Abstract
OBJECTIVE
Head and neck squamous cell carcinoma (HNSCC) is a complex disease process involving interactions with carcinoma associated fibroblasts and endothelial cells. We further investigated these relationships by suppressing stromal cell growth through the inhibition of fibroblast growth factor receptor (FGFR).
STUDY DESIGN
Preclinical investigation.
SUBJECTS AND METHODS
HNSCC cell lines (FADU, OSC19, Cal27, SCC1, SCC5, SCC22A), fibroblast (HS27) and endothelial cells (HUVEC) were cultured individually or in coculture. Proliferation was assessed following treatment with a range of physiologic concentrations of FGFR inhibitor PD173074. Mice bearing established HNSCC xenografts were treated with PD173074 (12 mg/kg) and tumor histology was analyzed for stromal composition, proliferation (Ki67 staining) and apoptosis (TUNEL staining).
RESULTS
In vitro, inhibition of FGFR with PD173074 dramatically reduced proliferation of fibroblasts and endothelial cells compared to untreated controls. However, HNSCC cell proliferation was not affected by inhibition of FGFR. When cocultured with fibroblasts, HNSCC cells proliferation increased by 15–80% (p<0.01). Furthermore, this fibroblasts enhanced tumor cell growth was suppressed by FGFR inhibition. Additionally, treatment of mice bearing HNSCC xenografts with PD173074 resulted in significant growth inhibition (p<0.001). Additionally, those tumors from mice treated with PD173074 had a smaller stromal component, decreased proliferation and increased apoptosis.
CONCLUSION
Targeting the FGFR pathway in head and neck cancer acts through the stromal components to decrease HNSCC growth in vivo and in vitro.
Level of Evidence
Not applicable.
Keywords: Head and neck squamous cell carcinoma, stroma fibroblast, fibroblast growth factor receptor
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) remains difficult to treat. Despite advances in treatment modalities, overall survival rates have not improved in the past 20 years. This resistance to therapy is likely the result of a complex relationship between oncogenic cells and the surrounding supporting cells, including fibroblasts and endothelial cells. Supporting cells provide cytokine signaling and ligand production that promotes oncogenic cell proliferation and resistance to apoptosis.1,2
There are four members of fibroblast growth factor receptor (FGFR) family. These transmembrane kinase receptors are involved in cell differentiation, proliferation and tumorigenesis.3 Activation of these receptors is achieved by fibroblast growth factor (FGF) ligands. Fibroblasts are important components of tumor stroma, allowing for increased tumor cell survival and proliferation.1,2 FGFR1/2/3 expression was found in about 12–100% of HNSCC specimens.4–8 Higher FGFR1/2/3 expression was found to correlate with earlier T classification and stage, suggesting FGFRs play a role in the transformation of normal mucosa into malignancy.5,9 While high FGFR1 expression has only been found in 11% of HNSCC cell lines, high FGFR2 and FGFR3 expression has been found in the majority of HNSCC cell lines.6,10 Furthermore, the FGFR1 signaling pathway plays a role in neovascularization.5 While a reduction of FGFR3 levels in HNSCC cell lines lead to a 35% decrease in proliferation in vitro.10 In addition, overexpression of FGFR3 was found to provide radiation resistance in vitro.10
The etiology of HNSCC is complex and disease progression relies on interactions between carcinoma cells and associated stromal cells, such as fibroblasts and endothelial cells. To improve our understanding of these relationships, we investigated the role of FGFRs in HNSCC and stromal cell proliferation and survival.
MATERIALS AND METHODS
Cell Culture and Reagents
SCC-1, SCC-5 and SCC-22A (University of Michigan), OSC-19 (University of Texas, MD Anderson), and FADU, Cal27 and HS27 (American Type Culture Collection, Rockville, MD), and endothelial cells (HUVEC) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin (100 U/mL), streptomycin (100 µg/mL). Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2. Inhibition of FGFR was achieved by treating with PD173074 (Sigma-Aldrick, St. Louis, MO), a small molecule tyrosine kinase inhibitor targeting FGFR1/2/3.3,11
Western Blot
Cells were grown to 70%–80% confluence, washed twice with cold PBS, and lysed in lysis buffer [50mM Tris-HCl (pH7.5), 150mM NaCl, 1%(v/v) NP40, 0.5 % (w/v) sodium deoxycholate, 1mM EDTA, 0.1% SDS and a protease inhibitor cocktail tablet (Roche Applied Science, Indianapolis, IN)]. The cleared lysates were collected by centrifugation at 12,000 × g for 20 minutes at 40°C. The protein concentrations were measured by BCA protein assay (Thermo Scientific, Rockford, IL). Lysates were resolved by SDS-PAGE and transferred to PVDF membranes. The membranes were incubated with the primary antibody, washed, incubated with horseradish peroxidase conjugated secondary antibodies and washed again. Following the final wash, the membrane was exposed to the Amersham ECL Western blotting detection system (GE healthcare, Buckinghamshire, UK). Following final analysis, the membranes were stripped and reprobed with horseradish peroxidase-conjugated mouse monoclonal antihuman β-actin to ensure equal protein loading.
Tumor cell proliferation assay
To assess cell proliferation, each cell line was seeded into 24 well plated at density of 104cells/per well and cultured for 96hr. The cells were then trypsinized and the cell numbers were counted by an accuri flow cytometer. To assess the effect of coculturing fibroblasts with HNSCC cells, HS27 cells were first labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) following the manufacture’s instruction (Invitrogen, Carlsbad, CA). Briefly, HS27 cells were cultured until they reached confluence. At which point, culture medium was replaced with prewarmed PBS containing 10 µM CFSE. Cells were then incubated at 37°C for 15 mins, followed by washing with DMEM 3 times and cultured at 37°C for an additional 24 hrs. The coculture of tumor and fibroblast was achieved by seeding 6.6×103 tumor cells with 3.3×103 CFSE-labeled fibroblast cells in 24 well plates. Following 96 hrs of incubation, the cells were trypsinized and counted by flow cytometry.
In vivo assessment of tumor cell growth
Athymic female nude mice aged 6 to 8 weeks (Charles River Laboratories and National Cancer Institute–Frederick) were obtained and housed in accordance with our institution's Institutional Animal Care and Use Committee (IACUC) guidelines. Animals were inoculated s.c. on the right flank with 2×106 tumor cells (SCC-5) in 0.2 mL of serum free DMEM. Animals were followed for 4 weeks after the tumor cell inoculation. Tumor measurement were converted to a calculated tumor weight (in mg) using the formula [width (mm)2 × length (mm)]/2.11 SCC-5 xenografts were treated with PD173074 (0.25mg/d) for 7 days and tumor growth was followed for 8 days following completion of treatment.
Assessing tumor cells and host stromal cells in tumor tissue by flow cytometry
In order to disaggregate cells, fresh tumors were cut into a small piece and incubated with PBS with 2mg/ml collagenase (Fisher Scientific, Waltham, MA) at 37°C for 2 hrs. Density centrifugation (800×g, 15min) using Ficoll-Paque Plus was used to remove dead cells and debris. Interface cells were resuspended in Hanks’ buffer (containing 3% FBS) then incubated with antibodies to mouse CD16/CD32 (eliminating nonspecific binding) and mouse H2k[d]-PE. PI negative cells were electronically gated and then analyzed with respect to mouse H2k[d]. The rest of tumor was fixed with formalin, subsequently embedded in paraffin and stained with hematoxylin and eosin as previously described.9
Assessing tumor cells and host stromal cells in tumor tissue by H&E, Ki67 and TUNEL staining
In vivo tumor specimens (SCC-5) were fixed with formalin, subsequently embedded in paraffin, cut at 5 µM, placed on treated slides and heated at 60°C for 2h. Tissue sections were deparafinnized with xylene and rehydrated with absolute enthanol (90 and 70%). Slides were stained with hematoxylin and eosin as previously described.12 In addition, SCC-5 xenografts were stained for Ki67 (Fisher Scientific,Waltham, MA) and TUNEL (Millipore, Billerica, MA).
Statistical analysis
Statistical analysis of tumor cell proliferation and tumor growth in mice for each of the studies was performed by a Student’s t-Test using Graph Pad Prism. P<0.05 was considered significant.
RESULTS
Inhibition of FGFR Decreased Proliferation of Fibroblasts and Endothelial Cells
To assess the effect of FGFR inhibition on receptor expression and down-stream enzyme expression, western blot analysis of untreated endothelial (HUVEC), fibroblasts (HS27) and HNSCC cells (SCC-1, SCC-5, OSC-19, and Cal27) were compared to those treated with PD173074 (100 nM; selective FGFR inhibitor) (Fig. 1). None of the cell lines investigated expressed FGFR-1 (data not shown). Interestingly, ERK-1/2 levels decreased in endothelial and fibroblasts cells following inhibition with PD173074, but increased in SCC-1, SCC-5, and Cal27 cells. FGFR3 levels were reduced in fibroblasts and SCC-5 cells, and remained unchanged in the remaining cell lines. Furthermore, AKT and FGFR2 levels were reduced in SCC-5 cells and remained unchanged in the remaining cell lines. Phosphorylated MAPK levels were reduced in endothelial cells, while phosphorylated AKT levels were reduced in OSC-19 cells.
Figure 1.
Western blot analysis of protein expression of untreated and treated endothelial, fibroblasts and HNSCC cells with PD173074.
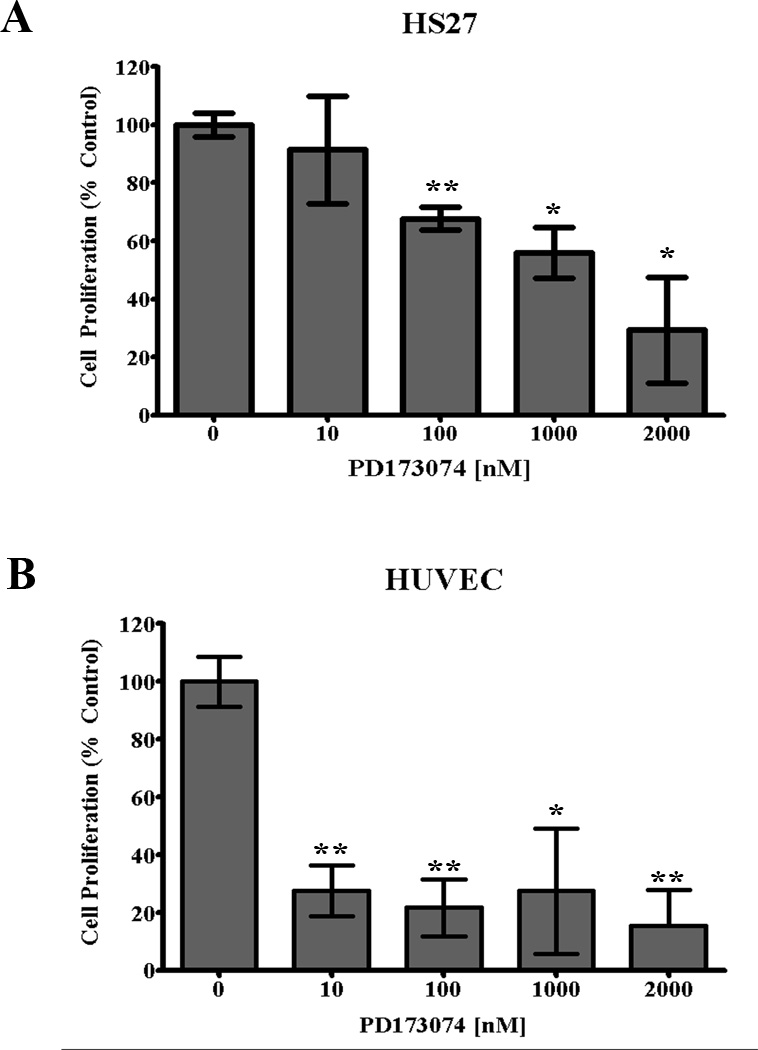
The effect of FGFR inhibition was assessed following treatment with PD173074 for 96 hrs in vitro. Fibroblasts (Fig. 2A) and endothelial cells (Fig. 2B) were cultured with a lower range of concentrations (0–2 µM) while HNSCC cells (Fig. 3) were cultured with a higher range concentrations (0–10 µM). Response to treatment was measured by changes in cell count and normalized to control values. A statistically significant reduction in proliferation was observed for endothelial cells (p<0.01) and fibroblasts (p<0.05). In contrast, inhibition of FGFR at or less than 1 µM PD173074 did not change proliferation of HNSCC cells.
Figure 2.
In vitro proliferation of endothelial cells (HUVEC) and fibroblasts (HS27) relative to controls following treatment with FGFR inhibitor (PD173074) for 72 hrs. (unpaired t-test; *p<0.05, **p<0.01).
Figure 3.
HNSCC cell proliferation was measured in vitro following treatment with a FGFR inhibitor (PD173074) for 72 hours.
Effects of Coculturing Fibroblasts with HNSCC Cells In Vitro
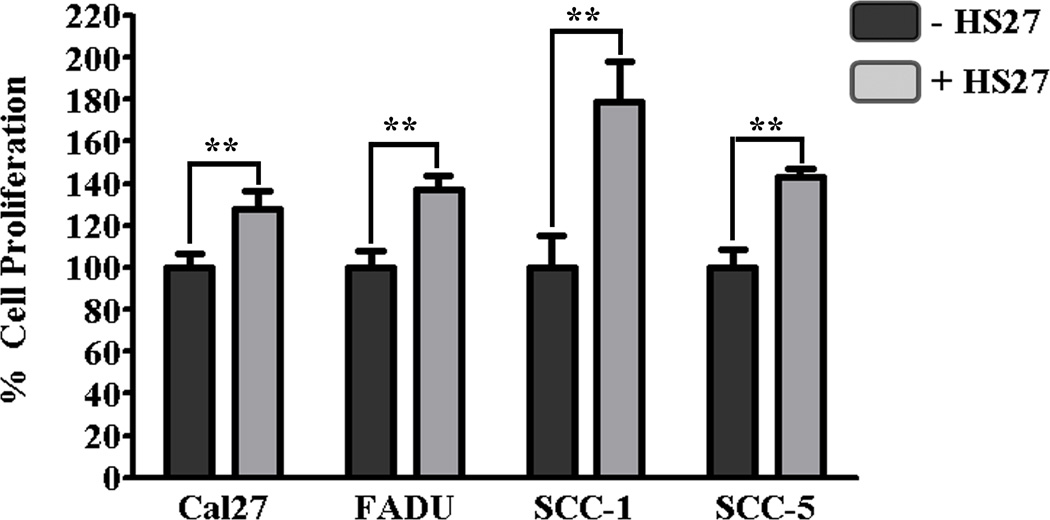
Culturing HNSCC cells with fibroblasts significantly increased proliferation (p≤0.01) for all cell lines except OSC-19 when compared to isolated cultures (Fig. 4). The cell line demonstrating the most significant increase in proliferation was SCC-1 for which proliferation almost doubled. Although FGFR inhibition (PD173074; 100nM) had no effect on HNSCC cell proliferation when cultured in isolation, it resulted in decreased proliferation of HNSCC cells when cocultured with fibroblasts (Fig. 5). Compared to untreated cocultures, inhibition of FGFR resulted in a trend towards decreased proliferation of Cal27 (p=0.09) and OSC-19 (p=0.08), and a significant decrease in proliferation of FADU (p=0.001), SCC-1 (p=0.004), and SCC-5 (p<0.0001) when cocultured with fibroblasts.
Figure 4.
In vitro proliferation of HNSCC cells cultured in isolation and cocultured with fibroblasts (HS27) for 72 hrs. (unpaired t-test; **p≤0.01).
Figure 5.
In vitro proliferation of HNSCC cells grown in coculture with fibroblasts (HS27) +/− treatment with FGFR inhibitor (PD173074; 100 nM) for 72 hrs. (unpaired t-test; **p<0.01, ***p≤0.001)
FGFR Promotes Tumor Growth In Vivo
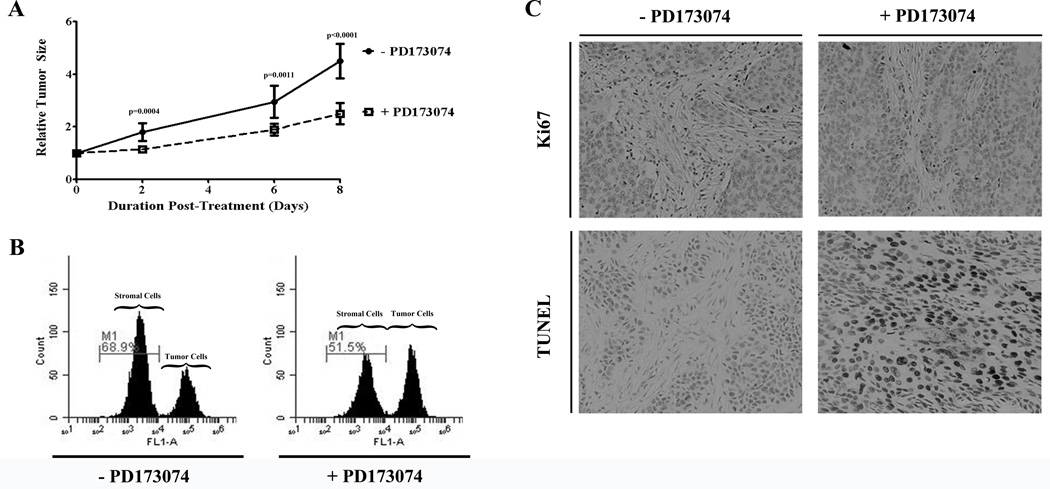
Tumor growth in untreated mouse xenografts (SCC-5) was compared to tumor growth in mouse xenografts treated with FGFR inhibitor PD173074 (Fig. 6A). Forty-eight hours following inhibition of FGFR, there was already a decrease rate of tumor growth compared to untreated controls (p<0.0004). On day 8 following treatment with the FGFR inhibitor, the rate of tumor growth remained decreased compared to untreated controls (p<0.0001). Tumors were harvested on day 8 following completion of treatment. On analysis by flow cytometry, the stromal component of the tumor was less for the group treated with the FGFR inhibitor (51.5%) compared to untreated controls (68.9%; Fig. 6B). On histologic analysis, the tumors harvested from mice treated with the FGFR inhibitor had reduced Ki67 staining (p=0.07) and increased TUNEL staining (p=0.017) when compared to untreated controls (Fig. 6C).
Figure 6.
Treatment of HNSCC xenografts with FGFR inhibitor (PD173074) resulted in decreased tumor size (A), a reduction in stromal components (B) and decreased cell proliferation (p=0.07) and increased apoptosis (p=0.017; C).
DISCUSSION
The proliferation, differentiation and survival of oncogenic cells rely on a symbiotic relationship with supporting cells.1,2,11,13–15 The result is a complex set of interactions involving multiple signaling pathways, including cytokine and ligand production and excretion.1,2 In this study we investigated the role of the FGFR pathways in promoting HNSCC.
We found that HSNCC cells grown in isolation were unaffected by inhibition of FGFRs. However, supporting cells such as fibroblasts and endothelial cells were extremely sensitive to blockade of this receptor. Suppression of FGFR activity resulted in decreased proliferation of fibroblasts and endothelial cells. Interestingly, ERK-1/2 levels decreased in endothelial and fibroblasts cells following inhibition of FGFR, but increased in SCC-1, SCC-5, and Cal27 cells. It could be hypothesized that treatment with PD173074 decreases ERK-1/2 expression in fibroblasts and endothelial cells, resulting in decreased proliferation.
When coculturing fibroblasts with HNSCC cells, proliferation of HNSCC cells increased relative to proliferation rates of isolated cultures. We hypothesize that fibroblasts are secreting growth factors and cytokines which stimulate HNSCC cells, resulting in increased HNSCC cell proliferation. In addition, there was a variation in the percentage of proliferation increase seen for the different cell lines. It is known that oncogenic cells behave differently depending on the patient. Similarly, in this study we observed a variance in HNSCC cell responsiveness to stimulation from factors secreted by the fibroblasts.
On further investigation, we found that when cocultures were treated with an FGFR inhibitor, there was a reduction in HNSCC cell proliferation compared to untreated coculture proliferation rates. Given the profound effect FGFR inhibition had on fibroblasts proliferation cultured in isolation, it can be extrapolated that the inhibition of FGFR decreased fibroblasts proliferation, which decreased the quantity of fibroblasts available to stimulate HNSCC cell growth and differentiation. Therefore, it could be concluded that when HNSCC cells are isolated from supporting cells, they are not dependent on FGFR activity for survival and proliferation.
Increased evidence for variation among HNSCC cell lines was found on western blot and proliferation analysis. These analyses revealed differences in the responsiveness of cell lines to FGFR inhibition. When examining the coculture environment more closely, the proliferation of the SCC-5 cell line was most profoundly affected by inhibition of FGFR. Western blot analysis demonstrated decreased AKT levels, FGFR-1 and FGFR-3 levels following treatment with the FGFR inhibitor. It could be hypothesized that decreased receptor and down-stream enzyme expression in both the SCC-5 and fibroblasts cells results in reduced signaling cascades normally involved in cell proliferation and differentiation. Of the HNSCC cell lines we investigated, only SCC-5 demonstrated that inhibition of FGFR may affect both the oncogenic cells and supporting stromal cells in the coculture setting.
As a result of these in vitro findings, we choose the SCC-5 cell line for implantation in vivo. Our study found inhibition of FGFR slowed the growth of HNSCC xenografts, however tumor regression was not observed. Further investigation found that targeting FGFR decreased the stromal component of the tumor, decreased tumor cell proliferation and increased tumor cell apoptosis. These in vivo results support our in vitro findings of FGFR inhibition reducing stromal cell proliferation.
CONCLUSIONS
HNSCC cells rely on supporting cells, such as fibroblast and endothelial cells, for survival and anti-apoptosis. There is a symbionic relationship between the oncogenic cells and supporting cells which relies on exchange of ligands and activation of various signaling cascades. Our study investigated the role of the FGFR pathway and resultant signaling cascade. We found inhibition of the FGFR in HNSCC cells grown in isolation had no effect on their survival, proliferation or differentiation. However, inhibition of the FGFR pathway had a profound effect on fibroblasts and endothelial cells. Furthermore, inhibition of the FGFR pathway in the coculture setting of HNSCC cells and fibroblast resulted in decreased HNSCC proliferation and survival. Finally, inhibition of the FGFR pathway in vivo reduced the stromal component of the tumor, decreased proliferation and increased apoptosis of tumor cells, and slowed tumor growth. We conclude the FGFR pathway is important in tumor-stromal interactions allowing for HNSCC cell proliferation and survival in this setting.
Acknowledgments
Financial Disclosures: This work was supported by the National Institutes of Health/National Cancer Institute grants 5R01CA142637, and T32 CA091078-09.
Footnotes
Conflicts of Interest: None.
References
- 1.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acevedo VD, Ittmann M, Spencer DM. Paths of FGFR-driven tumorigenesis. Cell Cycle. 2009;8:580–588. doi: 10.4161/cc.8.4.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamont FR, Tomlinson DC, Cooper PA, Shnyder SD, Chester JD, Knowles MA. Small molecule FGF receptor inhibitors block FGFR-dependent urothelial carcinoma growth in vitro and in vivo. Br J Cancer. 2011;104:75–82. doi: 10.1038/sj.bjc.6606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hase T, Kawashiri S, Tanaka A, et al. Correlation of basic fibroblast growth factor expression with the invasion and the prognosis of oral squamous cell carcinoma. J Oral Pathol Med. 2006;35:136–139. doi: 10.1111/j.1600-0714.2006.00397.x. [DOI] [PubMed] [Google Scholar]
- 5.Freier K, Schwaenen C, Sticht C, et al. Recurrent FGFR1 amplification and high FGFR1 protein expression in oral squamous cell carcinoma (OSCC) Oral Oncol. 2007;43:60–66. doi: 10.1016/j.oraloncology.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Marshall ME, Hinz TK, Kono SA, et al. Fibroblast growth factor receptors are components of autocrine signaling networks in head and neck squamous cell carcinoma cells. Clin Cancer Res. 2011;17:5016–5025. doi: 10.1158/1078-0432.CCR-11-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakulich C, Jackson-Boeters L, Daley TD, Wysocki GP. Immunohistochemical localization of growth factors fibroblast growth factor-1 and fibroblast growth factor-2 and receptors fibroblast growth factor receptor-2 and fibroblast growth factor receptor-3 in normal oral epithelium, epithelial dysplasias, and squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:573–579. doi: 10.1067/moe.2002.124461. [DOI] [PubMed] [Google Scholar]
- 8.Drugan CS, Paterson IC, Prime SS. Fibroblast growth factor receptor expression reflects cellular differentiation in human oral squamous carcinoma cell lines. Carcinogenesis. 1998;19:1153–1156. doi: 10.1093/carcin/19.6.1153. [DOI] [PubMed] [Google Scholar]
- 9.Vairaktaris E, Ragos V, Yapijakis C, et al. FGFR-2 and -3 play an important role in initial stages of oral oncogenesis. Anticancer Res. 2006;26:4217–4221. [PubMed] [Google Scholar]
- 10.Henson BJ, Gollin SM. Overexpression of KLF13 and FGFR3 in oral cancer cells. Cytogenet Genome Res. 2010;128:192–198. doi: 10.1159/000308303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Hartman YE, Warram JM, et al. Fibroblast growth factor receptor mediates fibroblast-dependent growth in EMMPRIN-depleted head and neck cancer tumor cells. Mol Cancer Res. 2011;9:1008–1017. doi: 10.1158/1541-7786.MCR-11-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenthal EL, Shreenivas S, Peters GE, Grizzle WE, Desmond R, Gladson CL. Expression of extracellular matrix metalloprotease inducer in laryngeal squamous cell carcinoma. Laryngoscope. 2003;113:1406–1410. doi: 10.1097/00005537-200308000-00027. [DOI] [PubMed] [Google Scholar]
- 13.Knights V, Cook SJ. De-regulated FGF receptors as therapeutic targets in cancer. Pharmacol Ther. 2010;125:105–117. doi: 10.1016/j.pharmthera.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Jeffers M, LaRochelle WJ, Lichenstein HS. Fibroblast growth factors in cancer: therapeutic possibilities. Expert Opin Ther Targets. 2002;6:469–482. doi: 10.1517/14728222.6.4.469. [DOI] [PubMed] [Google Scholar]
- 15.Korc M, Friesel RE. The role of fibroblast growth factors in tumor growth. Curr Cancer Drug Targets. 2009;9:639–651. doi: 10.2174/156800909789057006. [DOI] [PMC free article] [PubMed] [Google Scholar]