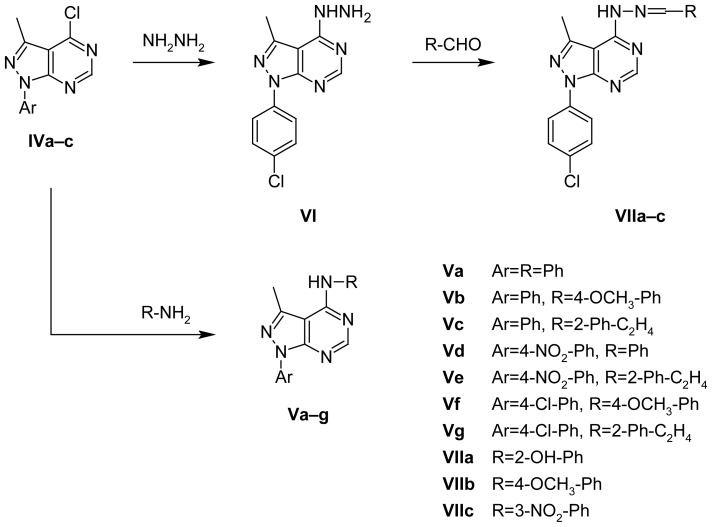
The synthesis of Va–g and VIIa–c was outlined in scheme 3. First, Va–g were obtained by the reflux of IVa–c with the appropriate amine using triethylamine as a catalyst, and the formed derivatives were confirmed by 1H NMR, which revealed appearance of the singlet D2O exchangeable signal corresponding to NH, and appearance of other signals characterizing the introduced groups. The structures of some of these derivatives were additionally confirmed by mass spectra. 13C NMR was performed on compounds Va, Vc, and VIIa. Va showed distinct aromatic carbons where the carbon at position 3 of the pyrazole ring appeared at 101.76, while that of the pyrimidine ring at position 6 was the highest desheilded and appeared at 156.35ppm. On the other hand, the aromatic ring at position 1 of the pyrazole ring gives aromatic carbons, which are given the numbers 1′,2′,3′,4′,5′, and 6′, and that attached to the amine at the pyrimidine ring gives aromatic carbons marked as 1″, 2″, 3″, 4″, 5″ and 6″. Vc showed two distinct doublet signals belonging to the two CH2 groups attached to the NH group. The presence of the ethylene spacer was further confirmed by DEPT technique.
Second, VIIa-c were synthesized by the reflux of IVc with hydrazine, producing VI which was confirmed by 1H NMR, IR spectrum, microanalysis, and mass spectrum in which appearance of a singlet proton at δ 8.36 ppm in 1H NMR indicated slight shielding of the proton at position 6 of the pyrimidine ring, due to the removal of the chlorine atom, and a broad range of the D2O exchangeable signal of NH and NH2 groups at 3.82 and 4.95 ppm, and by the mass spectrum which shows M+, M+ + 2 at m/z 274, 276 as 100.00%, 49.15%.
Furthermore, VI was condensed with appropriate aldehydes producing VIIa–c, the structure of which was confirmed by 1H NMR, 13C NMR, IR spectra, microanalyses, and mass spectra. The IR spectrum shows the disappearance of the IR peak equivalent to NH2. In 1H NMR, the appearance of the D2O exchangeable broad singlet indicated the presence of NH, deshielded singlet of N=CH, in addition to different peaks equivalent to different substituents in each derivative. The OH group of compound VIIa appeared in the IR at 3369 cm−1 as an exchangeable peak in 1H NMR at10.20ppm. The OCH3 group of compound VIIb was detected by 1H NMR appearing more deshielded at 3.82 ppm than the CH3 group at position 3 at 2.79 ppm. Compound VIIc mainly showed the NO2 group in the IR at 1437, 1346 and the aromatic protons of the benzene ring 2″, 4″, 5″, and 6″ with a deshielded singlet signal equivalent to 2″ at 8.7–8.8, proving the m-substitution.
Antitumor activity
The antitumor activity was determined for the newly synthesized compounds at the NCI for in vitro one-dose testing and detection of IC50 of their antitumor activity on 60 different cell lines. Compound Vc, Vg, VIIa, and VIIc were found to have the highest inhibitory activity on many cell lines. The obtained results of the tested derivatives showed a distinctive potential pattern of selectivity, as well as broad-spectrum antitumor activity (Table 1). Compound VIIa was subjected to 5-dose testing, as it showed the highest activity among other derivatives showing inhibition of 57 different cell lines (Table 2).
Tab. 1.
Inhibition percent of the tested compounds (10−5 Molar) on different 60 cell lines
| Cell line type | Tested compounds and inhibition percent of cell lines | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Va | Vb | Vc | Vd | Ve | Vf | Vg | VIIa | VIIb | VIIc | ||
| Leukemia | CCRF-CEM | 19.43 | 4.00 | 6.94 | 2.25 | – | 2.45 | 0.33 | 93.59 | – | 79.59 |
| HL-60(TB) | 29.20 | 11.40 | 22.22 | 8.87 | 35.22 | – | 29.98 | 73.60 | – | 69.05 | |
| K-562 | 30.71 | 24.64 | 65.57 | 5.24 | 23.27 | – | 76.32 | 81.45 | 19.22 | 82.62 | |
| MOLT-4 | 30.65 | 32.39 | 53.25 | 21.34 | 35.41 | 12.37 | 43.41 | 90.57 | 20.53 | 84.60 | |
| RPMI-8226 | 23.57 | 9.63 | 12.33 | 16.39 | 3.17 | 19.90 | 17.27 | 62.86 | 6.07 | 33.66 | |
| SR | 36.93 | 41.72 | 53.71 | 28.86 | 37.77 | 20.94 | 34.91 | 95.20 | 18.44 | 48.73 | |
|
| |||||||||||
| Non-Small Cell Lung Cancer | A549/ATCC | – | 4.05 | 6.18 | 10.26 | 0.26 | 3.61 | – | 90.54 | – | – |
| EKVX | – | 5.68 | 8.75 | – | 47.45 | 13.84 | 19.40 | 61.94 | – | 15.99 | |
| HOP-62 | – | 2.91 | 9.60 | – | – | 19.97 | 7.30 | 92.07 | – | – | |
| HOP-92 | 34.14 | 8.10 | 11.46 | 6.51 | 8.78 | 28.98 | 51.17 | 55.93 | 12.52 | 79.55 | |
| NCI-H226 | – | – | – | – | 2.27 | 10.33 | 3.81 | 47.25 | – | – | |
| NCI-H23 | – | – | – | – | 4.22 | 10.44 | 4.32 | 63.00 | – | 5.70 | |
| NCI-H322M | – | – | – | – | – | _ | – | 66.66 | – | 3.60 | |
| NCI-H460 | – | – | – | 10.24 | 8.52 | 4.20 | 3.49 | 94.96 | – | 22.17 | |
| NCI-H 522 | 8.39 | 9.21 | 17.72 | 19.25 | – | 28.80 | 4.48 | _ | – | – | |
|
| |||||||||||
| Colon Cancer | COLO 205 | 4.67 | – | – | – | – | 8.75 | 6.22 | 81.33 | – | 4.75 |
| HCC-2998 | – | – | – | – | – | – | _ | 68.95 | – | 5.17 | |
| HCT-116 | – | 0.89 | 6.87 | 33.53 | 7.57 | 8.26 | 5.93 | 73.26 | – | – | |
| HCT-15 | 3.74 | 9.57 | 7.60 | – | 4.73 | 6.88 | 6.10 | 68.08 | – | – | |
| HT29 | 12.25 | 18.87 | 5.24 | 10.25 | – | 14.96 | 4.20 | 79.92 | 0.97 | 9.36 | |
| KM12 | – | – | – | – | 4.92 | 5.48 | – | 71.56 | – | 33.45 | |
| SW-620 | 0.79 | – | – | – | 0.72 | 2.59 | – | 64.89 | – | 11.24 | |
|
| |||||||||||
| CNS Cancer | SF-268 | 5.96 | – | – | 3.91 | 6.41 | – | – | 90.33 | – | 41.99 |
| SF-295 | – | – | 2.42 | – | – | – | – | 75.51 | – | – | |
| SF-539 | 3.88 | 0.50 | – | 1.85 | 0.58 | 9.76 | – | 85.97 | – | – | |
| SNB-19 | – | – | – | 2.95 | 2.93 | 1.78 | – | 70.42 | – | 3.66 | |
| SNB-75 | 0.60 | 10.60 | – | – | 12,15 | 10.17 | – | 36.41 | 0.88 | 0.87 | |
| U251 | – | – | – | 0.50 | – | – | – | 86.92 | 0.73 | 17.78 | |
|
| |||||||||||
| Melanoma | LOX IMVI | – | 1.66 | 7.32 | 15.16 | – | 19.19 | – | 64.88 | 0.69 | 6.68 |
| MALME-3M | – | – | – | – | – | _ | – | 51.30 | – | 2.87 | |
| MDAMB435 | – | – | 6.98 | – | 5.22 | – | – | 72.73 | – | 2.94 | |
| SK-MEL-2 | – | – | – | – | – | 10.73 | 5.94 | 87.67 | 0.78 | 1.14 | |
| SK-MEL-28 | – | – | – | – | – | – | – | 74.82 | – | – | |
| SK-MEL-5 | – | – | 2.47 | – | – | 3.25 | – | 16.17 | – | – | |
| UACC-257 | – | – | – | – | – | – | – | 74.83 | – | – | |
| UACC-62 | – | 1.44 | 10.31 | 0.78 | 9.62 | 18.24 | 14.27 | 86.54 | – | – | |
|
| |||||||||||
| Ovarian Cancer | IGROV1 | – | – | 6.94 | – | 0.87 | _ | – | 76.08 | – | 5.25 |
| OVCAR-3 | – | – | – | – | – | – | – | 65.08 | – | 91.83 | |
| OVCAR-4 | – | 2.38 | 1.30 | – | – | 8.86 | – | 80.50 | – | 5.56 | |
| OVCAR-5 | 5.33 | 15.68 | 8.84 | – | – | 4.24 | 7.88 | 52.48 | – | – | |
| OVCAR-8 | – | 3.30 | 1.97 | 0.91 | 1.68 | 6.25 | – | 85.94 | – | 0.43 | |
| NCI/ADR-RES | – | 7.90 | 21.95 | – | – | – | 5.66 | 80.90 | – | – | |
| SK-OV-3 | – | – | – | – | – | 24.57 | 7.19 | 95.56 | – | – | |
|
| |||||||||||
| Renal Cancer | 786-0 | 3.00 | 8.93 | 11.46 | – | – | – | – | 91.01 | – | 3.71 |
| A498 | 18.12 | 12.14 | 7.44 | – | 18.37 | – | – | 21.46 | 8.64 | 14.62 | |
| ACHN | 0.89 | 6.27 | 5.86 | – | 0.28 | 13.55 | 7.13 | 83.54 | – | – | |
| CAKI-1 | – | – | 23.73 | – | 12.87 | — | 29.75 | 86.47 | 4.83 | 24.71 | |
| RXF 393 | – | – | – | – | – | 2.16 | 2.27 | 74.27 | – | – | |
| SN12C | – | – | – | 1.51 | – | 7.73 | – | 76.94 | – | – | |
| TK-10 | – | 5.94 | 5.32 | 70.00 | – | 16.23 | 5.88 | 83.60 | 2.97 | 6.83 | |
| UO-31 | – | 4.94 | 12.46 | 0.50 | 20.39 | — | 13.47 | 64.77 | 9.87 | 27.79 | |
|
| |||||||||||
| Prost. | PC-3 | 6.67 | 7.99 | 1.59 | 8.91 | 11.43 | 24.61 | 22.41 | 58.41 | 10.93 | 61.01 |
| Canc. | DU-145 | – | – | – | – | – | – | – | 80.91 | – | 31.32 |
|
| |||||||||||
| Breast Cancer | MCF7 | – | 4.97 | – | 6.68 | 6.98 | 15.10 | 1.80 | 91.72 | 4.60 | – |
| MDA-MB-231/ATCC | – | – | – | 4.83 | – | 24.17 | 14.77 | 67.43 | 2.75 | – | |
| HS578T | – | – | – | – | – | – | – | 19.00 | 0.98 | 0.81 | |
| BT-549 | – | – | – | – | – | – | – | 63.98 | – | – | |
| T-47D | – | – | 12.42 | 3.82 | 6.98 | 23.74 | 2.13 | 86.73 | – | – | |
| MDA-MB-468 | – | – | – | – | – | – | – | 47.74 | – | – | |
Tab. 2.
Values detected from 5 dilutions for compound (VIIa) on 60 cell lines
| Cell line type | GI50 (μM) | TGI (μM) | LC50 (μM) | |
|---|---|---|---|---|
| Leukemia | CCRF-CEM | 0.326 | — | >100 |
| HL-60(TB) | 0.522 | 48.6 | >100 | |
| K-562 | 1.37 | >100 | >100 | |
| MOLT-4 | 0.625 | >100 | >100 | |
| RPMI-8226 | 0.760 | >100 | >100 | |
| SR | 0.663 | 53.4 | >100 | |
|
| ||||
| Non-Small Cell Lung Cancer | A549/ATCC | 2.22 | 17.8 | 95.7 |
| EKVX | 1.50 | 52.0 | >100 | |
| HOP-62 | 0.559 | 24.0 | >100 | |
| HOP-92 | 0.383 | 30.9 | >100 | |
| NCI-H226 | 4.23 | 62.9 | >100 | |
| NCI-H23 | 2.97 | >100 | >100 | |
| NCI-H322M | 0.335 | 22.0 | >100 | |
| NCI-H460 | 0.398 | 3.61 | 45.0 | |
| NCI-H 522 | 1.79 | >100 | >100 | |
|
| ||||
| CNS Cancer | SF-268 | 0.646 | 23.0 | >100 |
| SF-295 | 1.67 | 20.0 | >100 | |
| SF-539 | 0.522 | 8.84 | 96.9 | |
| SNB-19 | 1.55 | 64.6 | >100 | |
| SNB-75 | 8.66 | 53.0 | >100 | |
| U251 | 0.595 | 16.9 | 95.1 | |
|
| ||||
| Melanoma | LOX IMVI | 0.389 | 11.9 | 57.1 |
| MALME-3M | 1.19 | 9.56 | >100 | |
| M14 | 0.726 | 2.68 | 8.83 | |
| MDAMB435 | 1.25 | >100 | >100 | |
| SK-MEL-2 | 2.97 | 19.0 | >100 | |
| SK-MEL-28 | 3.70 | >100 | >100 | |
| SK-MEL-5 | 1.06 | 2.55 | 6.11 | |
| UACC-257 | 3.49 | 24.7 | >100 | |
| UACC-62 | 1.75 | 7.78 | >100 | |
|
| ||||
| Ovarian Cancer | IGROV1 | 0.546 | 10.7 | >100 |
| OVCAR-3 | 0.427 | 23.5 | 97.1 | |
| OVCAR-4 | 0.578 | 73.4 | >100 | |
| OVCAR-5 | 3.66 | >100 | >100 | |
| OVCAR-8 | 0.477 | 36.1 | >100 | |
| NCI/ADR-RES | 0.419 | 22.6 | >100 | |
| SK-OV-3 | 0.601 | 37.4 | >100 | |
|
| ||||
| Renal Cancer | 786-0 | 1.58 | 15.8 | >100 |
| A498 | 17.0 | 68.3 | >100 | |
| ACHN | 0.350 | 10.8 | 92.9 | |
| CAKI-1 | 0.360 | 10.5 | >100 | |
| RXF 393 | 4.31 | 30.7 | >100 | |
| SN12C | 0.983 | 84.0 | >100 | |
| TK-10 | 2.60 | 46.9 | >100 | |
| UO-31 | 0.348 | 3.51 | >100 | |
|
| ||||
| Prostate | PC-3 | 1.36 | 92.3 | >100 |
| Cancer | DU-145 | 0.601 | 16.7 | >100 |
|
| ||||
| Breast Cancer | MCF7 | 1.56 | >100 | >100 |
| MDA-MB-231/ATCC | 0.719 | 42.7 | >100 | |
| HS578T | 7.34 | 77.6 | >100 | |
| BT-549 | 3.05 | 78.3 | >100 | |
| T-47D | 2.58 | >100 | >100 | |
| MDA-MB-468 | 2.15 | 7.81 | >100 | |
|
| ||||
| Colon Cancer | COLO 205 | 1.27 | >100 | >100 |
| HCC-2998 | 0.656 | >100 | >100 | |
| HCT-116 | 0.373 | 24.9 | >100 | |
| HCT-15 | 0.409 | >100 | >100 | |
| HT29 | 2.94 | >100 | >100 | |
| KM12 | 0.686 | >100 | >100 | |
| SW-620 | 2.31 | >100 | >100 | |
Experimental
Chemistry
All melting points were determined on the Stuart apparatus and the values given are uncorrected. The IR spectra were determined on the Shimadzu IR 435 spectrophotometer (KBr, cm−1), Faculty of Pharmacy, Cairo University, Egypt. The 1H NMR and 13C NMR spectra were recorded on the Varian Gemini 75 MHz spectrophotometer using TMS as the internal standard. Chemical shift values are recorded in ppm on the δ scale, Microanalysis Center, Cairo University, Egypt. Mass spectra were recorded on a Hewlett Packard 5988 spectrometer, Microanalysis Center, Cairo University, Egypt. Elemental analyses were carried out at the Microanalysis Center, Cairo University, Egypt; the values found were within ±0.35% of the theoretical ones. Progress of the reactions was monitored using TLC sheets precoated with the UV fluorescent silica gel, Merck 60F 254, and were visualized using a UV lamp.
The synthesis of the target compounds is outlined in Schemes 1–3. Compounds Ia [16], Ib [17], IIa–c [18–20], IIIa [21], and Iva [22] were prepared as reported in the literature.
Procedures for the synthesis of compounds IIIb and IIIc
Method 1
A suspension of the appropriate derivative Ia,b (0.01 mol) in formamide (30 ml) was stirred at 145°C for 3 h; the solution was then cooled by being poured on ice-cold water, filtered, washed with water, dried, and finally crystallized from formic acid.
Method 2
A suspension of the appropriate derivative IIa–c (0.01 mol) in 85% formic acid (40 ml), was heated under reflux for 7 h; the reaction mixture was then cooled, filtered, washed with water, dried, and crystallized from formic acid.
3-Methyl-1-(4-nitrophenyl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (IIIb)
Yield: 2.6 g (96% by method 2); M.p.: >300°C; 1H NMR (300 MHz, DMSO-d6): δ = 3.15 (s, 3H, CH3); 8.20 (s, 1H, C6-H); 8.34 (d, J = 9.3 Hz, 2H, ArH C2’,6’); 8.39 (d, J = 9.6 Hz, 2H, ArH C3’,5’); 12.51 (s, 1H, NH, D2O exchangeable) ppm; IR (cm−1): 3370(NH), 3075, 3034 (CH aromatic), 2911 (CH aliphatic),1686 (C=O), 1589 (C=N),1441,1337(NO2); MS (70 ev): m/z 271 (M+, 100%). Anal. Calcd for C12H9 N5O3 (271.23): C, 53.14; H, 3.34; N, 25.82. Found: C, 53.30; H, 3.64; N, 26.36
1-(4-Chlorophenyl)-3-methyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (IIIc)
Yield: 1.94 g (74% by method 2); M.p.: >300°C; 1H NMR (300 MHz, DMSO-d6): δ = 3.30 (s, 3H, CH3); 7.60 (d, J = 9.0 Hz, 2H, ArH C3’,5’); 8.08 (d, 2H, J = 9.0 Hz, ArH C2’,6’); 8.15 (s, 1H, C6-H); 12.37 (s, 1H, NH, D2O exchangeable) ppm; IR (cm−1): 3422 (NH), 3121, 3040 (CH aromatic) 2974 (CH aliphatic),1670 (C=O), 1589 (C=N); MS (70 ev): m/z 261 (M+ + 1, 9.24%). Anal. Calcd for C12H9ClN4O (260.68): C, 55.29; H, 3.48; N, 21.49. Found: C, 55.36; H, 3.69; N, 21.41.
General procedure for the synthesis of compounds IVb and IVc
A suspension of the appropriate derivative IIIa–c (0.01 mol) in phosphorus oxychloride (80 ml) was heated under reflux for 3 h; the solution was cooled and then poured onto ice-cold water. The precipitated product was filtered, dried, and crystallized from ethanol.
4-Chloro-3-methyl-1-(4-nitrophenyl)-1H-pyrazolo[3,4-d]pyrimidine (IVb)
Yield: 2.03 g (70%), M.p.: 210–212°C; 1H NMR (200 MHz, DMSO-d6): δ = 2.71 (s, 3H, CH3); 8.37 (d, J = 9.4 Hz, 2H, ArH C2’,6’); 8.41 (d, J = 9.4 Hz, 2H, ArH C3’,5’); 8.96 (s, 1H, C6-H) ppm; IR (cm−1): 3120, 3080 (CH aromatic), 2905 (CH aliphatic), 1597,1576 (C=N), 1445, 1341 (NO2); MS (70 ev): m/z 289 (M+, 100%). Anal. Calcd for C12H8ClN5O2 (289.68): C, 49.75; H, 2.78; N, 24.18. Found: C, 49.60; H, 2.90; N, 24.22.
4-Chloro-1-(4-chlorophenyl)-3-methyl-1H-pyrazolo[3,4-d]pyrimidine (IVc)
Yield: 1.48 g (53%), M.p.: 189–190°C; 1H NMR (300 MHz, DMSO-d6): δ = 3.40 (s, 3H, CH3); 7.65 (d, J = 9.0 Hz, 2H, ArH C3’,5’); 8.16 (d, 2H, J = 9.0 Hz, ArH C2’,6’); 8.92 (s, 1H, C6-H) ppm; IR (cm−1): 3095, 3065 (CH aromatic), 2924 (CH aliphatic), 1589,1574 (C= N); MS (70 ev): m/z 278 (M+, 87.22). Anal. Calcd for C12H8Cl2N4 (279.12): C, 51.64; H, 2.89; N, 20.07. Found: C, 51.43; H, 3.01; N, 19.81.
General procedure for the synthesis of compounds Va–g
A suspension of the appropriate derivative IVa–c (0.01 mol) and the appropriate amine (0.01 mol) in ethanol (30 ml), triethylamine (0.3 g, 0.03 mol), was added and the reaction mixture was heated under reflux for 2–7 h; (the reaction was monitored using TLC until the starting materials were consumed in the reaction). The reaction mixture was allowed to cool leading to separation of the product, and then the crude product was filtered, dried, and crystallized from the appropriate solvent.
3-Methyl-N,1-diphenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine (Va)
Yield: 0.15 g (49%), M.p.: 144–145°C (ethanol/water); 1H NMR (200 MHz, CDCl3): δ = 2.88 (s, 3H, CH3); 7.00 (s, 1H, NH); 7.20–7.35 (m, 3H, ArH C3”,4”,5”); 7.40–7.55 (m, 3H, ArH C3’,4’,5’); 7.72 (d, J = 9.0 Hz, 2H, ArH C2”,6”); 8.20 (d, J = 9.0 Hz, 2H, ArH C2’,6’); 8.55 (s, 1H, C6-H) ppm.; 13C NMR (75 MHz, CDCl3): δ = 15.17 (q, 1C, CH3); 101.76 (s, 1C, C 3a); 121.48 (d, 2C, ArC 2”,6”); 121.82 (d, 2C, ArC 2’,6’); 124.84 (d, 1C, ArC 4”); 126.38 (d, 1C, ArC 4’); 129.15 (d, 2C, ArC 3’,5’); 129.22 (d, 2C, ArC 3”,5”); 137.73 (s, 1C, ArC 1’); 138.73 (s, 1C, ArC 1”); 140.73 (s, 1C, C 3); 154.30 (s, 1C, C 7a); 155.60 (s, 1C, C 4); 156.35 (d, 1C, C 6) ppm; IR (cm−1): 3455 (NH), 3080, 3020 (CH aromatic), 2930 (CH aliphatic); MS (70 ev): m/z 301 (M+, 78.78%). Anal. Calcd for C18H15N5 (301.35): C, 71.74; H, 5.02; N, 23.24. Found: C, 72.00; H, 4.97; N, 23.07.
N-(4-Methoxyphenyl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine (Vb)
Yield: 0.20 g (59%), M.p.: 121–122°C (ethanol/water); 1H NMR (200 MHz, DMSO-d6): δ = 2.77 (s, 3H, CH3); 3.80 (s, 3H, OCH3); 6.99 (d, J = 6.6 Hz, 2H, ArH C3”,5”); 7.32 (t, J = 6.6 Hz, 1H, ArH C4’); 7.55 (d, J = 6.0 Hz & m, 4H, ArH C2”,6”,3’,5’); 8.20 (d, J = 8.2 Hz, 2H, ArH C2’,6’); 8.38 (s, 1H, C6-H); 8.76 (s, 1H, NH, D2O exchangeable) ppm; IR (cm−1): 3439 (NH), 3080, 3028 (CH aromatic), 2976 (CH aliphatic). Anal. Calcd for C19H17N5O (331.37): C, 68.87; H, 5.17; N, 21.13. Found: C, 68.90; H, 5.00; N, 21.01
3-Methyl-1-phenyl-N-(2-phenylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (Vc)
Yield: 0.10 g (30%), M.p.: 151–152°C (ethanol/water); 1H NMR (200 MHz, CDCl3): δ = 2.45 (s, 3H, CH3); 3.05 (t, J = 9.0 Hz, 2H, CH2); 3.88 (t, J = 9.0 Hz, 2H, CH2); 5.20 (s, 1H, NH); 7.25–7.40 (m, 5H, ArH”); 7.45–7.55 (m, 3H, ArH C3’,4’,5’); 8.18 (d, J = 9.0 Hz, 2H, ArH C2’,6’); 8.47 (s, 1H, C6-H) ppm; 13C NMR (75 MHz, CDCl3): δ = 14.73 (q, 1C, CH3); 35.33 (t, 1C, CH2); 41.70 (t, 1C, CH2); 101.33 (s, 1C, ArC C 3a); 121.38 (d, 2C, ArC C 2’,6’); 126.14 (d, 2C,ArC C4’, C4”); 126.89 (d, 2C, ArC C2”,6”); 128.89 (d, 2C, ArC C3”,5”); 129.08 (d, 2C, ArC C3’,5’); 138.56 (s,1C, ArC C 1”); 138.89 (s, 1C, ArC C 1’); 141.17 (s, 1C, ArC C 3); 153.99 (s, 1C, ArC C7a); 156.66 (s, 1C, ArC C6); 157.53 (s, 1C, ArC C4) ppm; IR (cm−1): 3428 (NH), 3030 (CH aromatic), 2940, 2922 (CH aliphatic). MS (70 ev): m/z 329 (M+, 16.33%). Anal. Calcd for C20H19N5 (329.40): C, 72.93; H, 5.81; N, 21.26. Found: C, 72.73; H, 5.90; N, 21.11.
3-Methyl-1-(4-nitrophenyl)-N-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine (Vd)
Yield: 0.28 g (80%), M.p.: 259–260°C (benzene); 1H NMR (300 MHz, DMSO-d6): δ = 2.78 (s, 3H, CH3); 7.20 (t, J = 7.2 Hz, 1H, ArH C4”); 7.40 (t, J = 7.8 Hz, 2H, ArH C3”,5”); 7.68 (d, J = 6.6 Hz, 2H, ArH C2”,6”); 8.40 (d, J = 7.2 Hz, 2H, ArH C2’,6’); 8.49 (s, 1H, C6-H); 8.58 (d, J = 7.2 Hz, 2H, ArH C3’,5’); 8.88 (s, 1H, NH, D2O exchangeable) ppm; IR (cm−1): 3431 (NH), 3040 (CH aromatic), 2900 (CH aliphatic), 1443, 1344 (NO2). Anal. Calcd for C18H14N6O2 (346.34): C, 62.42; H, 4.07; N, 24.27. Found: C, 62.59; H, 3.99; N, 24.57.
3-Methyl-1-(4-nitrophenyl)-N-(2-phenylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (Ve)
Yield: 0.13 g (36%), M.p.: 214°C (benzene); 1H NMR (200 MHz, DMSO-d6): δ = 2.63 (s, 3H, CH3); 2.96 (t, J = 7.0 Hz, 2H, CH2); 3.80 (t, J = 7.0 Hz, 2H, CH2); 7.20–7.39 (m, 5H, ArH”); 7.53 (s, 1H, NH, D2O exchangeable); 8.39 (d, J = 9.4 Hz, 2H, ArH C3’,5’); 8.45 (s, 1H, C6-H); 8.57 (d, J = 9.4 Hz, 2H, ArH C2’,6’) ppm; IR (cm−1): 3439 (NH), 3080, 30210 (CH aromatic), 2940, 2920 (CH aliphatic), 1441, 1346 (NO2).; MS (70 ev): m/z 374 (M+, 13.47%). Anal. Calcd for C20H18N6O2 (374.4): C, 64.16; H, 4.85; N, 22.45. Found: C, 64.27; H, 4.89; N, 22.66.
1-(4-Chlorophenyl)-N-(4-methoxyphenyl)-3-methyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine (Vf)
Yield: 0.26 g (70%), M.p.: 198–200°C (ethanol); 1H NMR (300 MHz, DMSO-d6): δ = 2.74 (s, 3H, CH3); 3.78 (s, 3H, OCH3); 6.97 (d, J = 7.2 Hz, 2H, ArH C3”,5”); 7.53 (d, J = 7.0 Hz, 2H, ArH C2”,6”); 7.58 (d, J = 6.6 Hz, 2H, ArH C3’,5’); 8.25 (d, J = 6.9 Hz, 2H, ArH C2’,6’); 8.36 (s, 1H, C6-H); 8.74 (s, 1H, NH, D2O exchangeable) ppm; IR (cm−1): 3443 (NH), 3010 (CH aromatic), 2934, 2915 (CH aliphatic); MS (70 ev): m/z 365 (M+, 24.61%). Anal. Calcd for C19H16ClN5O (365.82): C, 62.38; H, 4.41; N, 19.14. Found: C, 65.59; H, 4.43; N, 18.95.
1-(4-Chlorophenyl)-3-methyl-N-(2-phenylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (Vg)
Yield: 0.24 g (65%), M.p.: 123–125°C (ethanol/water); 1H NMR (300 MHz, DMSO-d6): δ = 2.61 (s, 3H, CH3); 2.95 (t, J = 7.8 Hz, 2H, CH2); 3.76 (t, J = 7.5 Hz, 2H, CH2); 7.21–7.34 (m, 5H, ArH”); 7.34 (s, 1H, NH, D2O exchangeable); 7.56 (d, J = 9.0 Hz, 2H, ArH C3’,5’); 8.24 (d, J = 9.0 Hz, 2H, ArH C2’,6’); 8.39 (s, 1H, C6-H) ppm; IR (cm−1): 3418 (NH), 3100, 3026 (CH aromatic), 2924, 2860 (CH aliphatic). Anal. Calcd for C20H18ClN5 (363.84): C, 66.02; H, 4.99; N, 19.25. Found: C, 66.13; H, 5.15; N, 19.19.
Synthesis of 1-(4-chlorophenyl)-4-hydrazino-3-methyl-1H-pyrazolo[3,4-d]pyrimidine (VI)
Hydrazine hydrate (0.1 mol) was added to a suspension of IVc (0.01 mol) in ethanol (35 ml), the reaction mixture was heated under reflux for 2.5 h; the precipitated product was filtered, washed with ethanol, dried, and crystallized from ethanol.
Yield: 2.55 g (93%), M.p.: 244–246°C; 1H NMR (300 MHz, DMSO-d6): δ = 2.62 (s, 3H, CH3); 3.82 (s, 1H, NH, D2O exchangeable); 4.95 (broad s, 2H, NH2, D2O exchangeable); 7.56 (d, J = 9.0 Hz, 2H, H3’,5’); 8.22 (d, J = 9.0 Hz, 2H, H2’,6’); 8.36 (s, 1H, H6) ppm; IR (cm−1): 3294 (NH), 3278 & 3199 (NH2); MS (70 ev): m/z 276 (M+ + 2); 274 (100%) (M+). Anal. Calcd for C12H11ClN6 (274.71): C, 52.47; H, 4.04; N, 30.59. Found: C, 52.67; H, 4.24; N, 30.59.
General procedure for the synthesis of compounds VIIa–c
A suspension of VI (1 mmol), and the appropriate aldehyde (1 mmol) in ethanol (35 ml), was heated under reflux with stirring for 3–4 h; the reaction mixture was then cooled and the separated precipitate was filtered, dried, and crystallized from ethanol.
2-({2-[1-(4-Chlorophenyl)-3-methyl-1H-pyrazolo[3,4-d]pyrimidin-4-yl]hydrazinylidene}-methyl)phenol (VIIa)
Yield: 0.24 g (63%), M.p.: 269–270°C; 1H NMR (300 MHz, DMSO-d6): δ = 2.76 (s, 3H, CH3); 6.88–6.95 (m, 1H, H5”); 7.28–7.33 (m, 1H, H3”); 7.39–7.43 (m, 1H, H4”); 7.58–7.63 (m, 1H, H6”); 7.80 (d, J = 9.0 Hz, 2H, H3’,5’); 8.04 (d, J = 9.0 Hz, 2H, H2’,6’); 8.23 (s, 1H, HC=N); 8.60 (s, 1H, H6); 10.20 (broad s, 1H, OH, D2O exchangeable); 11.90 (broad s, 1H, NH, D2O exchangeable) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 14.10 (q, 1C, CH3); 102.00 (s, 1C, ArC C3a); 117.80 (s,1C, ArC C 3”); 119.80 (d, 2C, ArC C2’,6’); 125.38 (s,1C, ArC C5”); 127.80 (s,1C, ArC C6”); 129.00 (d,2C, ArC C3’,5’); 131.50 (s,1C, ArC C4’); 132.90 (s,1C, ArC C 4”); 136.54 (s, 1C, ArC C 1”); 138.26 (s,1C, ArC C 1’); 144.93 (s, 1C,C=N); 147.85 (s, 1C, ArC C3); 149.50 (s, 1C, ArC C7a); 153.57 (s, 1C, ArC C6); 157.47 (s, 1C, ArC C2”); 157.71 (s, 1C, ArC C4) ppm; IR (cm−1): 3433 (NH), 3369 (OH); MS (70 ev): m/z 380 (M+ + 2); 378 (M+); 243 (100%). Anal. Calcd for C19H15ClN6O (378.82): C, 60.24; H, 3.99; N, 22.19. Found: C, 60.50; H, 3.89; N, 22.44.
1-(4-Chlorophenyl)-4-[2-(4-methoxybenzylidene)hydrazinyl]-3-methyl-1H- pyrazolo[3,4-d]pyrimidine (VIIb)
Yield: 0.32 g (82%), M.p.: 247–248°C; 1H NMR (300 MHz, DMSO-d6): δ = 2.79 (s, 3H, CH3); 3.82 (s, 3H, OCH3); 7.00 (d, J = 8.7 Hz, 2H, H3”,5”); 7.60 (d, J = 9.0 Hz, 2H, H3’,5’); 7.65 (d, J = 8.7 Hz, 2H, H2”,6”); 7.95 (d, J = 9.0 Hz, 2H, H2’,6’); 8.20 (s, 1H, HC=N); 8.35 (s, 1H, H6); 11.87 (broad s, 1H, NH, D2O exchangeable) ppm; IR (cm−1): 3220 (NH); MS (70 ev): m/z 393 (M+ + 1); 392 (100%). Anal. Calcd for C20H17ClN6O (392.84): C, 61.15; H, 4.36; N, 21.39. Found: C, 61.39; H, 4.51; N, 21.10.
1-(4-Chlorophenyl)-3-methyl-4-[2-(2-nitrobenzylidene)hydrazinyl]-1H- pyrazolo[3,4-d]pyrimidine (VIIc)
Yield: 0.35 g (86%), M.p.: 286–288°C; 1H NMR (300 MHz, DMSO-d6): δ = 2.77 (s, 3H, CH3); 7.56 (d, J = 9.0 Hz, 2H, H3’,5’); 7.78–7.84 (m, 2H, H5”,6”); 8.07 (d, J = 9.0 Hz, 2H, H2’,6’); 8.08–8.13 (m, 1H, H4”); 8.33 (s, 1H, HC=N); 8.53 (s, 1H, H6); 8.75–8.80 (m, 1H, H2”); 12.12 (broad s, 1H, NH, D2O exchangeable) ppm; IR (cm−1): 3327 (NH), 1437, 1346 (NO2). Anal. Calcd for C19H14ClN7O2 (407.81): C, 55.96; H, 3.46; N, 24.04. Found: C, 56.20; H, 3.56; N, 23.96.
Antitumor activity
The compounds were prepared in DMSO: glycerol 9:1 at a concentration of 4 mM for the single-dose assay. The solution was diluted 1:400, giving a test concentration of 10 μM. The human tumor cell lines of the cancer screening panel were prepared according to the standard procedure of the American National Cancer Institute (NCI), and the tests were performed at the American National Cancer Institute (NCI) [23, 24].
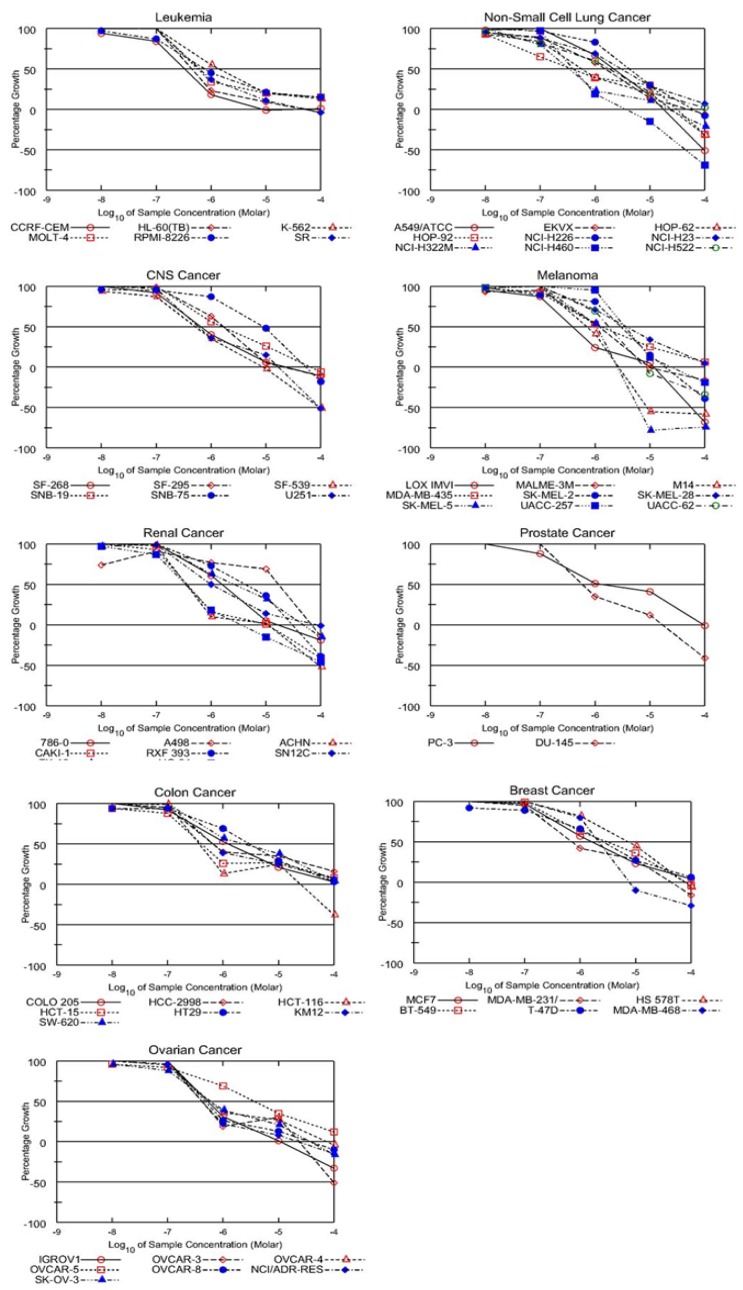
Compound VIIa, which was subjected to a 5-dose assay, was prepared at a concentration 40 mM. The solution was diluted 1:400, giving a Test concentration of 100 μM.
The human tumor cell lines of the cancer screening panel were prepared according to the standard procedures of the NCI. The results are summarized in Tables 1 and 2, and Figure 2.
Fig. 2.
Dose response curves for compound VIIa.
Conclusion
The objective of this study was to synthesize and investigate the anticancer inhibition activity of selected pyrazolopyrimidines with the hope of discovering new structure leads to serve as potential anticancer agents. The newly synthesized compounds showed good inhibitory activity on different cell lines at a concentration of 10μM, making them leading chemical entities for further modification to render them as clinically useful therapeutic agents. Compound Vc showed 65.57% and 53.25% on the k-562 and molt-4 cell lines of leukemia. Vg produced 76.32 and 51.72 percentage inhibition on the K-562 and HOP-92 cell lines of leukemia and small lung cancer, VIIc showed 90% inhibition on the ovcar-3 cell line of ovarian cancer, and 82% and 84% inhibition on the k-562 and molt-4 cell lines of leukemia. The highest activity was presented by compound VIIa, which showed good inhibitory activity against 57 cell lines, and was the reason for performing 5-dose testing on this compound and measuring its GI50 (growth inhibition)and LC50 (lethal concentration), which further revealed its high potency against most of the cell lines.
Acknowledgement
We would like to thank the American National Cancer Institute (NCI) team for their help in carrying out the antitumor screening of the newly synthesized compounds.
Footnotes
Authors’ Statement
Competing Interests
The authors declare no conflict of interest.
References
- 1.Zhang X, Lin Q, Zhong P. A Facile One-pot Synthesis of 1-Arylpyrazolo[3,4-d]pyrimidin-4-ones. Molecules. 2010;15:3079–3086. doi: 10.3390/molecules15053079. http://dx.doi.org/10.3390/molecules15053079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim DC, Lee YR, Yang B, Shin KJ, Kim DJ, Chung BY, Yoo KH. Synthesis and biological evaluations of pyrazolo[3,4-d]pyrimidines as cyclin-dependent kinase 2 inhibitors. Eur J Med Chem. 2003;38:525–532. doi: 10.1016/s0223-5234(03)00065-5. http://dx.doi.org/10.1016/S0223-5234(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 3.Markwalder JA, Arnone MR, Benfield PA, Boisclair M, Burton CR, Chang C, Cox SS, Czerniak PM, Dean CL, Doleniak D, Grafstrom R, Harrison BA, Kaltenbach RF, Nugiel DA, Rossi KA, Sherk SR, Sisk LM, Stouten P, Trainor GL, Worland P, Seitz SP. Synthesis and Biological Evaluation of 1-Aryl-4,5-dihydro-1H-pyrazolo[3,4-d]pyrimidin-4-one Inhibitors of Cyclin-Dependent Kinases. J Med Chem. 2004;47:5894–5911. doi: 10.1021/jm020455u. http://dx.doi.org/10.1021/jm020455u. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim DA, El-Metwally AM, Al-Arab EE. Structure-based design of a new class of highly selective pyrazolo[3,4-d]pyrimidines based inhibitors of cyclin dependent kinases. Arkivoc. 2009;7:12–25. [Google Scholar]
- 5.Schenone S, Brullo C, Bruno O, Bondavalli F, Mosti L, Maga G, Crespan E, Carraro F, Manetti F, Tintori C, Botta M. Synthesis, biological evaluation and docking studies of 4-amino substituted 1H-pyrazolo[3,4-d]pyrimidines. Eur J Med Chem. 2008;43:2665–2676. doi: 10.1016/j.ejmech.2008.01.034. http://dx.doi.org/10.1016/j.ejmech.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 6.Peat AJ, Boucheron JA, Dickerson SH, Garrido D, Mills W, Peckham J, Preugschat F, Smalley T, Schweiker SL, Wilson JR, Wang TY, Zhou HQ, Thomson SA. Novel pyrazolopyrimidine derivatives as GSK-3 inhibitors. Bioorg Med Chem Lett. 2004;14:2121–2125. doi: 10.1016/j.bmcl.2004.02.036. http://dx.doi.org/10.1016/j.bmcl.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 7.Smalley TL, Peat AJ, Boucheron JA, Dickerson S, Garrido D, Preugschat F, Schweiker SL, Thomson SA, Wang TY. Synthesis and evaluation of novel heterocyclic inhibitors of GSK-3. Bioorg Med Chem Lett. 2006;16:2091–2094. doi: 10.1016/j.bmcl.2006.01.057. http://dx.doi.org/10.1016/j.bmcl.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 8.Dessalew N, Patel DS, Bharatam PV. 3D-QSAR and molecular docking studies on pyrazolopyrimidine derivatives as glycogen synthase kinase-3 inhibitors. J Mol Graphics Modell. 2007;25:885–895. doi: 10.1016/j.jmgm.2006.08.009. http://dx.doi.org/10.1016/j.jmgm.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Settimo FD, Primofiore G, Motta CL, Taliani S, Simorini F, Marini AM, Mugnaini L, Lavecchia A, Novellino E, Tuscano D, Martini C. Novel, Highly Potent Adenosine Deaminase Inhibitors Containing the Pyrazolo[3,4-d]pyrimidine Ring System. Synthesis, Structure–Activity Relationships, and Molecular Modeling Studies. J Med Chem. 2005;48:5162–5174. doi: 10.1021/jm050136d. http://dx.doi.org/10.1021/jm050136d. [DOI] [PubMed] [Google Scholar]
- 10.Hubbard RD, Bamaung NY, Palazzo F, Zhang Q, Kovar P, Osterling DJ, Hu X, Wilsbacher JL, Johnson EF, Bouska J, Wang J, Bell RL, Davidsen SK, Sheppard GS. Pyrazolo[3,4-d]pyrimidines as potent inhibitors of the insulin-like growth factor receptor (IGF-IR) Bioorg Med Chem Lett. 2007;17:5406–5409. doi: 10.1016/j.bmcl.2007.07.037. http://dx.doi.org/10.1016/j.bmcl.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Rodrigues LM, Esteves AP, Oliveira-Campos AMF, Nascimento MSJ, Nazareth N, Cidade H, Neves MP, Fernandes E, Pinto M, Cerqueira NMFSA, Bras N. Synthesis of N-aryl-5-amino-4-cyanopyrazole derivatives as potent xanthine oxidase inhibitors. Eur J Med Chem. 2008;43:771–780. doi: 10.1016/j.ejmech.2007.06.002. http://dx.doi.org/10.1016/j.ejmech.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Abdelrazek HA, Abdelwahab AE. Synthesis and Biological Evaluation of Some Novel Fused Pyrazolopyrimidines as Potential Anticancer and Antimicrobial Agents. Arch Pharm Chem Life Sci. 2011;11:184–196. doi: 10.1002/ardp.201000188. http://dx.doi.org/10.1002/ardp.201000188. [DOI] [PubMed] [Google Scholar]
- 13.Schenone S, Bruno O, Bondavalli F, Ranise A, Mosti L, Menozzi G, Fossa P, Donnini S, Santoro A, Ziche M, Manetti F, Botta M. 1-Aryl-4-amino-1H-pyrazolo[3,4-d]pyrimidine derivatives toward the human epidermoid carcinomaA431 cell line. Eur J Med Chem. 2004;39:939–946. doi: 10.1016/j.ejmech.2004.07.010. http://dx.doi.org/10.1016/j.ejmech.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Ghorab MM, Ragab FA, Alqasoumi SI, Alafeefy AM, Aboulmagd SA. Synthesis of some new pyrazolo[3,4-d]pyrimidine derivatives of expected anticancer and radioprotective activity. Eur J Med Chem. 2010;45:171–178. doi: 10.1016/j.ejmech.2009.09.039. http://dx.doi.org/10.1016/j.ejmech.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 15.Curran KJ, Verheijen JC, Kaplan J, Richard DJ, Toral-Barza L, Hollander I, Lucas J, Ayral-Kaloustian S, Yu K, Zask A. Pyrazolopyrimidines as highly potent and selective, ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): Optimization of the 1-substituent. Bioorg Med Chem Lett. 2010;20:1440–1444. doi: 10.1016/j.bmcl.2009.12.086. http://dx.doi.org/10.1016/j.bmcl.2009.12.086. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Zhu Y, Wang X, Yang H. Synthesis and Herbicidal Activities of a Series of Di(aminopyrazoly) Ketone Derivatives. J Heterocyclic Chem. 2007;44:749–755. http://dx.doi.org/10.1002/jhet.5570440401. [Google Scholar]
- 17.O’Halloran N, James JP, Downey CA, O’Malley P, Duff T, Bertrand S. Inter- and intra-molecular cyclisation reactions of azoacetates derived from aryl hydrazones of ethyl acetoacetate and acetoacetanilides. Heterocycles. 2008;75:2681–2701. http://dx.doi.org/10.3987/COM-08-11433. [Google Scholar]
- 18.Cheng CC, Robins RK. Potential Purine Antagonists. VI. Synthesis of 1-Alkyl- and 1-Aryl-4-substituted Pyrazolo[3,4-d]pyrimidine. J Org Chem. 1956;21:1240–1256. http://dx.doi.org/10.1021/jo01117a010. [Google Scholar]
- 19.Kreutzberger A, Burgwitz K. Antimycotic drugs. XI: 4-Amino-3-methylpyrazolo[3,4-d]pyrimidines. Arch Pharm. 1980;313:906–912. http://dx.doi.org/10.1002/ardp.19803131103. [Google Scholar]
- 20.Silvestri R, Cascio MG, Regina GL, Piscitelli F, Lavecchia A, Brizzi A, Pasquini S, Botta M, Novellino E, Marzo VD, Corelli F. Synthesis, Cannabinoid Receptor Affinity, and Molecular Modeling Studies of Substituted 1-Aryl-5-(1H-pyrrol-1-yl)-1H-pyrazole-3-carboxamides. J Med Chem. 2008;51:1560–1576. doi: 10.1021/jm070566z. http://dx.doi.org/10.1021/jm070566z. [DOI] [PubMed] [Google Scholar]
- 21.Davoodnia A, Rahimizadeh M, Rivadeh S, Bakavoli M, Roshani M. Synthesis of new substituted pyrazolo[3,4-d]pyrimidin-4-ones under microwave irradiation. Indian J Heterocycl Chem. 2006;16:151–154. [Google Scholar]
- 22.Davoodnia A, Zhiani R, Tavakoli-Hoseini N. Synthesis of pyrazolo[4,3-e][1,2,4]triazolo[4,3-c]pyrimidines. Monatsh Chem. 2008;139:1405–1407. http://dx.doi.org/10.1007/s00706-008-0939-8. [Google Scholar]
- 23.Boyd MR, Paull KD. Some Practical Considerations and Applications of the National Cancer Institute In-Vitro Anticancer Drug Discovery Screen. Drug Dev Res. 1995;34:91–109. http://dx.doi.org/10.1002/ddr.430340203. [Google Scholar]
- 24.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JR, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxic assay for anticancer drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. http://dx.doi.org/10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]