Abstract
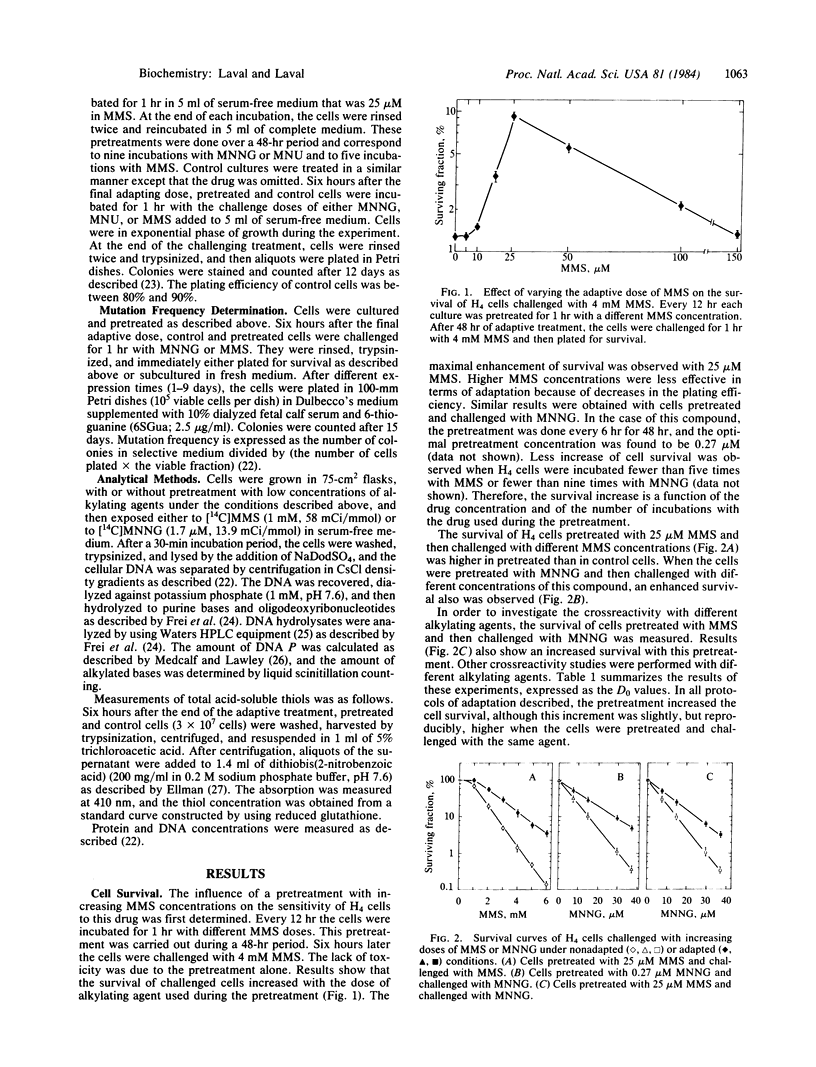
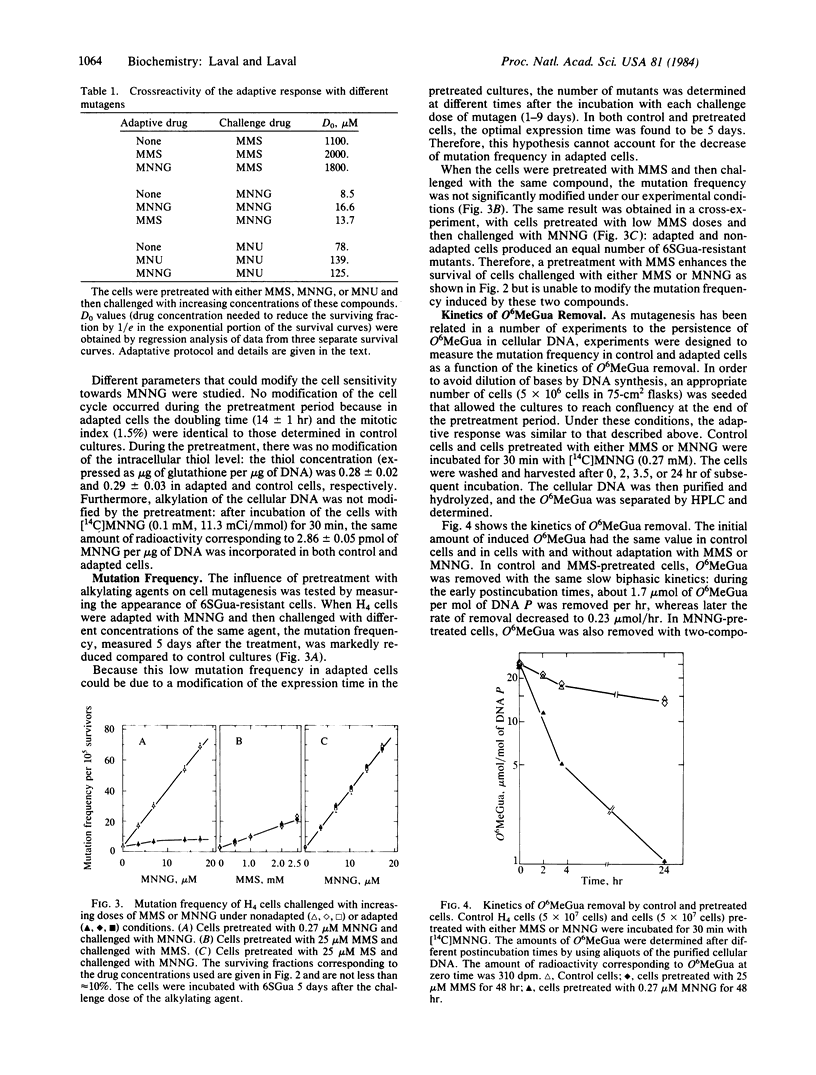
Pretreatment of H4 (rat hepatoma) cells for 48 hr with low nontoxic doses of alkylating agents [methyl methanesulfonate (MMS), N-methyl-N'-nitro-N-nitrosoguanidine (MNNG), and N-methyl-N-nitrosourea] renders the cells more resistant to the toxic effect of these compounds. Crossreactivity for survival is also observed with the different alkylating agents tested. Pretreatment with MNNG enables the cells to be less mutated than control cultures during a subsequent challenge with high doses of this compound. However, pretreatment with MMS does not modify the mutation frequency of cells challenged with either MMS or MNNG. The adaptive response to mutagenesis is correlated with a faster and more efficient removal of O6-methylguanine in MNNG-pretreated cells as compared to control cultures, whereas the disappearance of this lesion is not modified in MMS-pretreated cells. As MMS produces less methylation at the O6 position of guanine and more methylation at the N7 position in comparison to MNNG, the results suggest that: (i) N7-methylguanine is not implicated in the adaptive response and (ii) adaptation to mutagenesis can be correlated with the amount of O6-methylguanine induced during the pretreatment. The effect of pretreatment on other O-alkylated derivatives is not known.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cairns J., Robins P., Sedgwick B., Talmud P. The inducible repair of alkylated DNA. Prog Nucleic Acid Res Mol Biol. 1981;26:237–244. doi: 10.1016/s0079-6603(08)60408-0. [DOI] [PubMed] [Google Scholar]
- Crathorn A. R., Shackleton J. Repair processes and the response of dividing and non-dividing cells to methyl methanesulphonate and dimethyl sulphate. Chem Biol Interact. 1976 Oct 2;15(2):117–130. doi: 10.1016/0009-2797(76)90157-5. [DOI] [PubMed] [Google Scholar]
- Demple B., Jacobsson A., Olsson M., Robins P., Lindahl T. Repair of alkylated DNA in Escherichia coli. Physical properties of O6-methylguanine-DNA methyltransferase. J Biol Chem. 1982 Nov 25;257(22):13776–13780. [PubMed] [Google Scholar]
- Durrant L. G., Margison G. P., Boyle J. M. Pretreatment of Chinese hamster v79 cells with MNU increases survival without affecting DNA repair or mutagenicity. Carcinogenesis. 1981;2(1):55–60. doi: 10.1093/carcin/2.1.55. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Evensen G., Seeberg E. Adaptation to alkylation resistance involves the induction of a DNA glycosylase. Nature. 1982 Apr 22;296(5859):773–775. doi: 10.1038/296773a0. [DOI] [PubMed] [Google Scholar]
- Foote R. S., Mitra S., Pal B. C. Demethylation of O6-methylguanine in a synthetic DNA polymer by an inducible activity in Escherichia coli. Biochem Biophys Res Commun. 1980 Nov 28;97(2):654–659. doi: 10.1016/0006-291x(80)90314-9. [DOI] [PubMed] [Google Scholar]
- Frei J. V., Swenson D. H., Warren W., Lawley P. D. Alkylation of deoxyribonucleic acid in vivo in various organs of C57BL mice by the carcinogens N-methyl-N-nitrosourea, N-ethyl-N-nitrosourea and ethyl methanesulphonate in relation to induction of thymic lymphoma. Some applications of high-pressure liquid chromatography. Biochem J. 1978 Sep 15;174(3):1031–1044. doi: 10.1042/bj1741031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham J. W., Greenberg D. S., Kaufman D. G., Smith G. J. Cycle-related toxicity and transformation in 10T1/2 cells treated with N-methyl-N'-nitro-N-nitrosoguanidine. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4813–4817. doi: 10.1073/pnas.77.8.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G., Lawley P. D., Olsen I. Mode of action of methylating carcinogens: comparative studies of murine and human cells. Carcinogenesis. 1981;2(5):403–411. doi: 10.1093/carcin/2.5.403. [DOI] [PubMed] [Google Scholar]
- Jeggo P., Defais T. M., Samson L., Schendel P. An adaptive response of E. coli to low levels of alkylating agent: comparison with previously characterised DNA repair pathways. Mol Gen Genet. 1977 Nov 29;157(1):1–9. doi: 10.1007/BF00268680. [DOI] [PubMed] [Google Scholar]
- Kaina B. Enhanced survival and reduced mutation and aberration frequencies induced in V79 chinese hamster cells pre-exposed to low levels of methylating agents. Mutat Res. 1982 Mar;93(1):195–211. doi: 10.1016/0027-5107(82)90135-x. [DOI] [PubMed] [Google Scholar]
- Karran P., Hjelmgren T., Lindahl T. Induction of a DNA glycosylase for N-methylated purines is part of the adaptive response to alkylating agents. Nature. 1982 Apr 22;296(5859):770–773. doi: 10.1038/296770a0. [DOI] [PubMed] [Google Scholar]
- Laval F. Effect of uncouplers on radiosensitivity and mutagenicity in x-irradiated mammalian cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2702–2705. doi: 10.1073/pnas.77.5.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laval F., Little J. B. Enhancement of survival of X-irradiated mammalian cells by the uncoupler of oxidative phosphorylation, m-chloro carbonyl cyanide phenylhydrazone. Radiat Res. 1977 Sep;71(3):571–578. [PubMed] [Google Scholar]
- Laval J., Pierre J., Laval F. Release of 7-methylguanine residues from alkylated DNA by extracts of Micrococcus luteus and Escherichia coli. Proc Natl Acad Sci U S A. 1981 Feb;78(2):852–855. doi: 10.1073/pnas.78.2.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Thatcher C. J. Methylation of deoxyribonucleic acid in cultured mammalian cells by N-methyl-N'-nitro-N-nitrosoguanidine. The influence of cellular thiol concentrations on the extent of methylation and the 6-oxygen atom of guanine as a site of methylation. Biochem J. 1970 Feb;116(4):693–707. doi: 10.1042/bj1160693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc J. P., Martin B., Cadet J., Laval J. Uracil-DNA glycosylase. Purification and properties of uracil-DNA glycosylase from Micrococcus luteus. J Biol Chem. 1982 Apr 10;257(7):3477–3483. [PubMed] [Google Scholar]
- Lewis J. G., Swenberg J. A. Differential repair of O(6)-methylguanine in DNA of rat hepatocytes and nonparenchymal cells. Nature. 1980 Nov 13;288(5787):185–141. doi: 10.1038/288185a0. [DOI] [PubMed] [Google Scholar]
- Margison G. P., Pegg A. E. Enzymatic release of 7-methylguanine from methylated DNA by rodent liver extracts. Proc Natl Acad Sci U S A. 1981 Feb;78(2):861–865. doi: 10.1073/pnas.78.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medcalf A. S., Lawley P. D. Time course of O6-methylguanine removal from DNA of N-methyl-N-nitrosourea-treated human fibroblasts. Nature. 1981 Feb 26;289(5800):796–798. doi: 10.1038/289796a0. [DOI] [PubMed] [Google Scholar]
- Montesano R. Alkylation of DNA and tissue specificity in nitrosamine carcinogenesis. J Supramol Struct Cell Biochem. 1981;17(3):259–273. doi: 10.1002/jsscb.380170307. [DOI] [PubMed] [Google Scholar]
- Montesano R., Brésil H., Margison G. P. Increased excision of O6-methylguanine from rat liver DNA after chronic administration of dimethylnitrosamine. Cancer Res. 1979 May;39(5):1798–1802. [PubMed] [Google Scholar]
- Olsson M., Lindahl T. Repair of alkylated DNA in Escherichia coli. Methyl group transfer from O6-methylguanine to a protein cysteine residue. J Biol Chem. 1980 Nov 25;255(22):10569–10571. [PubMed] [Google Scholar]
- Pegg A. E., Wiest L., Foote R. S., Mitra S., Perry W. Purification and properties of O6-methylguanine-DNA transmethylase from rat liver. J Biol Chem. 1983 Feb 25;258(4):2327–2333. [PubMed] [Google Scholar]
- Renard A., Verly W. G. Kinetic analysis of O6-ethylguanine disappearance from DNA catalyzed by the chromatin factor of rat liver. FEBS Lett. 1980 Dec 29;122(2):271–274. doi: 10.1016/0014-5793(80)80454-6. [DOI] [PubMed] [Google Scholar]
- Samson L., Cairns J. A new pathway for DNA repair in Escherichia coli. Nature. 1977 May 19;267(5608):281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- Samson L., Schwartz J. L. Evidence for an adaptive DNA repair pathway in CHO and human skin fibroblast cell lines. Nature. 1980 Oct 30;287(5785):861–863. doi: 10.1038/287861a0. [DOI] [PubMed] [Google Scholar]
- Schwartz J. L., Samson L. Mutation induction in Chinese hamster ovary cells after chronic pretreatment with MNNG. Mutat Res. 1983 Mar;119(3):393–397. doi: 10.1016/0165-7992(83)90191-4. [DOI] [PubMed] [Google Scholar]
- Sedgwick B., Robins P. Isolation of mutants of Escherichia coli with increased resistance to alkylating agents: mutants deficient in thiols and mutants constitutive for the adaptive response. Mol Gen Genet. 1980;180(1):85–90. doi: 10.1007/BF00267355. [DOI] [PubMed] [Google Scholar]
- Singer B., Brent T. P. Human lymphoblasts contain DNA glycosylase activity excising N-3 and N-7 methyl and ethyl purines but not O6-alkylguanines or 1-alkyladenines. Proc Natl Acad Sci U S A. 1981 Feb;78(2):856–860. doi: 10.1073/pnas.78.2.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Kuśmierek J. T. Chemical mutagenesis. Annu Rev Biochem. 1982;51:655–693. doi: 10.1146/annurev.bi.51.070182.003255. [DOI] [PubMed] [Google Scholar]
- Singer B. The chemical effects of nucleic acid alkylation and their relation to mutagenesis and carcinogenesis. Prog Nucleic Acid Res Mol Biol. 1975;15(0):219–284. [PubMed] [Google Scholar]
- Sklar R., Brady K., Strauss B. Limited capacity for the removal of O6-methylguanine and its regeneration in a human lymphoma line. Carcinogenesis. 1981;2(12):1293–1298. doi: 10.1093/carcin/2.12.1293. [DOI] [PubMed] [Google Scholar]
- Thomas L., Yang C. H., Goldthwait D. A. Two DNA glycosylases in Escherichia coli which release primarily 3-methyladenine. Biochemistry. 1982 Mar 16;21(6):1162–1169. doi: 10.1021/bi00535a009. [DOI] [PubMed] [Google Scholar]
- Waldstein E. A., Cao E. H., Setlow R. B. Adaptive increase of O6-methylguanine-acceptor protein in HeLa cells following N-methyl-N'-nitro-N-nitrosoguanidine treatment. Nucleic Acids Res. 1982 Aug 11;10(15):4595–4604. doi: 10.1093/nar/10.15.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]



