Abstract
Seven potassium Boc-protected secondary aminomethyltrifluoroborates were prepared in a standardized two step process. The Suzuki–Miyaura cross-coupling reaction was studied with this new class of nucleophiles, and a large variety of aryl and hetaryl chlorides provided the desired products in good to excellent yields, thereby allowing easy access to secondary aminomethyl substructures.
Aminomethylated arenes are readily found in many biologically active compounds and active pharmaceutical ingredients, with the secondary aminomethyl moiety of particular interest.
Secondary aminomethyated arenes can be prepared by several different synthetic methods. One of the most popular ways to install this moiety is the reductive amination using the corresponding aldehyde and a primary amine (Scheme 1, path a).1 However, many functional groups cannot be embedded in desirable substrates because of the harsh reduction conditions required for such transformations. Therefore, this pathway is unsuitable in many cases. Another conventional approach is the N-alkylation of primary amines (Scheme 1, path b).2 One of the drawbacks of this pathway is polyalkylation, which is frequently hard to control. Alkylation methods often give a mixture of secondary amines, tertiary amines, and even ammonium salts.2a,b
Scheme 1.
A more straightforward route to build secondary aminomethyl substructures would be transition metal catalyzed cross-coupling reactions of aminomethylmetallic reagents.3 Cross-coupling reactions, especially Suzuki–Miyaura coupling reactions, are normally more functional group tolerant compared to the reductive amination to install aminomethyl subunits. Therefore, the reactions are not limited by sensitive functional groups and can be much more general. Additionally, there is a much greater diversity of commercially available aryl and hetaryl halides for cross-coupling than of corresponding substrate partners for reductive amination or N-alkylation. To the best of our knowledge, only one cross-coupling to install secondary aminomethyl moieties reaction has been reported, and that approach employed secondary ammoniomethyl trifluoroborates as nucleophiles (Scheme 2, path a).4 Even though this method proved to be a good synthetic pathway to access the secondary aminomethyl moiety, the scope of the coupling reactions was limited to bromides as the electrophile, which are more expensive and appear in a less diverse range of substructures compared to chlorides. Furthermore, this previously developed method proved effective for only a few select hetaryl bromides.
Scheme 2.
To overcome these limitations and in continuation of our studies on aminomethylating agents, Boc-protected secondary aminomethyltrifluoroborates were imagined as good candidates for the synthesis of the secondary aminomethyl moieties (Scheme 2, path b).5 Boc-Protected primary aminomethyltrifluoroborate has already proven to be an excellent nucleophilic coupling partner in Suzuki–Miyaura cross-coupling reactions with a wide variety of aryl and hetaryl chlorides5a as well as mesylates.5b After deprotection of the Boc protecting group, the corresponding aminomethylated arenes are readily accessed.6 Therefore, we expected the development of Boc-protected secondary aminomethyltrifluoroborates would also be useful to synthesize secondary aminomethylated arenes.
In this contribution, we disclose the synthesis of potassium Boc-protected secondary aminomethyltrifluoroborates and their application as nucleophiles in the Suzuki–Miyaura cross-coupling reaction with a large variety of aryl and hetaryl chlorides.
Based on the development of Boc-protected primary aminomethyltrifluoroborate,5 we first tried to synthesize the secondary version through a ‘one-pot’ process. However, all methods tried were unsuccessful. As an alternative, the expected secondary aminomethyltrifluoroborates were synthesized over two steps: N-alkylation of Boc-protected amines with iodomethylpinacolboronate7 followed by addition of KHF2 (Table 1). Seven different Boc-protected secondary aminomethyltrifluoroborates were prepared in moderate to good yields. The acetal derivative 2g was successfully synthesized in 75% yield. Initial attempts to convert the pinacol boronate to the trifluoroborate failed because of the acidic reaction media. By adding 1 equivalent of K2CO3 before addition of KHF2, the desired acetal 3g was obtained in 53% isolated yield (Table 1, entry 7). Unfortunately, we were unable to access aniline derivatives under these conditions (Table, entry 8). Attempts to generate such substrates provided a mixture of the desired trifluoroborate 3h as well as Boc-deprotected material. These two trifluorobroates were inseparable.
Table 1.
Synthesis of Potassium Boc-Protected Secondary Aminomethyltrifluoroborates 3a–g
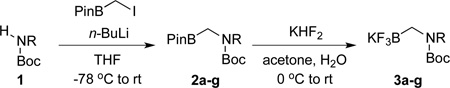 | |||
|---|---|---|---|
| entry | R | 2, yield (%) | 3, yield (%) |
| 1 | n-Bu | 2a, 65 | 3a, 67 |
| 2 | i-Pr | 2b, 69 | 3b, 71 |
| 3 | 2c, 68 | 3c, 87 | |
| 4 | 2d, 53 | 3d, 90 | |
| 5 | Bn | 2e, 41 | 3e, 74 |
| 6 | 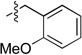 |
2f, 60 | 3f, 88 |
| 7 | 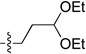 |
2g, 75 | 3g, 53a |
| 8 | Ph | 2h, 64 | 3h, -a,b |
1 equiv of K2CO3 was added before KHF2 was added.
A mixture of 3h and Boc-deprotected 3h.
With these aminomethyltrifluoroborates in hand, the Suzuki–Miyaura cross-coupling reaction was studied. n-Butyl Boc-protected aminomethyltrifluoroborate 3a and 4-chloroanisole were chosen as coupling partners to optimize the reaction conditions. Cross-coupling reactions were screened extensively with many different palladium catalysts, ligands, bases, solvents, concentrations, temperatures, and time. Buchwald’s second generation preformed catalyst A8 proved to be the most efficient catalyst, and thus the best conditions found were the combination of 4 mol % of XPhos-Pd-G2 A and 3 equiv of Cs2CO3 in toluene/H2O (4:1, 0.5 M) at 85 °C, allowing the reactions to go to completion in only 3 h.
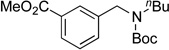
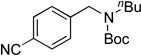
We applied these optimized conditions to various substituted aryl chlorides (Table 2). A large range of aryl chlorides was successfully cross-coupled to give the corresponding products in good to excellent yields. In general, aryl chlorides possessing electron neutral-entries 1–3) and electron donating groups (entries 4–6) on the aromatic ring were slightly better substrates than those containing electron withdrawing groups (entries 7–11). Interestingly, electrophiles that are sterically hindered at the ortho position gave even higher yields than less hindered ones (entries 3 and 5). Several functional groups, such as esters, nitriles, and nitro groups, were compatible with the reaction conditions, providing the desired products in high yields. When the reaction was performed on a larger scale (4 mmol of 4-chloroanisole), the catalyst loading was lowered to 2 mol %, affording the desired product 4d in 93% isolated yield (entry 4).
Table 2.
Cross-Coupling of Various Aryl Chlorides with Secondary Aminomethyltrifluoroborate 3a
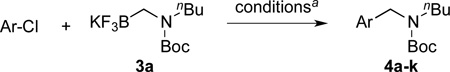 | ||||
|---|---|---|---|---|
| entry | Ar-Cl | product | yield (%) | |
| 1 | 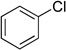 |
 |
4a | 91 |
| 2 | 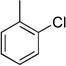 |
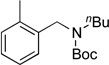 |
4b | 89 |
| 3 | 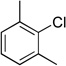 |
 |
4c | 95 |
| 4 |  |
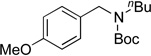 |
4d | 97(93)b |
| 5 |  |
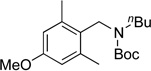 |
4e | 100 |
| 6 | 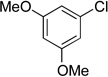 |
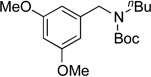 |
4f | 96 |
| 7 | 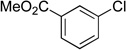 |
 |
4g | 97 |
| 8 | 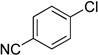 |
 |
4h | 93 |
| 9 | 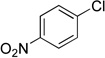 |
 |
4i | 86 |
| 10 |  |
 |
4j | 80 |
| 11 |  |
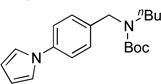 |
4k | 94 |
Reaction conditions: 1.0 equiv of aryl chloride 1.05 equiv of trifluoroborate, 4 mol % of XPhos-Pd-G2 A, 3 equiv of Cs2CO3, toluene/H2O = 4:1 (0.5 M), 85 °C, 3 h.
4 mmol scale of 4-chloroanisole, 2 mol % of XPhos-Pd-G2 A.
Next, the scope of the electrophiles was expanded to hetaryl chlorides (Table 3). Sulfur, oxygen, and nitrogen containing hetaryl chlorides proved to be good coupling partners with secondary aminomethyltrifluoroborates under the same set of reaction conditions. In some cases, a longer reaction time (18 h instead of 3 h) was required to complete the reactions (entries 4, 8, 9 and 11). Pyridine derivatives gave interesting results. The desired product was obtained in 85% yield with 3-chloropyridine and 92% yield with 5-chloro-2-methoxypyridine (entries 6 and 7). However, when 2-chloropyridine was tested, the product was not formed at all. Interestingly, 2-chloropyridine with a methoxy substituent on the ring gave the expected product in 49% isolated yield (entry 8). In the case of indoles, a protecting group on the nitrogen was required (entries 12 and 13). The desired products were not detected without the Boc protecting group. Additionally, in the cross-coupling of primary aminomethyltrifluoroborate, indole derivatives did not require a protecting group.6a Again, sensitive functional groups such as esters and aldehydes were tolerated throughout the coupling reactions.
Table 3.
Cross-Coupling of Various Hetaryl Chlorides with Secondary Aminomethyltrifluoroborate 3a
 | ||||
|---|---|---|---|---|
| entry | HetAr-Cl | product | yield (%) | |
| 1 | 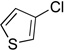 |
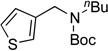 |
5a | 94 |
| 2 | 5b | 80 | ||
| 3 | 5c | 91 | ||
| 4 | 5d | 91a | ||
| 5 | 5e | 91 | ||
| 6 | 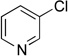 |
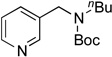 |
5f | 85a |
| 7 | 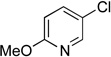 |
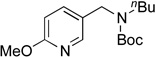 |
5g | 92 |
| 8 | 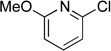 |
 |
5h | 49a |
| 9 |  |
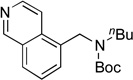 |
5i | 98a |
| 10 | 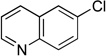 |
 |
5j | 99 |
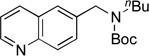
| 11 | 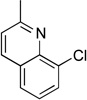 |
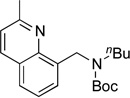 |
5k | 77a |
| 12 | 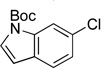 |
 |
5l | 82 |
| 13 |  |
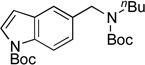 |
5m | 93 |
Reaction conditions: 1.0 equiv of hetaryl chloride 1.05 equiv of trifluoroborate, 4 mol % of XPhos-Pd-G2 A, 3 equiv of Cs2CO3, toluene/H2O = 4:1 (0.5 M), 85 °C, 3 h.
18 h.
We then examined the coupling reactions with seven different secondary aminomethyltrifluoroborates and 4-chloroanisole using the same set of reaction conditions developed (Table 4). All of them provided the desired product in good to excellent yields. Aliphatic alkyl-(entries 1 and 2) and cyclic alkyl groups on the nitrogen (entries 3 and 4) were good coupling partners, yielding the products in 69–97% yield. A benzyl group on the amine nitrogen gave the product in 90% yield (entry 5). With an electron donating group on the benzyl group, however, the yield dropped significantly to 73% (entry 6). The acetal derivative 3g also proved to be a good nucleophilic coupling partner, affording the expected product in 85% isolated yield (entry 7).
Table 4.
Cross-Coupling of 4-Chloroanisole with Various Secondary Aminomethyltrifluoroborates 3a–g
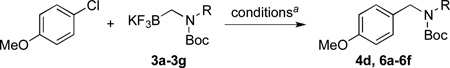 | ||||
|---|---|---|---|---|
| entry | R | product | yield (%) | |
| 1 | n-Bu |  |
4d | 97 |
| 2 | i-Pr |  |
6a | 83 |
| 3 |  |
6b | 69 | |
| 4 | 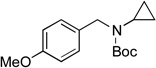 |
6c | 81 | |
| 5 | Bn | 6d | 90 | |
| 6 | 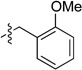 |
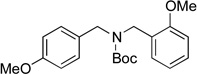 |
6e | 73 |
| 7 |  |
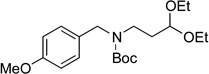 |
6f | 85 |
Reaction conditions: 1.0 equiv of 4-chloroanisole 1.05 equiv of trifluoroborate, 4 mol % of XPhos-Pd-G2 A, 3 equiv of Cs2CO3, toluene/H2O = 4:1 (0.5 M), 85 °C, 3 h.
To expand the scope of the electrophile, mesylates were tested as the coupling partners (eq 1). However, these reactions did not proceed using the same set of reaction conditions and only the starting mesylates were detected by 1H NMR after 3 h and 18 h stirring at 85 °C. Thus a different set of conditions will have to be developed for successful coupling of these electrophiles.
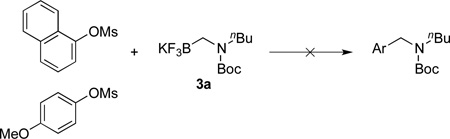 |
(1) |
In conclusion, a method has been developed to synthesize potassium Boc-protected secondary aminomethyltrifluoroborates, and seven such derivatives have been prepared. The Suzuki–Miyaura cross-coupling reaction of these aminomethyltrifluoroborates with various aryl and hetaryl chlorides was investigated. Employing this method, secondary aminomethylated arenes could be readily prepared after Boc deprotection.
Supplementary Material
Figure 2.
Pd preformed catalyst A and XPhos.
Acknowledgment
We thank NIGMS (1 R01 GM081376) for their support of this research. We are grateful to Johnson Matthey for providing the catalysts for this study. We also acknowledge Dr. Rakesh Kohli (University of Pennsylvania) for obtaining HRMS data.
Footnotes
Supporting Information Available Experimental procedures and spectral data of all compounds synthesized. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Mićović IV, Ivanović MD, Roglić GM, Kiricojević VD, Popović JB. J. Chem. Soc. Perkin Trans. 1995;1:265. [Google Scholar]; (b) Liao W, Chen Y, Liu Y, Duan H, Petersen JL, Shi X. Chem. Commun. 2009:6436. doi: 10.1039/b915361f. [DOI] [PubMed] [Google Scholar]; (c) Sato S, Sakamoto T, Miyazawa E, Kikugawa Y. Tetrahedron. 2004;60:7899. [Google Scholar]; (d) Menche D, Arikan F, Li J, Rudolph S. Org. Lett. 2007;9:267. doi: 10.1021/ol062715y. [DOI] [PubMed] [Google Scholar]
- 2.(a) Salvatore RN, Yoon CH, Jung KW. Tetrahedron. 2001;57:7785. [Google Scholar]; (b) Ikawa T, Fujita Y, Mizusaki T, Betsuin S, Takamatsu H, Maegawa T, Monguchi Y, Sajiki H. Org. Biomol. Chem. 2012;10:293. doi: 10.1039/c1ob06303k. [DOI] [PubMed] [Google Scholar]; (c) Ruano JLG, Parra A, Alemán J, Yuste F, Mastranzo VM. Chem. Commun. 2009:404. doi: 10.1039/b816846f. [DOI] [PubMed] [Google Scholar]; (d) Guillena G, Ramon DJ, Yus M. Chem. Rev. 2010;110:1611. doi: 10.1021/cr9002159. [DOI] [PubMed] [Google Scholar]
- 3.(a) Molander GA, Fleury-Brégeot N, Hiebel M-A. Org. Lett. 2011;13:1694. doi: 10.1021/ol200202g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Molander GA, Hiebel M-A. Org. Lett. 2010;12:4876. doi: 10.1021/ol102039c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tanaka K. PCT Int. Appl. 2008 WO 2008007670. [Google Scholar]; (d) Devulapally R, Fleury-Brégeot N, Molander GA, Seapy DG. Tetrahedron Lett. 2012;53:1051. doi: 10.1016/j.tetlet.2011.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Murai N, Miyano M, Yonaga M, Tanaka K. Org. Lett. 2012;14:2818. doi: 10.1021/ol301037s. [DOI] [PubMed] [Google Scholar]
- 4.Fleury-Brégeot N, Raushel J, Sandrock DL, Dreher SD, Molander GA. Chem. - Eur. J. 2012 doi: 10.1002/chem.201200831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Molander GA, Shin I. Org. Lett. 2011;13:3956. doi: 10.1021/ol2014768. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Molander GA, Shin I. Org. Lett. 2012;14:3138. doi: 10.1021/ol301221p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Greene TW, Wuts PGM. Protective Groups in Organic Synthesis. 3rd ed. New York: John Wiley & Sons; 1999. [Google Scholar]; (b) Kocienski PJ. Protecting Groups. 3rd ed. Stuttgart, New York: Georg Thieme; 2005. [Google Scholar]; (c) du Vigneaud V, Behrens OK. J. Biol. Chem. 1937;117:27. [Google Scholar]; (d) Kharasch MS, Priestley HM. J. Am. Chem. Soc. 1939;61:3425. [Google Scholar]; (e) Snyder HR, Heckert RE. J. Am. Chem. Soc. 1952;74:2006. [Google Scholar]; (f) Li S, Gortler LB, Waring A, Battisti A, Bank S, Closson WD, Wriede P. J. Am. Chem. Soc. 1967;89:5311. [Google Scholar]
- 7.(a) Smoum R, Rubinstein A, Srebnik M. Bioorg. Chem. 2003;31:464. doi: 10.1016/j.bioorg.2003.08.004. [DOI] [PubMed] [Google Scholar]; (b) Mattesson DS. Chem. Rev. 1989;89:1535. [Google Scholar]
- 8.Kinzel T, Zhang Y, Buchwald SL. J. Am. Chem. Soc. 2010;132:14073. doi: 10.1021/ja1073799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






